Abstract
This study was investigated to know whether pachymic acid (PA), one of the predominant triterpenoids in Poria cocos (Hoelen) has the sedative-hypnotic effects, and underlying mechanisms are mediated via γ-aminobutyric acid (GABA)-ergic systems. Oral administration of PA markedly suppressed locomotion activity in mice. This compound also prolonged sleeping time, and reduced sleep latency showing synergic effects with muscimol (0.2 mg/kg) in shortening sleep onset and enhancing sleep time induced by pentobarbital, both at the hypnotic (40 mg/kg) and sub-hypnotic (28 mg/kg) doses. Additionally, PA elevated intracellular chloride levels in hypothalamic primary cultured neuronal cells of rats. Moreover, Western blotting quantitative results showed that PA increased the amount of protein level expression of GAD65/67 over a broader range of doses. PA increased α- and β-subunits protein levels, but decreased γ-subunit protein levels in GABAA receptors. The present experiment provides evidence for the hypnotic effects as PA enhanced pentobarbital-induced sleeping behaviors via GABAA-ergic mechanisms in rodents. Taken together, it is proposed that PA may be useful for the treatment of sleep disturbed subjects with insomnia.
Keywords: Poria cocos (Hoelen), Pachymic acid (PA), Insomnia, Pentobarbital (PENT), GABAA receptors, Glutamic acid decarboxylase (GAD)
INTRODUCTION
Poria cocos (Hoelen) (Fam. Polyporaceae) is one of the most important well-known traditional Chinese medicines (TCM) that has been practiced for treating a range of sleep disorders such as insomnia, either in single herb or in herbal formula in Asian countries (Chen et al., 2011). It grows around the roots of pine trees in China, Japan, Korea, and North America (Lee et al., 2012). It has traditionally been used as diuretic and sedative agents (Spelman et al., 2006) in Chinese herbal prescriptions. In addition, it has also been used for the improvement of appetite, exclusion of sputum and edema, and the treatment of palpitation and insomnia (Kaminaga et al., 1996). Earlier studies have shown that the main ingredients of this herb medicine are a group of triterpenoid compounds. Pachymic acid (PA), a lanostane type triterpenoid possess anti-emetic, anti-inflammatory, and anti-cancer properties (Tai et al., 1995; Cuella et al., 1996; Kaminaga et al., 1996; Giner et al., 2000; Li et al., 2004; Gapter et al., 2005).
Insomnia has high prevalence rates and is associated with significant personal and socio-economic burden, yet it remains largely under recognized and inadequately treated. Medical science has exploited natural resources since time immemorial and the success of modern medical science as the potential sources of drugs to prevent and cure human as well as veterinary health problems. The sedative-hypnotic me dications, including benzodiazepines and non-benzodiazepines, are the most common treatments for insomnia. How ever, concerns regarding patterns of inappropriate use, dependence and adverse effects have led to caution in prescribing those sedative-hypnotic medications. Patients with insomnia are generally subdivided into three categories: sleep onset insomnia, sleep maintenance insomnia and terminal insomnia (early-morning awakening coupled with an inability to return to sleep) (Rosenberg, 2006). It has been shown that sleep deprivation has a great impact on the everyday life of healthy subjects, affecting alertness, attention, concentration, cognitive abilities, memory, mood, and pain.
Pharmacological evidence suggests that the molecular target of medicinal plants those having sedative-hypnotic activity has been mainly focused on the benzodiazepine site of GABAA (GABAA-BZD) receptors (Abourashed et al., 2004). GABAA receptors are the principal inhibitory neurotransmitter in the vertebrate CNS, which are mainly located postsynaptically and mediate the majority of fast synaptic inhibition in the brain. They also represent the major sites of action for both benzodiazepines and barbiturates. These receptors are Cl− sensitive ligand gated ion channels that can be assembled from seven subunit classes: αl-6, βl-3, γl-3, δ, ρθl-3, θ, π and ε of GABAA R structure (Sieghart and Sperk, 2002; Rudolph and Mohler, 2004, 2006). They play an important role in neuronal firing patterns and activity of neuronal networks and serves as targets for numerous classes of drugs, used both in clinical practice and as research tools.
