Abstract
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide and results in an economic and social burden that is both substantial and increasing. The natural history of COPD is punctuated by exacerbations which have major short- and long-term implications on the patient and healthcare system. Evidence-based guidelines stipulate that early detection and prompt treatment of exacerbations are essential to ensure optimal outcomes and to reduce the burden of COPD. Several factors can identify populations at risk of exacerbations. Implementing prevention measures in patients at risk is a major goal in the management of COPD.
Keywords: bronchodilators, chronic bronchitis, chronic obstructive pulmonary disease, emphysema, exacerbation, prevention, treatment
Introduction
Chronic obstructive pulmonary disease (COPD), a common preventable and treatable disease, is characterized by persistent airflow limitation that is usually progressive and that is caused by an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases [GOLD, 2013]. COPD is a major cause of morbidity and mortality worldwide and results in an economic and social burden that is both substantial and increasing [Lopez et al. 2006]. COPD prevalence, morbidity and mortality vary across countries. In the USA, COPD affects approximately 24 million Americans, results in about 120,000 deaths a year and is now the third leading cause of death [Kochanek et al. 2011].
The natural history of COPD is punctuated by exacerbations which have major implications on the patient and healthcare system. In this review we provide a concise overview of COPD exacerbations and their impact, outlining the population at risk, etiology and current management and preventive strategies.
Impact of COPD exacerbations
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines a COPD exacerbation as an event in the natural course of the disease that is characterized by a change in the patient’s baseline dyspnea, cough and sputum that is beyond normal day-to-day variations, is acute in onset and warrants a change in regular medication [GOLD, 2011].
Exacerbations of COPD impose a substantial burden on healthcare systems worldwide; they are a major cause of morbidity, mortality and poor health status [Seemungal et al. 1998]. Furthermore, they account for the majority of hospital admissions. More than 50% of the total cost of COPD is accounted for by services related to exacerbations. For instance, in the UK, they are the most common cause of medical hospital admission, accounting for 15·9% of hospital admissions, at a cost to the National Health System of over £253 million a year [British Thoracic Society, 2006].
Exacerbations of COPD have short- and long-term clinical implications. The time course of recovery of symptoms during an acute exacerbation was evaluated by one study which showed that in 50% of community-treated exacerbations, patients recovered to baseline symptoms within 7 days. However, in 14% of these events patients’ symptoms did not return to baseline 35 days following the onset and in some patients symptoms never returned to the baseline level [Seemungal et al. 2000].
Recurrent exacerbations are associated with accelerated decline in lung function that is the hallmark of COPD. In one study, frequent exacerbators had a decline in forced expiratory volume in 1 s (FEV1) of 40.1 ml/year [95% confidence interval (CI) 38–42] versus 32.1 ml/year (95% CI 31–33) in those with no or infrequent exacerbations (p < 0.05) [Donaldson et al. 2002]. More recently, a 3-year longitudinal cohort study demonstrated that exacerbations experienced during the study were associated with an excess decline in lung function (FEV1) with a mean loss of 2 ml per year per exacerbation (p < 0.001) [Vestbo et al. 2011].
Furthermore, frequent exacerbations are associated with reduced physical activity [Donaldson et al. 2005], poorer quality of life and even an increased risk of death [Soler-Cataluña et al. 2005] (Figure 1).
Figure 1.
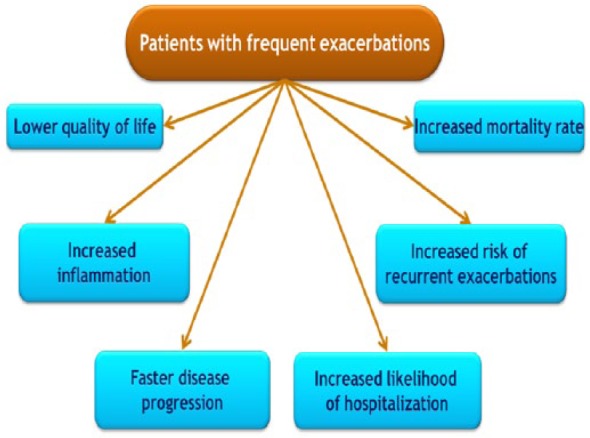
Impact of chronic obstructive pulmonary disease exacerbations.
Population at risk
Table 1 shows risk factors associated with increased COPD exacerbation. An ‘exacerbator’ phenotype for COPD has recently been described using data from large COPD cohorts, such as the COPD Gene study and the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study. Hurst and colleagues demonstrated that COPD exacerbations are not random events but cluster together in time such that there is a high-risk period for recurrent exacerbations in the 8-week period after the initial exacerbation [Hurst et al. 2009]. Furthermore, analysis of exacerbations in 2138 patients enrolled in the ECLIPSE study demonstrated that exacerbations were more frequent (two or more) and more severe (associated with hospitalization) with increased severity of the disease defined using spirometry measures. Overall, 22% of patients with stage II disease, 33% with stage III and 47% with stage IV had frequent exacerbations in the first year of follow up. In the same study, the single best predictor of exacerbations, across all GOLD stages, was a past history of exacerbations. Other predictors included health status, presence of gastro-esophageal reflux and increased white blood cells (WBCs) [Hurst and Vestbo, 2010]. In patients seeking medical care, dyspnea may in fact be defined as chronic cough and phlegm production for more than 3 months/year for 2 consecutive years, was associated with worse respiratory symptoms and more exacerbations in patients with COPD [Kim et al. 2011].
Table 1.
Risk factors for chronic obstructive pulmonary disease (COPD) exacerbations [Miravitles et al. 2000; Niewoehner et al. 2007; Anzueto et al. 2007].
| Age | Prior use of COPD medications |
| Severity of airway obstruction | Bacterial colonization |
| Chronic bronchial mucus production | Comorbid conditions like cardiovascular disease |
| Longer duration of COPD | Poor health-related quality of life |
| Productive cough and wheezing | Prior history of exacerbations |
| Antibiotic or systemic steroid use |
Etiology of COPD exacerbation
It is estimated that 70–80% of COPD exacerbations are triggered by viral or bacterial respiratory infections (Table 2) [Sethi and Murphy, 2008]. The remaining 20–30% are associated with exposure to environmental pollution or have an unknown etiology [Sapey and Stockley, 2006]. COPD exacerbations may be mimicked by other medical conditions [Beghé et al. 2013]. Occasionally, the presence of congestive heart failure and pneumonia may be difficult to distinguish from an acute exacerbation because, in severe disease, the characteristic radiologic features of these conditions may be masked. Moreover, these two conditions as well as other multiple comorbid conditions may complicate an exacerbation [Clini et al. 2013; Roca et al 2013]. The patients seeking medical care with symptom of dyspnea may in fact be related to their multiple comorbid condition and not necessarily a true COPD exacerbation. Hence, this should always be kept in mind when managing these patients with COPD exacerbations.
Table 2.
