Abstract
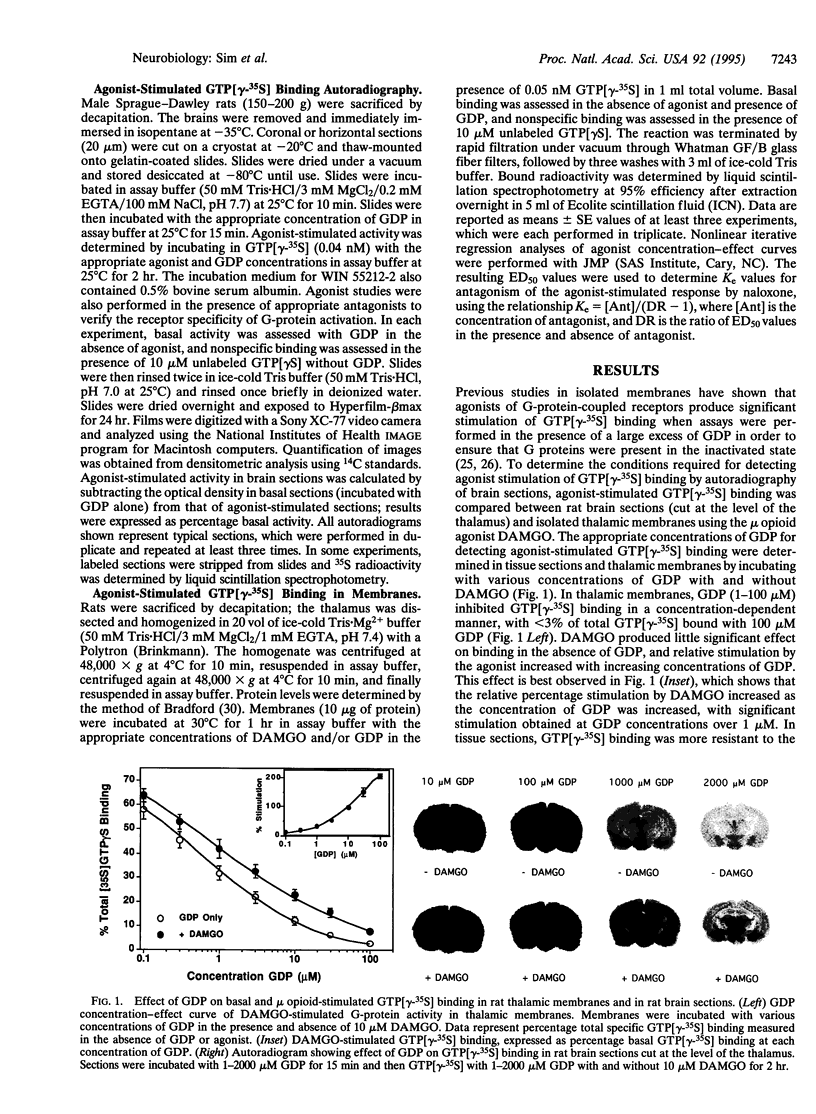
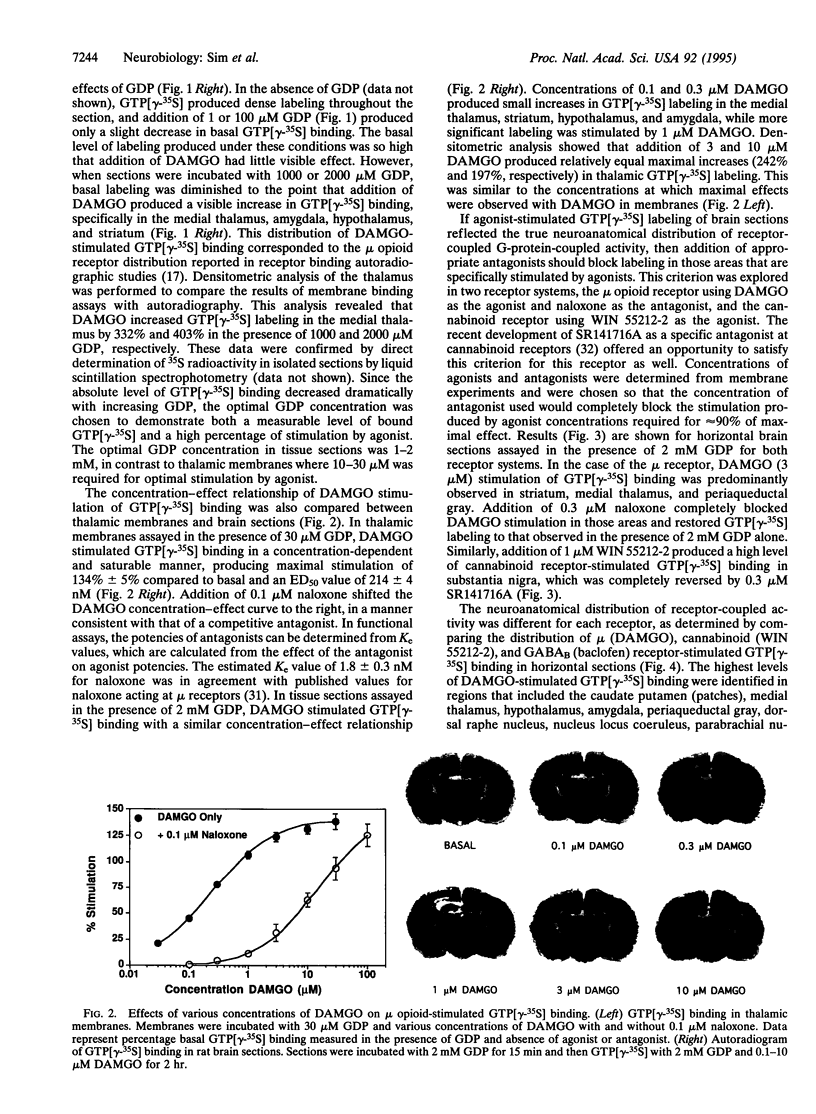
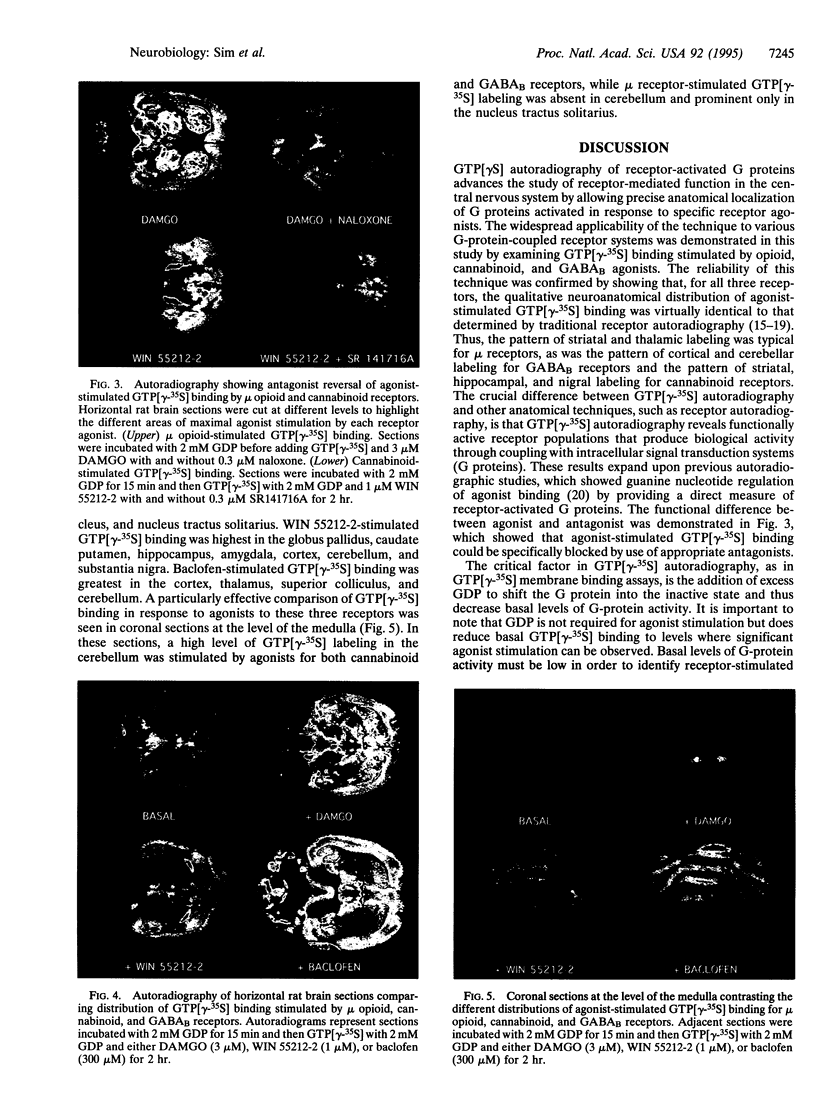
Agonists stimulate guanylyl 5'-[gamma-[35S]thio]-triphosphate (GTP[gamma-35S]) binding to receptor-coupled guanine nucleotide binding protein (G proteins) in cell membranes as revealed in the presence of excess GDP. We now report that this reaction can be used to neuroanatomically localize receptor-activated G proteins in brain sections by in vitro autoradiography of GTP[gamma-35S] binding. Using the mu opioid-selective peptide [D-Ala2,N-MePhe4,Gly5-ol]enkephalin (DAMGO) as an agonist in rat brain sections and isolated thalamic membranes, agonist stimulation of GTP[gamma-35S] binding required the presence of excess GDP (1-2 mM GDP in sections vs. 10-30 microM GDP in membranes) to decrease basal G-protein activity and reveal agonist-stimulated GTP[gamma-35S] binding. Similar concentrations of DAMGO were required to stimulate GTP[gamma-35S] binding in sections and membranes. To demonstrate the general applicability of the technique, agonist-stimulated GTP[gamma-35S] binding in tissue sections was assessed with agonists for the mu opioid (DAMGO), cannabinoid (WIN 55212-2), and gamma-aminobutyric acid type B (baclofen) receptors. For opioid and cannabinoid receptors, agonist stimulation of GTP[gamma-35S] binding was blocked by incubation with agonists in the presence of the appropriate antagonists (naloxone for mu opioid and SR-141716A for cannabinoid), thus demonstrating that the effect was specifically receptor mediated. The anatomical distribution of agonist-stimulated GTP[gamma-35S] binding qualitatively paralleled receptor distribution as determined by receptor binding autoradiography. However, quantitative differences suggest that variations in coupling efficiency may exist between different receptors in various brain regions. This technique provides a method of functional neuroanatomy that identifies changes in the activation of G proteins by specific receptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki C., Go C. G., Wu K., Siekevitz P. Light and electron microscopic localization of alpha subunits of GTP-binding proteins, G(o) and Gi, in the cerebral cortex and hippocampus of rat brain. Brain Res. 1992 Nov 20;596(1-2):189–201. doi: 10.1016/0006-8993(92)91547-r. [DOI] [PubMed] [Google Scholar]