Researchers admit that neuropsychopharmacology plays an important role in insomnia therapy, targeting several transmitter and peptide systems, including γ-aminobutyric acid (GABA)-ergic and serotonergic, as well as histaminergic and hypocretinergic (also known as orexinergic) systems (Wafford and Ebert, 2008). The GABA neurotransmitter receptors, in particular the type A subtype (GABAA receptors), have received considerable attention as the site of action for drugs acting as anxiolytics, sedatives, anticonvulsants, and muscle relaxants. These clinically beneficial effects are exhibited by the benzodiazepines, which act by allosterically binding to GABAA receptors and enhancing the ability of GABA to increase chloride conductance. Research into psychoactive plants that may affect the central nervous system (CNS) has flourished, with an abundance of preclinical in vitro and in vivo studies validating many phytotherapies. However, as with the development of many nascent pharmacological strategies, there is still limited information on the pharmacological studies of PA in the sleep disorder treatments such as insomnia. Therefore, the present study was to determine the sedative-hypnotic effects of PA on pentobarbital-induced sleeping behaviors. In addition, we defined the mediation of γ-aminobutyric acid (GABA)-ergic systems to understand the possible mechanisms.
MATERIALS AND METHODS
Reagents and chemicals
Pachymic acid (Fig. 1, purity HPLC, 98%) was purchased from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, Sichuan, China). Muscimol (Tocris Bioscience, Bristol, UK), pentobarbital sodium (Hanlim Pharm. Co. Ltd., Seoul, Korea), diazepam (Samjin Pharm. Seoul, Korea), dimethyl sulfoxide (DMSO, Amresco, Solon, Ohio, USA) were also purchased, respectively. Fetal bovine serum (FBS) and Dulbecco’s Modified Eagle Medium (DMEM) were obtained from GIBCO (Grand Island, NY, USA). The Cl− sensitive fluorescence probe N-(ethoxycarbonyl-methyl)-6-methoxyquinolinium bromide (MQAE) was purchased from Sigma-Aldrich Co. (St Louis, MO, USA). The specific rabbit polyclonal antibodies against GABAA receptors subunits or GAD65/67 and the corresponding conjugated anti-rabbit immunoglobulin G-horseradish peroxidase were obtained from Abcam Inc. (Cambridge, UK). Chemiluminescent HRP substrate was purchased from Millipore Co. (Billerica, MA, USA).
Fig. 1.
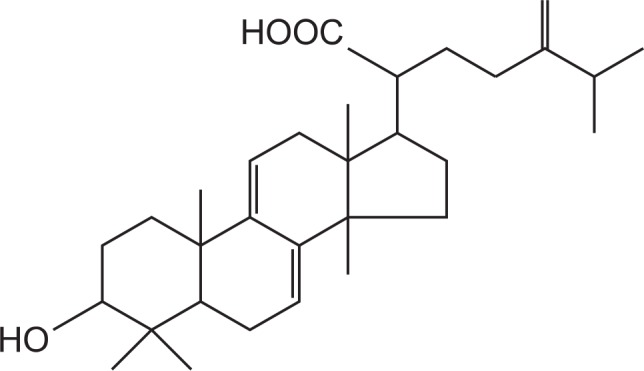
Chemical structure of the pachymic acid.
Animals
The animals used for behavioral experiments were ICR ma le mice (purchased from Samtako, Osan, Korea), weighing 20–25 g, in groups of 10–15. Animals were housed in acrylic cages (45×60×23 cm) with water and food available ad libitum under an artificial 12-h light/dark cycle (light on at 7:00 am), at the relative humidity (50–52%) and at a constant temperature (22 ± 2°C). To ensure adaptation to the new environment, the mice were kept in the departmental holding room for 1 week before the experiment. All the behavioral experiments were performed between 10:00 and 17:00 h. All of the experiments involving animals were carried out in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1985), and the Institutional Animal Care and Use Committee of Chungbuk National University approved the protocol.