Pathogens responsible for chronic obstructive pulmonary disease exacerbations.
| Microbe | Role in exacerbations |
|---|---|
| Bacteria | |
| Haemophilus influenzae | 20–30% |
| Streptococcus pneumoniae | 10–15% |
| Moraxella catarrhalis | 10–15% |
| Psuedomonas aeruginosa | 5–10% |
| Enterobacteriaceae | Undefined |
| H. hemolyticus | Undefined |
| H. parainfluenza | Undefined |
| Staphylococcus aureus | Undefined |
| Viruses | |
| Rhinovirus | 10–25% |
| Parainfluenza virus | 5–10% |
| Influenza virus | 5–10% |
| Respiratory syncytial virus | 5–10% |
| Adenovirus | 3–5% |
| Coronavirus | 3–5% |
| Human metapneumovirus | 3–5% |
| Atypical bacteria | |
| Chlamydophilia pneumoniae | 3–5% |
| Mycoplasma pneumoniae | 1–2% |
| Fungi | |
| Pneumocystis jeroveci | Undefined |
Limited studies suggest deep venous thrombosis and pulmonary embolism are associated with acute exacerbations [Erelel et al. 2002]. The relationship between COPD exacerbation and pulmonary embolism was illustrated by a meta-analysis of five observational studies [Rizkallah et al. 2009]. Among the 550 patients having a COPD exacerbation, the prevalence of pulmonary embolism was 20%. The prevalence was even higher (25%) among those hospitalized.
Pathophysiologic consequences of COPD exacerbations
In the past decade, the understanding of COPD has evolved from a disease limited to the airways to a more complex disease frequently associated with systemic inflammation and other chronic comorbidities [Clini et al. 2013]. COPD exacerbation has been associated with a proinflammatory and prothrombotic state. A recent study demonstrated elevated levels of interleukin 6, von Willebrand’s factor, D dimer and prothrombin fragment 1+2 being surrogate markers for inflammation, endothelial damage and clotting activation respectively during an exacerbation of COPD with a return of these levels to baseline with recovery [Polosa et al. 2011].
Management strategies of COPD exacerbations
The goals of management of COPD exacerbation are to minimize the impact of the current exacerbation and prevent the development of subsequent exacerbations. Depending on the severity, an exacerbation can be managed in an outpatient or inpatient setting. Most of the time outpatient therapy is sufficient with pharmacologic therapies, including bronchodilators, corticosteroids and antibiotics.
Based on available evidence, early detection and aggressive prompt management of exacerbations are warranted to ensure optimal outcome. Unfortunately, many patients with COPD fail to report their exacerbations to their healthcare providers. It is therefore imperative to educate patients about the signs and symptoms of exacerbations in an attempt to build a self-management plan that may help them seek advice early in the course of exacerbations [American Thoracic Society/European Respiratory Society, 2010].
Pharmacological interventions
Several pharmacological interventions outlined below are used in the management of a COPD exacerbation.
Inhaled bronchodilators
Short-acting inhaled β2 agonists (SABAs) and anticholinergic agents (short-acting muscarinic antagonists, SAMAs) remain the mainstay in the treatment of symptoms and airflow obstruction during exacerbations. SABAs such as albuterol act by increasing the concentration of cyclic adenosine monophosphate [Johnson and Rennard, 2001], while SAMAs such as ipratropium bromide are nonselective muscarinic antagonists [McCrory and Brown, 2002].
There is no evidence of a difference between classes of short-acting bronchodilators in terms of improvement in lung function (improvement in FEV1 range from 150 to 250 ml) at 90 min [Karpel et al. 1990]. When inhaled, the effects of SABAs begin within 5 min with maximum peaks at 30 min. In contrast, ipratropium bromide begins to take effect after 10–15 min, with a peak at 30–60 min. The effects of these two classes of bronchodilators decline after 2–3 h but can last as long as 4–6 h, depending on their individual properties.
The efficacy of combinations of short-acting bronchodilators remains controversial in the acute management of COPD. Unlike stable COPD where the simultaneous concurrent administration of SABAs and SAMAs has been shown to be more efficacious than either agent given alone [COMBIVENT Inhalation Solution Study Group, 1997], a combination of short-acting bronchodilators given sequentially in exacerbations does not provide additional benefit [Moayeddi et al. 1995; McCrory and Brown, 2002].
A systematic review of the route of delivery of short-acting bronchodilators demonstrated no significant differences in FEV1 improvement between the use of handheld metered dose inhaler (MDI) with a good inhaler technique (with or without a spacer device) and nebulizers [Turner et al. 1997]. However, nebulizer delivery of short-acting bronchodilators is used in very ill patients as the patients may not be able to use the MDI properly.
The use of long-acting bronchodilators in COPD has been restricted for maintenance treatment of stable disease. However, formoterol, a rapid-acting long-acting inhaled β2 agonist (LABA), has also been proposed for use in a cumulative manner for the management of exacerbations [Malolepszy et al. 2001]. Despite the fact that high doses of formoterol are well tolerated, it remains uncertain whether its use can replace the need for short-acting agents during exacerbations.
Antibiotics
More than half of the acute exacerbations of COPD are triggered by bacterial infection caused by pathogens that commonly colonize the respiratory tract, such as Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis [Sethi, 1999]. The use of antibiotics routinely in treatment of exacerbations remains unsettled. There is evidence supporting the use of antibiotics in exacerbations when patients have clinical signs of a bacterial infection, for example an increase in sputum production. A systematic review of the very few available placebo-controlled studies showed that antibiotics reduced the risk of short-term mortality by 77%, treatment failure by 53% and sputum purulence by 44% [Ram et al. 2006].
A large number of oral antimicrobial agents have been approved for treating acute COPD exacerbations. Treatment is usually empirical and not based on sputum cultures. Sputum gram stain provides semiquantitative information on the number of bacteria in the sputum; culture provides information only on the identity of the organisms [Murray and Washington, 1975] and cannot separate colonization from infection.
The choice of antibiotics is influenced by the severity of exacerbation, prior use of antibiotics and systemic steroids, and the presence of underlying structural lung disease such as bronchiectasis. For a simple exacerbation in patients who are not at high risk of resistant organisms, macrolides or β-lactam agents can be used. However, because more than 50% of H. influenzae and M. catarrhalis species are β-lactamase producing, the use of β-lactam antibiotics should be coupled with β-lactamase inhibitors. The choice of antibiotics for treatment of acute exacerbation has recently been challenged by the rise in prevalence of resistant organisms, especially in patients with severe exacerbations and those with prior therapy with antibiotics and oral corticosteroids. In this situation, broader-spectrum antibiotics such as new fluoroquinolones that will be effective against resistant strains of H. influenzae and S. pneumonia are recommended. In the presence of underlying structural lung disease such as bronchiectasis, antibiotics targeting Pseudomonas species should be considered. If systemic symptoms such as fever are prominent, the presence of pneumonia should be ruled out and treatment with a broad-spectrum antibiotic is recommended [Celli et al. 2004]. A meta-analysis of the duration of antibiotic treatment therapy in an exacerbation of COPD demonstrated that a short course of antibiotic treatment (<5 days) is as effective as the traditional longer treatment in patients with mild to moderate exacerbations of CB and COPD.
There has been some recent interest in using inhaled antibiotics for treatment and prevention of COPD. However, so far, none of the studies have led to conclusive evidence to incorporate them in the guidelines for COPD exacerbation management. Some data exist regarding the use of inhaled tobramycin in patients with severe COPD (GOLD 3–4) who have colonization of their respiratory tracts with multidrug-resistant Pseudomonas [Dal Negro et al. 2008]. However, the study only showed reduction in sputum inflammatory markers and reduction in colony counts of the bacteria with no change in other outcomes.