- Asano T., Pedersen S. E., Scott C. W., Ross E. M. Reconstitution of catecholamine-stimulated binding of guanosine 5'-O-(3-thiotriphosphate) to the stimulatory GTP-binding protein of adenylate cyclase. Biochemistry. 1984 Nov 6;23(23):5460–5467. doi: 10.1021/bi00318a013. [DOI] [PubMed] [Google Scholar]
- Asano T., Shinohara H., Morishita R., Kato K. Immunochemical and immunohistochemical localization of the G protein Gi1 in rat central nervous tissues. J Biochem. 1990 Dec;108(6):988–994. doi: 10.1093/oxfordjournals.jbchem.a123326. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Ionic channels and their regulation by G protein subunits. Annu Rev Physiol. 1990;52:197–213. doi: 10.1146/annurev.ph.52.030190.001213. [DOI] [PubMed] [Google Scholar]
- Chen Y., Mestek A., Liu J., Hurley J. A., Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol. 1993 Jul;44(1):8–12. [PubMed] [Google Scholar]
- Childers S. R. Opioid receptor-coupled second messenger systems. Life Sci. 1991;48(21):1991–2003. doi: 10.1016/0024-3205(91)90154-4. [DOI] [PubMed] [Google Scholar]
- Chu D. C., Albin R. L., Young A. B., Penney J. B. Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neuroscience. 1990;34(2):341–357. doi: 10.1016/0306-4522(90)90144-s. [DOI] [PubMed] [Google Scholar]
- Devane W. A., Dysarz F. A., 3rd, Johnson M. R., Melvin L. S., Howlett A. C. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988 Nov;34(5):605–613. [PubMed] [Google Scholar]
- Florio V. A., Sternweis P. C. Mechanisms of muscarinic receptor action on Go in reconstituted phospholipid vesicles. J Biol Chem. 1989 Mar 5;264(7):3909–3915. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Snyder S. H., Kuhar M. J., Young W. S., 3rd Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Lynn A. B., Johnson M. R., Melvin L. S., de Costa B. R., Rice K. C. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991 Feb;11(2):563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987 Oct;23(1):1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Pert C. B. Light microscopic localization of brain opiate receptors: a general autoradiographic method which preserves tissue quality. J Neurosci. 1982 Aug;2(8):1129–1149. doi: 10.1523/JNEUROSCI.02-08-01129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. D., Sekura R. D., Codina J., Iyengar R., Manclark C. R., Birnbaumer L. Stimulation and inhibition of adenylyl cyclases mediated by distinct regulatory proteins. Nature. 1983 Apr 21;302(5910):706–709. doi: 10.1038/302706a0. [DOI] [PubMed] [Google Scholar]
- Jansen E. M., Haycock D. A., Ward S. J., Seybold V. S. Distribution of cannabinoid receptors in rat brain determined with aminoalkylindoles. Brain Res. 1992 Mar 13;575(1):93–102. doi: 10.1016/0006-8993(92)90428-c. [DOI] [PubMed] [Google Scholar]
- Keith D. E., Jr, Anton B., Evans C. J. Characterization and mapping of a delta opioid receptor clone from NG108-15 cells. Proc West Pharmacol Soc. 1993;36:299–306. [PubMed] [Google Scholar]
- Kuster J. E., Stevenson J. I., Ward S. J., D'Ambra T. E., Haycock D. A. Aminoalkylindole binding in rat cerebellum: selective displacement by natural and synthetic cannabinoids. J Pharmacol Exp Ther. 1993 Mar;264(3):1352–1363. [PubMed] [Google Scholar]
- Lewis E. J., Harrington C. A., Chikaraishi D. M. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen A., Fuss M., Vogt H., Schwabe U. Measurement of guanine nucleotide-binding protein activation by A1 adenosine receptor agonists in bovine brain membranes: stimulation of guanosine-5'-O-(3-[35S]thio)triphosphate binding. Mol Pharmacol. 1993 Jul;44(1):115–123. [PubMed] [Google Scholar]
- Matsuda L. A., Bonner T. I., Lolait S. J. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993 Jan 22;327(4):535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990 Aug 9;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Nijssen P. C., Sexton T., Childers S. R. Opioid-inhibited adenylyl cyclase in rat brain membranes: lack of correlation with high-affinity opioid receptor binding sites. J Neurochem. 