Locomotor activity
Spontaneous locomotor activity was measured automatically with a tilting-type ambulometer (AMB-10, O’Hara, Japan). Each mouse was placed in the activity cage (20 cm in diameter and 18 cm in height) and after an adaptation period of 10 min, the test compound administration protocol was implemented (Park et al., 2005). Diazepam (2.0 mg/kg, p.o) and PA (1, 3 and 5 mg/kg, p.o) were administered to mice 30 and 60 min prior to the experiment, respectively. The ambulatory activity was measured for 1 h after the administration of the agents (Morton et al., 2011).
Pentobarbital-induced sleep
Ten to twelve mice were used for each treatment group. All experiments were carried out between 1:00 and 5:00 pm. Animals were fasted for 24 h, prior to the experiment. Pentobarbital sodium and muscimol were diluted in 0.9% physiological saline and administered to each mouse intraperitoneally (i.p.) to induce sleep. PA was suspended and dissolved in 0.1% DMSO and administered orally to the mice (0.1 ml/10 g). Muscimol was administered as a reference drug 30 min prior to administration of pentobarbital. Pentobarbital was administered to animals placed in a box 1 h after the oral administration of test drugs. Those animals that stopped moving in the box within 15 min after pentobarbital injection were immediately transferred to another box. Those individuals that stayed immobile for more than 3 min were judged to be asleep. The time that elapsed from receiving pentobarbital until an animal, positioned delicately on its back, lost its righting reflex represented latency to onset of sleeping. The animals were observed constantly, and the time of awakening, characterized by righting of the animal, was noted. The sleeping time was defined as the time taken for the animal to regain spontaneous movements after being transferred to the second box. Animals that failed to fall asleep within 15 min after pentobarbital administration were excluded from the experiments (Wolfman et al., 1996; Darias et al., 1998).
Primary cell culture
Primary cultures of hypothalamus were prepared from of 8 days old Sprague-Dawley rats as previously described (Ma et al., 2007). After 8 days in culture, these cells express functional GABAA receptors, with an expression pattern similar to that of the hypothalamus during postnatal development, but different from the pattern observed in the adult rats. Briefly, hypothalamus cells were plated (1.0×105 cells per well) in 96 well microplates that had been coated with poly-L-lysine (50 μg/mL; Sigma, St. Louis, MO, USA), and were cultured DMEM nutrient and neurobasal A media supplemented with 10% heat-inactivated fetal bovine serum, glutamine (2.0 mM), gentamicin (100 μg/ml), antibiotic-antimycotic solution (10 μg/ml; Sigma), and potassium chloride (25 mM); such a high concentration of potassium was necessary to induce persistent depolarization, which promotes the survival of granule cells. The cells were incubated for 6–9 days in a humidified 5% CO2/95% air atmosphere at 37°C. Cytosine arabinofuranoside (final concentration, 10 μM; Sigma) was added to cultures 18–24 h after plating to inhibit the proliferation of non-neuronal cells.
Measurement of intracellular Cl− influx
The intracellular Cl− concentration ([Cl−]i) of hypothalamic neuron cells of rats was estimated using Cl− sensitive fluorescence probe MQAE according to the method of West and Molloy, with a slight modification (West and Molloy, 1996). The buffer (pH 7.4) contained the following components: 2.4 mM HPO42−, 0.6 mM H2PO4−, 10 mM HEPES, 10 mM D-glucose and 1 mM MgSO4. A variety of MQAE-loading conditions were assessed. The cells were incubated overnight in medium containing 10 mM MQAE. After loading, the cells were washed three times in the appropriate Cl− containing buffer or Cl− free buffer. The buffer was replaced with buffer with or without the compounds or with Cl− free buffer. Repetitive fluorescence measurements were initiated immediately using a SpectraMax M2e Multi-Mode Microplate Reader (Excitation wavelength: 320 nm, emission wavelength: 460 nm; Molecular Devices, Downingtown, PA, USA). The data is presented as the relative fluorescence F/F0, where F is the fluorescence as a function of each sample and F0 is the fluorescence without Cl− ions. The F/F0 values were directly proportional to [Cl−]i.