Corticosteroids
The role of systemic corticosteroids in the treatment of exacerbations also remains contentious. There is no strong evidence to guide appropriate patient selection, route of administration or duration of treatment. Systemic corticosteroids reduce recovery time and treatment failures when used to treat acute exacerbations [Albert et al. 1980; Niewoehner et al. 1999]. The optimal dose and duration of therapy with corticosteroids has not been well established. GOLD guidelines recommended a dose of 30–40 mg prednisolone equivalent per day, preferably by the oral route, for 10–14 days. In a randomized controlled trial of a 9-day tapering dose of oral prednisone versus placebo in COPD exacerbation, a more rapid improvement in FEV1 and in moderate hypoxemia (on days 3 and 10 compared with day 1) were observed in the treatment group compared with the placebo group [Thompson et al. 1996]. Similarly, a 10-day course of 40 mg of oral prednisone versus placebo reduced the overall relapse rate at 30 days (27% versus 43% with number needed to treat of 6), improved post-bronchodilator FEV1 (34% versus 15% and 300 ml versus 160 ml) and breathlessness, but did not affect hospital admission rate and mortality. The insomnia group reported more insomnia, increased appetite and weight gain and increased trend towards higher incidence of depression and anxiety [Aaron et al. 2003].
In the largest inpatient study of COPD exacerbation to date [Niewoehner et al. 1999], patients received either 125 mg intravenous methylprednisolone four times daily for 3 days followed by 2 or 8 weeks of a tapering dose of oral prednisone (starting with 60 mg once daily) or placebo. The steroid groups had a significantly lower treatment failure rate compared with the placebo group on days 30 (33% versus 23%) and day 90 (48% versus 37%) but not at 6 months. The length of hospital stay was shortened by 1 day and FEV1 improved more rapidly in the steroid group (by approximately 100 ml) from day 1 but did not differ at 2 weeks. Adverse events were more noticeable in the steroid treated groups (67% patients had diabetes), including hyperglycemia requiring treatment (15%) and a higher proportion of secondary infection in the group treated for 8 weeks. The duration of steroid treatment (2 versus 8 weeks) did not influence these outcomes.
The role of inhaled corticosteroids (ICS) in the treatment of acute COPD exacerbation is even less defined. A recent randomized controlled trial in inpatients [Maltais et al. 2002] compared nebulized budesonide 0.5 mg/ml (2 mg four times daily for 3 days followed by 2 mg per day for 7 days) with oral prednisolone (30 mg twice daily for 3 days followed by 40 mg once daily for 7 days) and with placebo in patients with severe COPD hospitalized for moderate to severe exacerbations. The ICS group showed the same improvement in FEV1 compared with the oral prednisolone group. The duration of hospitalization was also similar, with the oral steroids group having more hyperglycemic events. Another randomized trial examining outpatient therapy for COPD exacerbation compared inhaled budesonide/formoterol with prednisolone plus formoterol and demonstrated similar improvement in FEV1 and no differences in symptoms, quality of life, treatment failures, and the need for reliever medication in the follow-up period between the two groups [Ställberg et al. 2009].
No clinical, biochemical or functional markers can clearly identify patients who will respond better to corticosteroid treatment. Although no effects on airway cytokines have been demonstrated in patients with stable COPD [Keatings et al. 1997], two studies have reported reductions in airway eosinophilic inflammatory markers [Brightling et al. 2000] and in serum C-reactive protein after 2 weeks of treatment with oral steroids [Sin et al. 2004]. An increased number of eosinophils have been found in patients with mild to moderate COPD exacerbations [Saetta et al. 1994].
Oxygen
Oxygen therapy is of beneficial value in acute COPD exacerbation as patients are often hypoxemic. The primary objective is to treat hypoxemia without increasing ventilation/perfusion mismatch which may occur in patients with chronic hypoxemia when they receive high amounts of oxygen. During a severe exacerbation, arterial blood gases should be monitored for arterial oxygen and carbon dioxide tension and pH. Oxygen saturation should be monitored for trending and adjusting oxygen settings. The goal of inpatient oxygen therapy is to maintain a partial pressure of arterial oxygen (PaO2) of 60 mmHg or oxygen saturation of 90% in order to prevent tissue hypoxia and preserve cellular oxygenation. Increasing PaO2 to values much greater than 60 mmHg confers little added benefit and may increase the risk of carbon dioxide retention, which may lead to acute hypercapnic respiratory failure [Celli et al. 2004].
Methylxanthines
Intravenous methylxanthines (theophylline or aminophylline) are considered second-line therapy, only to be used in selected cases when there is insufficient response to short-acting bronchodilators [Mahon et al. 1999]. Side effects of methylxanthines are significant and their beneficial effects in terms of lung function and clinical endpoints are modest and inconsistent [Barr et al. 2003]. In general it is not advised that these agents be used in the early treatment of exacerbations.
Mucolytic agents
The use of mucolytics and antioxidant agents (ambroxol, erdosteine, carbocysteine, iodinated glycerol) was investigated in numerous studies with controversial results. Although a few patients with viscous sputum may benefit from mucolytics, the overall benefit seems to be very small; therefore, the widespread use of these agents is not recommended at the present time.
Adjunct therapies
Depending on the clinical condition of the patient, appropriate hydration with special attention to the administration of diuretics, anticoagulants, treatment of comorbidities and nutritional aspects should be considered. At all times, healthcare providers should strongly enforce stringent measures against active cigarette smoking. Use of medications that have sedative effects on the sensorium like narcotics, benzodiazepines and other sedative hypnotics should be restricted and these medications if needed should be used with extreme caution.
Nonpharmacological interventions
Chest physical therapy
Chest percussion therapy initially was thought to augment the sputum clearance and improve respiratory symptoms of patients admitted with COPD exacerbation. Although the use of this modality is very beneficial in patients with bronchiectasis and cystic fibrosis, its use in COPD exacerbations remains of questionable benefit and currently is not recommended by GOLD guidelines of COPD management.
Ventilatory support
The primary therapeutic goal of ventilatory support in patients with exacerbations with acute respiratory failure is to decrease both mortality and morbidity and to relieve symptoms, despite optimal medical treatment. Mechanical ventilation can be delivered noninvasively or invasively (conventionally) using different modes that are, in essence, positive pressure devices (negative ventilation is currently not recommended) for noninvasive ventilation using either a nasal or facial mask, or via an endotracheal tube or a tracheostomy for invasive ventilation.
A major advance in the treatment of acute exacerbations of COPD has been the implementation of noninvasive positive pressure ventilation (NIV). NIV has been shown to improve acute respiratory acidosis (increases pH and decreases partial pressure of arterial carbon dioxide), decrease respiratory rate, work of breathing, severity of breathlessness, complications such as ventilator associated pneumonia and length of stay. Not only can intubations be avoided, but mortality for severe COPD exacerbations is also substantially reduced. A recent randomized trial testing the benefit of a helium–oxygen mixture for use in noninvasive ventilatory support in COPD exacerbations did not show superiority [Maggiore et al. 2010].
It is common practice for this form of ventilatory support to be provided in an intensive care unit setting with trained staff. There are several contraindications to its use, including respiratory arrest, cardiac instability, high aspiration risk and inability to fit the device securely. If contraindications are present or if noninvasive ventilation is inadequate, patients may require intubation and invasive mechanical ventilatory support. Detailed discussion of invasive mechanical ventilation in the management of COPD exacerbation is beyond the scope of this review. However, important consideration to avoid hyperinflation to avoid barotrauma and volutrauma should be implemented. This can be achieved by properly sedating the intubated patient and implementing controlled hypoventilation awaiting the effects of other treatments to reduce airway obstruction.
Weaning patients from the ventilator can be a very difficult and prolonged process and the best method (pressure support of T-piece trial) remains a matter of debate. In patients with COPD in whom extubation fails, NIV facilitates weaning, prevents reintubation and reduces mortality [Esteban et al. 1995]. Early NIV after extubation reduces the risk of respiratory failure and lowers 90-day mortality in patients with hypercapnia during a spontaneous breathing trial [Ferrer et al. 2009].