1992 Dec;59(6):2251–2262. doi: 10.1111/j.1471-4159.1992.tb10118.x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M., Barth F., Héaulme M., Shire D., Calandra B., Congy C., Martinez S., Maruani J., Néliat G., Caput D. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994 Aug 22;350(2-3):240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Sim L. J., Selley D. E., Tsai K. P., Morris M. Calcium and cAMP mediated stimulation of Fos in cultured hypothalamic tyrosine hydroxylase-immunoreactive neurons. Brain Res. 1994 Aug 8;653(1-2):155–160. doi: 10.1016/0006-8993(94)90384-0. [DOI] [PubMed] [Google Scholar]
- Sim L. J., Selley D. E., Tsai K. P., Morris M. D2 inhibition of stimulated Fos immunoreactivity in cultured tyrosine hydroxylase-ir hypothalamic neurons. Brain Res. 1994 Jul 18;651(1-2):311–316. doi: 10.1016/0006-8993(94)90711-0. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Mansour A., Akil H., Watson S. J. Cloning and pharmacological characterization of a rat mu opioid receptor. Neuron. 1993 Nov;11(5):903–913. doi: 10.1016/0896-6273(93)90120-g. [DOI] [PubMed] [Google Scholar]
- Traynor J. R., Nahorski S. R. Modulation by mu-opioid agonists of guanosine-5'-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 1995 Apr;47(4):848–854. doi: 10.1016/S0026-895X(25)08634-1. [DOI] [PubMed] [Google Scholar]
- Wojcik W. J., Neff N. H. gamma-aminobutyric acid B receptors are negatively coupled to adenylate cyclase in brain, and in the cerebellum these receptors may be associated with granule cells. Mol Pharmacol. 1984 Jan;25(1):24–28. [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Van Dop C., Neer E. J., Snyder S. H. Go, a guanine nucleotide-binding protein: immunohistochemical localization in rat brain resembles distribution of second messenger systems. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4561–4565. doi: 10.1073/pnas.83.12.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbin M. A., Palacios J. M., Wamsley J. K., Kuhar M. J. Axonal transport of beta-adrenergic receptors. Antero- and retrogradely transported receptors differ in agonist affinity and nucleotide sensitivity. Mol Pharmacol. 1983 Sep;24(2):341–348. [PubMed] [Google Scholar]