Expression of GAD65/67 and GABAA receptors subunits
On 8 days of culture, the treatment of the primary cultured hypothalamic neurons with PA was initiated. PA was suspended and dissolved in 0.1% DMSO, and then diluted sequentially in culture medium to final concentrations of 5.0 and 50 μM respectively. The control group was treated with vehicle alone at the same dilution as that used for drug treatment. The culture medium was completely replaced with fresh medium containing the appropriate drug. After treatment of PA, the cells were harvested and treated with lysis buffer. The extracts were centrifuged at 13,000×g at 4°C for 10 min and the supernatant was recovered. The concentration of protein in the supernatant was determined, and then the supernatant was used for Western blot analysis. The concentration of total protein was determined by the modified Lowry method using bovine serum albumin as a standard. The samples were stored at −20°C for further use.
Equal amount of protein was added to each lane, and sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed using 10% polyacrylamide gels. Proteins were transferred to PVDF membranes (Hybond-P, GE Healthcare, Amersham, UK) using a semidry transfer system. The blots were blocked for 1 h at room temperature with 5% (w/v) BSA (applied to all primary antibodies excepting for GAPDH) and 5% (w/v) skim milk (only applied to GAPDH) in Tris-buffered saline solution (TBS) containing 0.1% Tween-20. The membrane was incubated with specific rabbit polyclonal antibodies against GABAA receptors subunits (diluted 1:2,500 in TBS containing 0.1% Tween-20, 5.0% BSA) and rabbit anti-GAD65/67 polyclonal antibody (diluted 1:2,500 in TBS containing 0.1% Tween-20, 5.0% BSA). Blots were then washed and incubated with the horseradish peroxidase conjugated secondary antibodies: goat anti-rabbit IgG (diluted 1:3,000 in TBS containing 0.1% Tween-20, 1.0% BSA). Immunoreactive bands were developed with a BM chemiluminescence detection kit (Roche Diagnostics, Mannheim, Germany). The quantitative analysis of detected bands was performed with densitometric scanning, and all values were normalized to the amount of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in the sample, which was measured as follows. All of the immunoblots were stripped, incubated with sheep anti-GAPDH (1:2,500 in TBS containing 0.1% Tween-20) subsequently incubated with anti-sheep IgG-conjugated secondary antibodies: rabbit anti-sheep IgG (1:3,000 in TBS containing 0.1% Tween-20), and developed to confirm equal protein loading.
Statistical analysis
All statistical analysis was conducted using SigmaStat software (SPSS Inc., Chicago, IL, USA). The data are expressed as mean ± S.E.M. The significance of the effects of the compounds was assessed by analysis of variance (ANOVA). In the case of significant variation, the individual values were compared using post hoc Dunnett’s test. For the subhypnotic pentobarbital dosage test, For statistical test of the sub-hypnotic pentobarbital dose, Chi square test was used to compare the proportion of sleep onset between the group treated with a sub-hypnotic dose of pentobarbital alone and each of the other groups. A p-value of less than 0.05 was considered to be significant.
RESULTS
Effects of PA and diazepam on locomotor activity test in mice
The locomotor activity of mice treated with different dose of PA or diazepam was compared with that of mice treated with vehicle. It was found that PA at a dose of 5 mg/kg significantly inhibited locomotor activity in mice. Moreover, diazepam (2 mg/kg) also showed significant decrease in locomotor activity in mice (Fig. 2).
Fig. 2.