Hospital discharge and follow up
Insufficient clinical data exist to establish the optimal duration of hospitalization in individual patients with an exacerbation of COPD, although units with more respiratory consultants and better organized care have lower mortality and reduced length of hospital stay following admission for an exacerbation [Price et al. 2006]. In the hospital, prior to discharge, patients should start optimum therapy for COPD, which will include long-acting bronchodilators, with or without ICS. Follow up after discharge should include counseling for smoking cessation, monitoring of the inhaler technique and effectiveness of medications, and monitoring changes in spirometric parameters [Gravil et al. 1998].
Preventive strategies of COPD exacerbations
The significant impact of COPD exacerbation on both clinical outcomes and financial burden makes it imperative for clinicians to try to prevent these exacerbations from occurring. Current guidelines stipulate that prevention of exacerbation is a major goal in management. Many modalities, both pharmacological and nonpharmacological, have been proposed to prevent exacerbations (Table 3). We provide a detail account of these modalities below.
Table 3.
Strategies to prevent chronic obstructive pulmonary (COPD) disease exacerbations.
| Nonpharmacological | Pharmacological |
|---|---|
| Smoking cessation | Bronchodilators |
| Vaccines | LAMAs: tiotoprium |
| Approved | LABAs: salmeterol, formoterol, Indacaterol |
| Influenza | Antioxidants |
| Pneumococcal | Carbocisteine |
| Experimental | Acetylcysteine |
| Killed Haemophilus influenzae | Anti-inflammatory |
| NTHi oral immunotherapy | ICS/LABA |
| (HI-164OV) | Roflumilast |
| Bacterial lysate | Antibiotics |
| Pulmonary rehabilitation | Systemic antibiotics |
| Disease management programs | Macrolides, fluoroquinolones |
| Inhaled antibiotics | |
| Drugs used to treat comorbidities of COPD: statins, β blockers, ACE inhibitors |
ACE, angiotensin-converting enzyme; ICS, inhaled corticosteroid; LABA, long-acting inhaled β2 agonist; LAMA, long-acting muscarinic antagonist; NTHi, nontypeable Haemophilus influenzae.
Nonpharmacological strategies
Smoking cessation
Smoking cessation is the intervention with the greatest capacity to influence the natural history of COPD. Evaluation of the smoking cessation component in a lung heath study indicates that if effective resources and time are dedicated to smoking cessation, 25% long-term quit rates can be achieved [Anthonisen et al. 1994]. A study conducted among 23,497 Veterans in the USA demonstrated that smoking cessation is associated with a reduced risk of COPD exacerbations and the magnitude of the reduced risk was dependent on duration of abstinence [Au et al. 2009]. A systemic review of all the available literature supports the conclusion that, even in severe COPD, smoking cessation slows the accelerated rate of lung function decline and improves survival compared with continued smoking [Godtfredsen et al. 2008]. A recent study looking at the cost effectiveness of a smoking cessation program showed that a high-intensity smoking cessation program was associated with a lower average number of exacerbations (0.38 versus 0.60) and hospital days (0.39 versus 1) per patient and a higher number of quitters (20 versus 9) at lower total costs [Christenhusz et al. 2012].
Vaccines
Several studies evaluated the use of influenza and pneumococcal vaccination, which are now routinely recommended for all patients with COPD of significant severity [Wongsurakiat et al. 2004; Wang et al 2007]. One study that reviewed the outcome of influenza vaccination in a cohort of older patients with chronic lung disease demonstrated that influenza vaccination is associated with significant health benefits with fewer outpatient visits, fewer hospitalizations and reduced mortality [Nichol et al. 1999].
A Cochrane database review of four studies in patients with COPD showed no evidence of efficacy for injectable antipneumococcal vaccines [Granger et al. 2006]; however, in another study of the 23 serotype pneumococcal polysaccharide vaccine in patients with COPD, Alfageme and colleagues demonstrated that the vaccine was effective in the prevention of community-acquired pneumonia compared with placebo in patients younger than 65 years or those with severe airflow obstruction [Alfageme et al.2006]. However, no difference in mortality between the groups was seen. Larger well designed studies are needed to examine the effects of pneumococcal vaccine in patients older than 65 years with COPD.
Immunostimulatory agents have also been reported to reduce COPD exacerbation frequency. A study of the immunostimulant OM-85, a detoxified oral immunoactive bacterial extract, reported a reduction in the severe complications of exacerbations and hospital admissions in patients with COPD, with a follow-up study confirming the economic benefits of using this agent [Collet et al. 1997]. A recent randomized study demonstrated benefits but the patients had heterogeneous pathology [Soler et al. 2007]. A systematic review of 13 trials involving 2066 patients reported no consistent evidence of a benefit, though the agent is currently in use in Europe [Sprenkle et al. 2005]. Further studies are needed to understand the mechanisms of action of this immunostimulant before its role in COPD can be defined.
Pulmonary rehabilitation
The evidence for the effectiveness of pulmonary rehabilitation is very strong, but its impact on exacerbation rate is less studied than other more direct outcomes, such as exercise performance and health status. Conducting such studies now will be difficult, given the ethics of withholding rehabilitation for long enough for an exacerbation to occur. Respiratory rehabilitation may improve prognosis in patients with COPD by addressing relevant risk factors for exacerbations, such as low exercise capacity, decreased anxiety and depression, central desensitization to dyspnea and reduction in dynamic hyperinflation.
The most convincing evidence comes from the prospective randomized control trial conducted some years ago in South Wales where patients who received pulmonary rehabilitation had on average 10.4 days in hospital compared with 21.0 days in those randomized to receive conventional medical treatment [Griffiths et al. 2000]. Combined analysis of results from six trials including 230 patients indicated that respiratory rehabilitation reduced the risk of hospital admissions (pooled relative risk = 0.26) and mortality (pooled relative risk = 0.45) [Puhan et al. 2005].
Disease management programs and patient education
Self-management interventions improve various outcomes for many chronic diseases. Studies conducted in a COPD population showed that establishing a simple disease management program and improving patient education about COPD exacerbation symptoms and seeking help early does reduce hospitalizations and emergency department visits and helps in reducing costs to the healthcare delivery systems [Bourbeau et al. 2003, 2004; Rice, 2010]. However, controversy was thrown on the above findings by a recent randomized trial which suggested that implementation of a home educational and management program for patients with COPD did not result in a reduction in admissions for acute exacerbations of COPD and, unexpectedly, showed increased overall mortality [Fan et al. 2012]. More studies are needed to clarify the role of disease management and patient education on reduction in COPD exacerbations.
Pharmacological strategies
Comparative data of these pharmacologic therapies and their impact on reduction of exacerbation rates is shown in Table 4. A detailed account of these modalities is discussed below.
Table 4.
Comparative data of pharmacologic therapies for reduction in chronic obstructive pulmonary exacerbation rates [Han and Martinez, 2011].
| Drug class | Reduction in exacerbation rates |
|---|---|
| LAMA | 14% reduction [Tashkin et al. 2008] |
| LABA | 15% reduction [Calverley et al. 2007] |
| ICS | 25% reduction [Burge et al. 2000] |
| ICS/LABA combination | 26–35% reduction [Calverley et al. 2007; Anzueto et al. 2009] |
| PDE4 inhibitors | 17% reduction [Calverley et al. 2009] |
| N acetylcysteine | 25% reduction [Gerrits et al. 2003] |
| Antibiotics (moxifloxacin) | 20% reduction [Sethi et al. 2010] |
ICS, inhaled corticosteroid; LABA, long-acting inhaled β2 agonist; LAMA, long-acting muscarinic antagonist; PDE4, phosphodiesterase 4.
Long-acting bronchodilators
With regard to long-acting muscarinic antagonists (LAMAs), the use of the long-acting anticholinergic, tiotropium, has demonstrated a significant effect on reduction in COPD exacerbations. Understanding the Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) Trial, which included a total of 5993 patients, 2987 in the tiotropium group and 3006 in the placebo group, showed that tiotropium was associated with a reduction in the mean number of exacerbations of 14% (p < 0.001) during the 4-year period [Tashkin et al. 2008]. The recently published Prevention of Exacerbations with Tiotropium POET-COPD trial, a 1-year, randomized, double-blind, double-dummy, parallel-group trial, compared the effect of treatment with 18 μg of tiotropium once daily with that of 50 μg salmeterol twice daily on the incidence of moderate or severe exacerbations in patients with moderate to very severe COPD and a history of exacerbations in the preceding year. The trial demonstrated superiority of tiotropium to salmeterol in prolonging time to first exacerbation [Vogelmeier et al. 2011].
The role of other long-acting anticholinergic agents (some of which are still in clinical development) such as aclidinium bromide, glycopyrronium and umeclidinium in the prevention of COPD exacerbations has not yet been well defined.
With regard to LABAs, the use of salmeterol, formoterol and indacaterol in the maintenance treatment of COPD has been shown to reduce COPD exacerbations. A recent meta-analysis reviewed 17 randomized trials to assess the effect of LABA on COPD exacerbations [Wang et al. 2012]. Salmeterol, formoterol and indacaterol significantly reduced COPD exacerbations compared with placebo. Salmeterol significantly reduced COPD exacerbations with both study arms exposed or not exposed to ICS. The summary ORs were 0·79 (95% CI 0·67–0·92; p < 0·01) and 0·80 (95% CI 0·65–0·99; p = 0·04).
Compared with twice-daily LABAs, new LABAs with ultra-long duration (ultra LABAs) could provide improvements in efficacy and compliance with fast onset of action, 24 h bronchodilation and a good safety profile. Several novel ultra LABAs showing once-daily delivery profiles are in development. The only licensed ultra LABA at this time is indacaterol. As more drugs get developed in this class and more safety and long-term data are available, their use in the management of COPD will increase [Malerba et al. 2012].
With regard to LABA/LAMA combinations, several of these are under development for maintenance therapy of COPD, including indacaterol/glycopyrronium, formoterol/aclidinium, vilanterol/umeclidinium and olodaterol/tiotropium. The role of these agents in the prevention of COPD exacerbation has not been well defined and need to be examined.
Inhaled corticosteroids
ICS have consistently resulted in about a 25% reduction in exacerbation frequency and are recommended in the GOLD guidelines for this purpose. The Inhaled Steroid in Obstructive Lung Disease in Europe (ISOLDE) trial studied the long-term effects of fluticasone on exacerbations in 751 patients with COPD. The median exacerbation rate was reduced by 25% from 1.32 a year on placebo to 0.99 a year with fluticasone propionate (p = 0.026) [Burge et al. 2000]. However, current guidelines do not recommend the use of ICS as standalone agents in the maintenance therapy of COPD as their effect is significantly improved when combined with a LABA.
ICS/LABA combination
Results from the TORCH study showed that all active treatments were significantly superior to placebo in decreasing the risk of moderate to severe exacerbations, and exacerbations requiring systemic steroids (all p < 0.05). Combination treatment and salmeterol were also significantly superior to placebo in decreasing the risk for exacerbations requiring hospitalization (both p < 0.05) [Calverley et al. 2007].
A study by Anzueto and colleagues comparing the effects of combination therapy of fluticasone propionate/salmeterol 250/50 µg versus salmeterol alone [Anzueto et al. 2009] demonstrated a statistically significant reduction in moderate/severe exacerbations, rescue albuterol use, night-time awakenings and dyspnea scores, supporting the use of combination therapies for COPD for prevention of exacerbations.
A recently published randomized, double-blind, double-dummy, parallel-group, 12-month multicenter study evaluated the effect of budesonide/formoterol pressurized MDI on COPD exacerbations. Both doses of budesonide/formoterol 320/9 and 160/9 reduced exacerbation rates (number per patient-treatment year) by 34.6% and 25.9% respectively versus formoterol (p ≤ 0.002) [Sharafkhaneh et al. 2012].
A review of four clinical trials assessing combination therapy with budesonide and formoterol indicates that in patients with moderate or severe COPD who have a history of a COPD exacerbation, combination therapy improves various COPD-related outcomes, including lung function and health-related quality of life, and reduces the incidence of exacerbations compared with the individual components alone or placebo [Sharafkhaneh and Mattewal, 2010].
A recent publication of two replicate double-blind parallel-group 1-year trials looking at the addition of fluticasone furoate to vilanterol showed that the addition of an ICS to vilanterol was associated with a decreased rate of moderate and severe exacerbations of COPD but was also associated with an increased pneumonia risk [Dransfield et al. 2013].
Phosphodiesterase 4 inhibitors
Although theophylline remains the most widely used nonspecific phosphodiesterase inhibitor, data about its effects on exacerbations are lacking and the situation is scarcely better for the more specific phosphodiesterase 4 inhibitors. These drugs have many attractive pharmacological properties which might potentially reduce the patient’s likelihood of exacerbating but, to date, there are few published data to support this action. Cilomilast has been studied in several unpublished clinical trials which can be accessed via the US Food and Drug Administration website (http://www.fda.gov). Overall there was a small (32 ml) improvement in lung function with the drug and no effect on exacerbation rates compared with placebo.
More recently a different drug in this class, roflumilast, has been shown to reduce the overall rate of moderate/severe exacerbation rates over 1 year of treatment in subjects with severe COPD, CB and a history of previous exacerbation. This was accompanied by improvements in FEV1 with an acceptable side-effect profile [Calverley et al. 2009].
Antioxidants and mucolytic agents
Carbocisteine, a mucolytic agent with anti-inflammatory and antioxidant properties, was studied in the PEACE trial for the effect on COPD exacerbations. The study demonstrated a significant decrease in the treatment arm. The 1-year cumulative number of exacerbations was 325 in the carbocisteine group and 439 in the placebo group (p = 0.004) [Zheng et al. 2008].
N acetylcysteine (NAC) has been shown in vivo to have a significant antioxidant effect and this provides a biologically attractive mechanism for exacerbation prevention in COPD. A systematic review of the existing evidence suggested that NAC would have an important effect on exacerbation prevention [Gerrits et al. 2003]. But a well performed prospective randomized control trial (BRONCHUS) conducted over 3 years in 523 patients which found that the exacerbation rate in those randomized to NAC was no different from that in the placebo group [Decramer et al. 2005]. A subgroup analysis suggested that patients who were not receiving concomitant ICS had fewer exacerbations if they were randomized to NAC (0.76 versus 1.11 moderate or severe exacerbations per year), but this conclusion requires prospective confirmation.
A recent 1-year double-blind placebo-controlled trial in Hong Kong showed significant improvement in forced expiratory flow (25% to 75%), a significant reduction in exacerbation frequency (0.96 versus 1.71 times/year) and a tendency towards reduction in admission rate (0.5 versus 0.8 times/year) with NAC versus placebo [Tse et al. 2013].