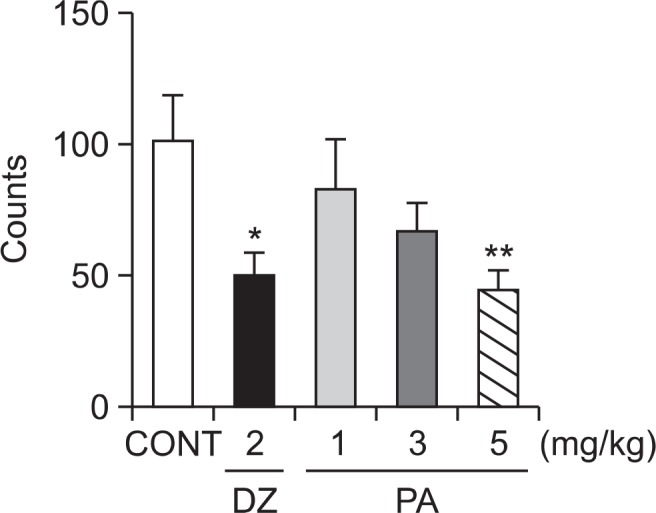
Effects of PA and diazepam on locomotor activity test in mice. Ambulation activity was measured for 1 h, 30 min after oral administration of diazepam and 1 h after the administration of PA. Each column represents the mean with S.E.M (n=10). The significance of the effects of the compounds was assessed using analysis of variance (ANOVA). Where there was significant variability, the individual values were compared using Dunnett’s- test. *p<0.05, **p<0.01, compared with that of control.
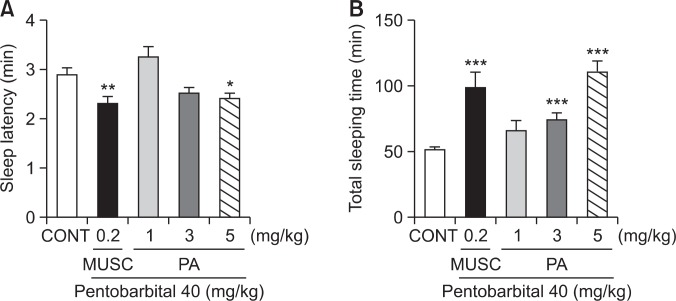
Effects of PA on the onset and duration of sleep in pentobarbital-treated mice
Oral administration of PA decreased the latency of sleep and increased total sleep time when given with hypnotic dose of pentobarbital (40 mg/kg). PA produced a dose-dependent prolongation of pentobarbital-induced sleep time at the doses of 3 and 5 mg/kg, respectively. PA (5 mg/kg) significantly reduced the latency of sleep. Pretreatment of mice with muscimol (0.2 mg/kg, i.p.) as a positive control, 30 min before the administration of pentobarbital also produced an increase in the sleeping time and a decrease in the sleep latency of sleep (Fig. 3).
Fig. 3.
Effects of PA on onset and duration of sleep in pentobarbital-treated mice. Mice were food deprived for 24 h prior to the experiment. Pentobarbital (40 mg/kg, i.p) was administered to mice following administration of muscimol or PA. The sleep latency (A) sleep time (B) were recorded. Each column represents the mean with S.E.M (n=10). The significance of the effects of the compounds was assessed using analysis of variance (ANOVA). Where there was significant variability, the individual values were compared using Dunnett’s- test. *p<0.05, **p<0.01, ***p<0.005, compared with that of control.
Effects of PA on sleep onset of mice treated by sub-hypnotic dosage of pentobarbital
PA reduced the sleep onset and increased the duration of sleep time induced by sub-hypnotic dose of pentobarbital (28 mg/kg. i.p.). Pretreatment of muscimol also increased the rate of sleep onset and prolonged the sleep time at sub-hypnotic dosage of pentobarbital (Table 1).
Table 1.