Antibiotics
An alternative approach to preventing infectious exacerbations of COPD is the use of antibiotics in a prophylactic manner. Among the antibiotic classes, macrolides (including erythromycin, azithromycin and clarithromycin) are especially attractive as prophylactic antibiotics in COPD. In addition to their direct antibacterial effects, they have also been shown to have potentially beneficial immunomodulatory and anti-inflammatory effects. Albert and colleagues report a major study with daily azithromycin for the prevention of exacerbations. In this multicenter, prospective, placebo-controlled, double-blind, randomized trial, the authors examined the use of azithromycin in 570 subjects with COPD compared with placebo in 572 subjects with similar severity of COPD. The median time to the first exacerbation was 266 days in the azithromycin group versus 174 days in the placebo group, which was significantly different. The authors also noted an exacerbation frequency of 1.48 exacerbations per patient year in the group receiving azithromycin versus 1.83 exacerbations per patient year in the placebo group, with a hazard ratio of 0.73. Patients with COPD with two or more exacerbations a year in spite of appropriate standard therapy are potential candidates for this therapeutic approach. However, optimal duration and dosing of macrolide prophylaxis for exacerbation remains uncertain [Albert et al. 2011].
In another recent study (PULSE) by Sethi and colleagues, the effect of pulsed therapy with moxifloxacin 400 mg daily for 5 days and repeated every 8 weeks for a total of six courses showed a statistical reduction in odds of an exacerbation by 20% and a 44% reduction in patients who exhibited purulent sputum at baseline [Sethi et al 2010]. Though PULSE demonstrates that intermittent treatment with moxifloxacin is an effective option for preventing acute exacerbations in patients with COPD, further studies are required to determine the optimal patient population as well as dosing regimen and therapy duration for this approach.
The above studies have also sparked interest in potentially using antibiotics via inhalation to reduce exacerbation risk. Several studies are ongoing to examine the effects of inhaled antibiotics in the prevention of COPD exacerbations.
Vitamin D
A recent randomized trial looking at the effect of vitamin D supplementation in patients with COPD did not show a statistically significant reduction in exacerbation rates [Lehouck et al. 2012]. A subgroup of patients with very low vitamin D levels did show some reduction in COPD exacerbation rates but further studies are needed to establish guideline for vitamin supplementation to reduce exacerbation rates.
Effect of cardiovascular drugs on COPD exacerbations
Multiple observational studies examined the effects of statins/β blockers and angiotensin-converting enzyme (ACE) inhibitors on prevention of exacerbations of COPD. There is evidence from observational studies and one randomized controlled trial that statins may reduce morbidity and mortality in patients with COPD [Dobler et al. 2009]. To clarify the effect of statins on COPD, a large prospective randomized controlled trial (STATCOPE) is underway and results are pending [ClinicalTrials.gov identifier: NCT01061671].
Multiple studies have shown a beneficial effect of β blockers on reducing the rate of COPD exacerbations, which was contrary to prevailing thought that β blockers may be harmful in COPD. Recent data show a reduction in mortality in patients with COPD exacerbations with β blockers, possibly due to dual cardiopulmonary protective properties [Short et al. 2011; Rutten et al. 2010]. These findings need further exploration in prospective trials.
Conclusion
COPD exacerbations are often triggered by airway infection and are an important cause of morbidity, impairment of health status and mortality. One of the main goals of management of COPD is to reduce the morbidity associated with exacerbations and thus improve the quality of life of patients with this disabling condition. Although many pharmacological and nonpharmacological interventions are available to prevent exacerbations, the degree of reduction of exacerbation frequency by such interventions is still restricted, underlining the need for novel interventions to be developed and studied in well designed and adequately powered randomized trials.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Hammad Qureshi, Section of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Baylor College of Medicine, Houston, TX, USA.
Amir Sharafkhaneh, Section of Pulmonary, Critical Care and Sleep Medicine, Medical Care Line, Michael E. DeBaKey VA Medical Center; and Department of Medicine, Baylor College of Medicine, Houston, TX, USA.
Nicola A. Hanania, Section of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Baylor College of Medicine, 1504 Taub Loop, Houston, TX 77030, USA
References
- Aaron S., Vandemheen K., Hebert P., Dales R., Stiell I., Ahuja J. (2003) Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Eng J Med 348: 2618–2625 [DOI] [PubMed] [Google Scholar]
- Albert R., Connett J., Bailey W., Casaburi R., Cooper J., Jr, Criner G. (2011) Azithromycin for prevention of exacerbations of COPD. N Eng J Med 365: 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert R., Martin T., Lewis S. (1980) Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Ann Intern Med 92: 753–758 [DOI] [PubMed] [Google Scholar]
- Alfageme I., Vazquez R., Reyes N., Muñoz J., Fernández A., Hernández M. (2006) Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax 61: 189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society/European Respiratory Society (2010) Standards for the diagnosis and management of patients with COPD. Version 1.2. Available at: www.thoracic.org/go/copd (accessed 13 September 2010).
- Anthonisen N., Connett J., Kiley J., Altose M., Bailey W., Buist A. (1994) Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. JAMA 272: 1497–1505 [PubMed] [Google Scholar]
- Anzueto A., Ferguson G., Feldman G. (2009) Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD 6: 320–329 [DOI] [PubMed] [Google Scholar]
- Anzueto A., Sethi S., Martinez F. (2007) Exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 4: 554–564 [DOI] [PubMed] [Google Scholar]
- Au D., Bryson C., Chien J., Udris E., Evans L., Bradley K. (2009) The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med 24: 457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr R., Rowe R., Camargo C. (2003) Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta-analysis of randomized trials. BMJ 327: 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghé B., Verduri A., Roca M., Fabbri L. (2013) Exacerbation of respiratory symptoms in COPD patients may not be exacerbations of COPD. Eur Respir J 41(4): 993–995 [DOI] [PubMed] [Google Scholar]
- Bourbeau J., Julien M., Maltais F., Rouleau M., Beaupré A., Bégin R. (2003) Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med 163: 585-591 [DOI] [PubMed] [Google Scholar]
- Bourbeau J., Nault D., Dang-Tan T. (2004) Self-management and behavior modification in COPD. Patient Educ Couns 52: 271–277 [DOI] [PubMed] [Google Scholar]
- Brightling C., Monteiro W., Ward R., Parker D., Morgan M., Wardlaw A., et al. (2000) Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 356: 1480-1485 [DOI] [PubMed] [Google Scholar]
- British Thoracic Society (2006) Burden of Lung Disease Report, 2nd edn. Available at: www.britthoracic.org.uk/copd/pubs_frameset.html (accessed 2 May 2007).
- Burge P., Calverley P., Jones P., Spencer S., Anderson J., Maslen T. (2000) Randomized, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 320: 1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley P., Anderson J., Celli B. (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356: 775–789 [DOI] [PubMed] [Google Scholar]
- Calverley P., Rabe K., Goehring U., Kristiansen S., Fabbri L., Martinez F. (2009) Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 374: 685–694 [DOI] [PubMed] [Google Scholar]
- Celli B., MacNee W., Agusti A., Anzueto A., Berg B., Buist A., et al. (2004) Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J 23: 932–946 [DOI] [PubMed] [Google Scholar]
- Christenhusz L., Prenger R., Pieterse M., Seydel E., Van der Palen J. (2012) Cost-effectiveness of an intensive smoking cessation intervention for COPD outpatients. Nicotine Tob Res 14: 657–663 [DOI] [PubMed] [Google Scholar]
- Clini E., Beghé B., Fabbri L. (2013) Chronic obstructive pulmonary disease is just one component of the complex multimorbidities in patients with COPD. Am J Respir Crit Care Med 187: 668–671 [DOI] [PubMed] [Google Scholar]
- Clini E., Crisafulli E., Radaeli A., Malerba M. (2013) COPD and the metabolic syndrome: an intriguing association. Intern Emerg Med 8: 283–289 [DOI] [PubMed] [Google Scholar]
- Collet J., Shapiro S., Ernst P. (1997) Effect of an immunostimulating agent on acute exacerbations and hospitalization in COPD patients. Am J Respir Crit Care Med 156: 1719–1724 [DOI] [PubMed] [Google Scholar]
- COMBIVENT Inhalation Solution Study Group (1997) Routine nebulized ipratropium and albuterol together are better than either alone in COPD. Chest 112: 1514–1521 [DOI] [PubMed] [Google Scholar]
- Dal Negro R., Micheletto C., Tognella S., Visconti M., Turati C. (2008) Tobramycin Nebulizer Solution in severe COPD patients colonized with Pseudomonas aeruginosa: effects on bronchial Inflammation. Adv Ther 25: 1019–1030 [DOI] [PubMed] [Google Scholar]
- Decramer M., Rutten-van Mölken M., Dekhuijzen P., Troosters T., van Herwaarden C., et al. (2005) Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomized placebo-controlled trial. Lancet 365: 1552–1560 [DOI] [PubMed] [Google Scholar]
- Dobler C., Wong K., Marks G. (2009) Associations between statins and COPD: a systematic review. BMC Pulmon Med 9: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson G., Seemungal T., Bhowmik A., Wedzicha J. (2002) Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 57: 847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson G., Wilkinson T., Hurst J., Perera W., Wedzicha J. (2005) Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 171: 446–452 [DOI] [PubMed] [Google Scholar]
- Dransfield M., Bourbeau J., Jones P., Hanania N., Mahler D., Vestbo J. (2013) Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Resp Med 234: 210–223 [DOI] [PubMed] [Google Scholar]
- Erelel M., Cuhadaroglu C., Ece T., Arseven O. (2002) The frequency of deep venous thrombosis and pulmonary embolus in acute exacerbation of chronic obstructive pulmonary disease. Respir Med 96: 515–518 [DOI] [PubMed] [Google Scholar]
- Esteban A., Frutos F., Tobin M., Alía I., Solsona J., Valverdu V. (1995) A comparison of four methods of weaning patients from mechanical ventilation. N Eng J of Med 332: 345–350 [DOI] [PubMed] [Google Scholar]
- Fan V., Gaziano J., Lew R., Bourbeau J., Adams S., Leatherman S. (2012) A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations a randomized, controlled trial. Ann Int Med 156: 673–683 [DOI] [PubMed] [Google Scholar]
- Ferrer M., Sellarés J., Valencia M., Carrillo A., Gonzalez G., Badia J. (2009) Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 374: 1082–1088 [DOI] [PubMed] [Google Scholar]
- Gerrits C., Herings R., Leufkens H., Lammers J. (2003) N-acetylcysteine reduces the risk of re-hospitalization among patients with chronic obstructive pulmonary disease. Eur Respir J 21: 795–798 [DOI] [PubMed] [Google Scholar]
- Godtfredsen N., Lam T., Hansel T., Leon M., Gray N., Dresler C. (2008) COPD-related morbidity and mortality after smoking cessation: status of the evidence. Eur Resp J 32: 844–853 [DOI] [PubMed] [Google Scholar]
- GOLD (2011) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Available at: http://www.goldcopd.org
- GOLD (2013) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. February. Available at: http://www.goldcopd.org
- Granger R., Walters J., Poole P. (2006) Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 4: CD001390. [DOI] [PubMed] [Google Scholar]
- Gravil J., Al-Rawas O., Cotton M., Flanigan U., Irwin A., Stevenson R. (1998) Home treatment of exacerbations of chronic obstructive pulmonary disease by an acute respiratory assessment service. Lancet 351: 1853–1855 [DOI] [PubMed] [Google Scholar]
- Griffiths T., Burr M., Campbell I. (2000) Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation. Lancet 355: 362–368 [DOI] [PubMed] [Google Scholar]
- Han M., Martinez F. (2011) Pharmacotherapeutic approaches to preventing acute exacerbations of chronic obstructive pulmonary disease. Proc ATS 8: 356–362 [DOI] [PubMed] [Google Scholar]
- Hurst J., Donaldson G., Quint J., Goldring J., Baghai-Ravary R., Wedzicha J. (2009) Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 179: 369–374 [DOI] [PubMed] [Google Scholar]
- Hurst J., Vestbo J. (2010) Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 363: 1128–1138 [DOI] [PubMed] [Google Scholar]
- Johnson M., Rennard S. (2001) Alternative mechanisms for long-acting beta(2)-adrenergic agonists in COPD. Chest 120: 258–270 [DOI] [PubMed] [Google Scholar]
- Karpel J., Pesin J., Greenberg D., Gentry E. (1990) A comparison of the effects of ipratropium bromide and metaproterenol sulfate in acute exacerbations of COPD. CHEST J 98: 835–839 [DOI] [PubMed] [Google Scholar]
- Keatings V., Jatakanon A., Worsdell Y. (1997) Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med 155: 542–548 [DOI] [PubMed] [Google Scholar]
- Kim V., Han M., Vance G., Make B., Newell J., Hokanson J., et al. (2011) The chronic bronchitic phenotype of COPD: an analysis of the COPD Gene study. Chest 140: 626–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek K., Xu J., Murphy S., Miniño A., Kung H. (2011) Deaths: final data for 2009. Natl Vital Stat Rep 60: 3–11 [PubMed] [Google Scholar]
- Lehouck A., Mathieu C., Carremans C., Baeke F., Verhaegen J., Van Eldere J., et al. (2012) High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 156: 105–114 [DOI] [PubMed] [Google Scholar]
- Lopez A., Shibuya K., Rao C., Mathers C., Hansell A., Held L., et al. (2006) Chronic obstructive pulmonary disease: current burden and future projections. ERS J 27: 397–412 [DOI] [PubMed] [Google Scholar]
- Maggiore S., Richard J., Abroug F., Diehl J., Antonelli M., Sauder P., et al. (2010) A multicenter, randomized trial of noninvasive ventilation with helium-oxygen mixture in exacerbations of chronic obstructive lung disease. Crit Care Med 38: 145–151 [DOI] [PubMed] [Google Scholar]
- Mahon J., Laupacis A., Hodder R., McKim D., Paterson N., Wood T., et al. (1999) Theophylline for irreversible chronic airflow limitation: a randomized study comparing n of 1 trials to standard practice. Chest 115: 38–48 [DOI] [PubMed] [Google Scholar]
- Malerba M., Radaeli A., Morjaria J. (2012) Therapeutic potential for novel ultra long-acting β-agonists in the management of COPD: biological and pharmacological aspects. Drug Discov Today 17: 496–504 [DOI] [PubMed] [Google Scholar]
- Malolepszy J., Boszormenyi N., Selroos O. (2001) Safety of formoterol Turbuhaler at cumulative dose of 90 microg in patients with acute bronchial obstruction. Eur Respir J 18: 928–934 [DOI] [PubMed] [Google Scholar]