Effects of PA on sleep onset of mice treated by subhypnotic dose of pentobarbital (28 mg/kg, i.p.)
| Group | Dose (mg/kg) | No. falling asleep/total | Sleep time (min) |
|---|---|---|---|
| Control | 0 | 8/15 | 30.4 ± 3.8 |
| Muscimol | 0.2 | 10/14*** | 42.9 ± 3.9* |
| PA | 1 | 11/14 | 37.0 ± 2.3 |
| 3 | 12/14 | 38.1 ± 3.0 | |
| 5 | 12/13*** | 79.1 ± 8.1*** |
Each value represents the mean (±S.E.M.) of 13–15 observations.
p<0 .05 and
p<0.005 compared to control.
Effects of PA on chloride ion influx in primary cultured hypothalamic neuronal cells
Intracellular Cl− influx in primary cultured hypothalamic neuron cells was measured. The measured data is presented as the relative fluorescence F/F0, where F is the fluorescence as a function of each sample and F0 is the fluorescence without chloride ions. The F/F0 values were directly proportional to intracellular chloride ion concentration. Treatment of hypothalamic neuron cells with PA at 1 μM and 10 μM produced a significant increase in intracellular chloride ion influx dose-dependently. Additionally, Pentobarbital (10 μM) also showed significant increase in the influx of Cl− in primary cultured hypothalamic neurons (Fig. 4).
Fig. 4.
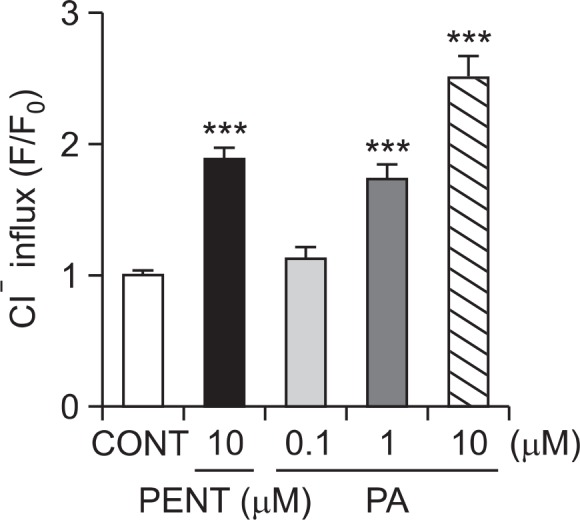
Effects of PA on chloride influx in primary cultured hypothalamic neuron cells. After the culture of hypothalamic neurons for 8 days, the cells were incubated with MQAE overnight, and then PA (0.1, 1 and 10 μM) and pentobarbital (PENT 10 μM) were added 1 h prior to measurement. Each column represents the mean with S.E.M (n=3). The significance of the effects of the compounds was assessed using analysis of variance (ANOVA). Where there was significant variability, the individual values were compared using Dunnett’s- test. ***p<0.005, compared with that of control.
Effects of PA on GAD65/67 expression
In order to confirm PA affects pentobarbital-induced sleeping behaviors through the synthesis of GABA, 8 days primary cultured neuronal cells were treated with broader range of PA concentration (5 and 50 μM, respectively) for 1 h and they were sacrificed to examine the effects of these drugs on the abundance of GAD65/67 in the hypothalamus. PA treatment at 50 μM significantly increased the protein content of GAD65/67 (Fig. 5).
Fig. 5.
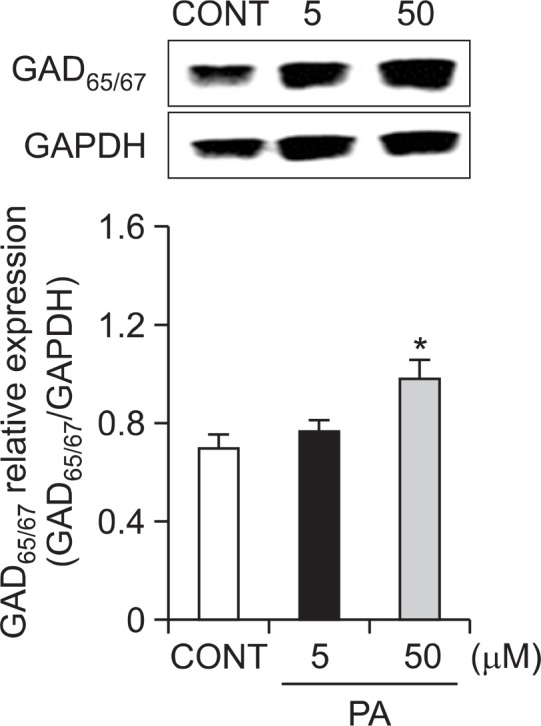
Effects of PA on the expression of GAD. Immunobolts of lysed neuronal cells which were treated for 1 h following PA are shown. GAPDH levels were need for the normalization of the protein expression. Each column represents the mean with S.E.M (n=3). The significance of the effects of the compounds was assessed using analysis of variance (ANOVA). Where there was significant variability, the individual values were compared using Dunnett’s- test. *p<0.05, compared with that of control.