- Maltais F., Ostinelli J., Bourbeau J. (2002) Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 165: 698–703 [DOI] [PubMed] [Google Scholar]
- McCrory D., Brown C. (2002) Anticholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 4: CD003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miravitlles M., Guerrero T., Mayordomo C., Sánchez-Agudo L., Nicolau F., Segú J. (2000) Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration 67: 495–501 [DOI] [PubMed] [Google Scholar]
- Moayyedi P., Congleton J., Page R., Pearson S., Muers M. (1995) Comparison of nebulised salbutamol and ipratropium bromide with salbutamol alone in the treatment of chronic obstructive pulmonary disease. Thorax 50: 834–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P., Washington J. (1975) Microscopic and bacteriologic analysis of expectorated sputum. Mayo Clin Proc 50: 339–344 [PubMed] [Google Scholar]
- Nichol K., Baken L., Nelson A. (1999) Relation between influenza vaccination and outpatient visits, hospitalization and mortality in elderly patients with chronic lung disease. Ann Intern Med 130: 397–403 [DOI] [PubMed] [Google Scholar]
- Niewoehner D., Erbland M., Deupree R., Collins D., Gross N., Light R., et al. (1999) Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Medi 340: 1941–1947 [DOI] [PubMed] [Google Scholar]
- Niewoehner D., Lokhnygina Y., Rice K. (2007) Risk indexes for exacerbations and hospitalizations due to COPD. Chest 131: 20–28 [DOI] [PubMed] [Google Scholar]
- Polosa R., Malerba M., Cacciola R., Morjaria J., Maugeri C., Prosperini G., et al. (2011) Effect of acute exacerbations on circulating endothelial, clotting and fibrinolytic markers in COPD patients. Intern Emerg Med 1–8: 567–674 [DOI] [PubMed] [Google Scholar]
- Price L., Lowe D., Hosker H., Anstey K., Pearson M., Roberts C. (2006) UK National COPD Audit 2003: impact of hospital resources and organization of care on patient outcome following admission for acute COPD exacerbation. Thorax 61: 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhan M., Scharplatz M., Troosters T., Steurer J. (2005) Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality – a systematic review. Respir Res 6: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram F., Rodriguez-Roisin R., Granados-Navarrete A., Garcia-Aymerich J., Barnes N. (2006) Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev CD004403. [DOI] [PubMed] [Google Scholar]
- Rice K. (2010) Disease management program for COPD. AJRCCM 182: 890–896 [DOI] [PubMed] [Google Scholar]
- Rizkallah J., Man S., Sin D. (2009) Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest 135: 786–793 [DOI] [PubMed] [Google Scholar]
- Roca M., Verduri A., Corbetta L., Clini E., Fabbri L., Beghé B. (2013) Mechanisms of acute exacerbation of respiratory symptoms in chronic obstructive pulmonary disease. Eur J Clin Invest 43: 510–521 [DOI] [PubMed] [Google Scholar]
- Rutten F., Zuithoff N., Hak E., Grobbee D., Hoes A. (2010) {beta}-Blockers may reduce mortality and risk of exacerbations in patients with chronic obstructive pulmonary disease. Arch Intern Med 170: 880–887 [DOI] [PubMed] [Google Scholar]
- Saetta M., Di Stefano A., Maestrelli P. (1994) Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med 150: 1646–1652 [DOI] [PubMed] [Google Scholar]
- Sapey E., Stockley R. (2006) COPD exacerbations.2: Aetiology. Thorax 61: 250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemungal T., Donaldson G., Bhowmik A., Jeffries D., Wedzicha J. (2000) Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 161: 1608–1613 [DOI] [PubMed] [Google Scholar]
- Seemungal T., Donaldson G., Paul E., Bestall J., Jeffries D., Wedzicha J. (1998) Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 151: 1418–1422 [DOI] [PubMed] [Google Scholar]
- Sethi S. (1999) Infectious exacerbations of chronic bronchitis: diagnosis and management. J Antimicrob Chemother 43(Suppl. A): 97–105 [DOI] [PubMed] [Google Scholar]
- Sethi S., Jones P., Theron M., Miravitlles M., Rubinstein E., Wedzicha J., et al. (2010) Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S., Murphy T. (2008) Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 359: 2355–2365 [DOI] [PubMed] [Google Scholar]
- Sharafkhaneh A., Mattewal A. (2010) Budesonide/formoterol combination in COPD: a US perspective. Int J Chron Obstruct Pulmon Dis 5: 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharafkhaneh A., Southard J., Goldman M., Uryniak T., Martin U. (2012) Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Resp Med 106: 257–268 [DOI] [PubMed] [Google Scholar]
- Short P., Lipworth S., Elder D., Schembri S., Lipworth B. (2011) Effect of β blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort study. BMJ 342: d2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin D., Lacy P., York E. (2004) Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 170: 760–765 [DOI] [PubMed] [Google Scholar]
- Soler M., Mutterlein R., Cozma G.; Swiss-German OM-85 Study Group (2007) Double-blind study of OM-85 in patients with chronic bronchitis or mild chronic obstructive pulmonary disease. Respiration 74: 26–32 [DOI] [PubMed] [Google Scholar]
- Soler-Cataluña J., Martínez-García M., Román Sánchez P., Salcedo E., Navarro M., et al. (2005) Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 60: 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenkle M., Niewoehner D., MacDonald R., Rutks I., Wilt T. (2005) Clinical efficacy of OM–85 BV in COPD and chronic bronchitis: a systematic review. COPD 2: 167–175 [DOI] [PubMed] [Google Scholar]
- Ställberg B., Selroos O., Vogelmeier C., Andersson E., Ekström T., Larsson K. (2009) Budesonide/formoterol as effective as prednisolone plus formoterol in acute exacerbations of COPD. A double-blind, randomised, non-inferiority, parallel-group, multicentre study. Respir Res 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin D., Celli B., Senn S., Burkhart D., Kesten S., Menjoge S., et al. (2008) A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 359: 1543–1554 [DOI] [PubMed] [Google Scholar]
- Thompson W., Nielson C., Carvalho P. (1996) Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 154: 407–412 [DOI] [PubMed] [Google Scholar]
- Tse H., Raiteri L., Wong K., Yee K., Ng L., Wai K. (2013) High-dose N-acetylcysteine in stable chronic obstructive pulmonary disease: the 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest 144: 106–118 [DOI] [PubMed] [Google Scholar]
- Turner M., Patel A., Ginsburg S., FitzGerald J. (1997) Bronchodilator delivery in acute airflow obstruction: a meta-analysis. Arch Int Med 157: 1736–1744 [PubMed] [Google Scholar]
- Wang C., Wang S., Lai C., Lin L., Chou P. (2007) Impact of influenza vaccination on major cause-specific mortality. Vaccine 26: 1196–1203 [DOI] [PubMed] [Google Scholar]
- Wang J., Nie B., Xiong W., Xu Y. (2012) Effect of long-acting beta-agonists on the frequency of COPD exacerbations: a meta-analysis. J Clin Pharm Ther 37: 204–211 [DOI] [PubMed] [Google Scholar]
- Wongsurakiat P., Maranetra K., Wasi C., Kositanont U., Dejsomritrutai W., Charoenratanakul S. (2004) Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest 25: 1971–1972 [DOI] [PubMed] [Google Scholar]
- Vestbo J, Lisa D., Scanlon P., Yates J., Agusti A., Bakke P., et al. (2011) Changes in forced expiratory volume in 1 second overtime in COPD. N Engl J Med 365: 1184–1192 [DOI] [PubMed] [Google Scholar]
- Vogelmeier C., Hederer B., Glaab T., Schmidt H., Rutten-van Mölken M., Beeh K. (2011) Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 364: 1093–1103 [DOI] [PubMed] [Google Scholar]
- Zheng J., Kang J., Huang S., Chen P., Yao W., Yang L., et al. (2008) Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled study. The Lancet 371: 2013–2018 [DOI] [PubMed] [Google Scholar]