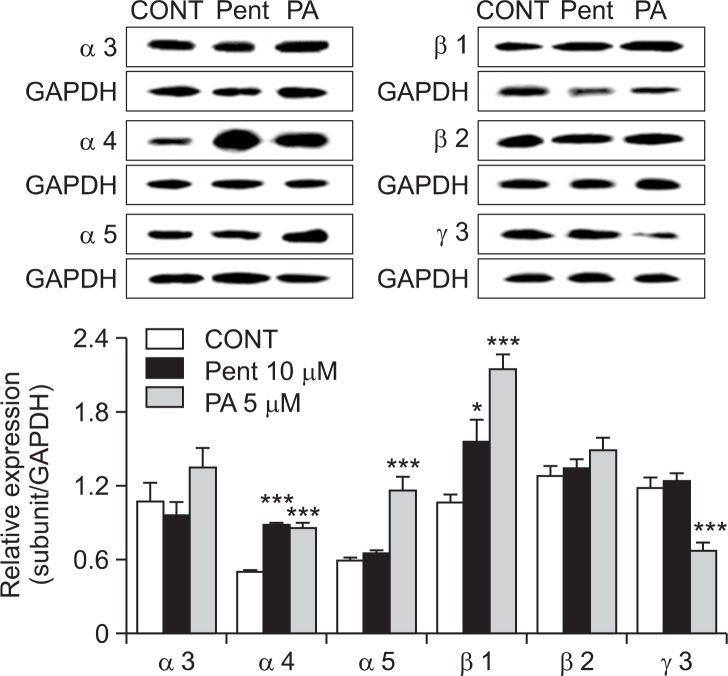
Effects of PA on of GABAA receptors subunits expression
Primary cultured neuronal cells of hypothalmus were treated with PA (5 μM) and pentobarbital (PENT 10 μM) for 1 h and they were sacrificed to examine the effects of these drugs on the abundance of GABAA receptor subunits in the cerebella (Fig. 6). We performed expression patterns on 3α-subunits (α3, α4 and α5), 2β-subunits (β1 and β2) and γ-subunit (γ3) in GABAA receptor in primary cultured cerebella granule cells in rat. Treatment of cells with PA produced over-expressed protein levels for α- and β-subunits in GABAA receptors in this culture model. However, PA treatment with cell decreased γ-subunits in GABAA receptors in rats. Pentobarbital (positive control) increased expression for α4, α5, β1, β2 and γ3-subunits, but decreased abundance for α3-subunits in GABAA receptors in rat.
Fig. 6.
Effects of PA on expression of GABAA receptors subunits. Immunobolts of lysed neuronal cells which were treated for 1 h following PA are shown. GAPDH levels were need for the normalization of the protein expression. Each column represents the mean with S.E.M (n=3). The significance of the effects of the compounds was assessed using analysis of variance (ANOVA). Where there was significant variability, the individual values were compared using Dunnett’s- test. *p<0.05, ***p<0.005, compared with that of control.
DISCUSSION
PA, a lanostane type triterpenoid, one of the major constituents from Hoelen, has been reported to possess anti-emetic, anti-inflammatory, and anti-cancer properties (Tai et al., 1995; Cuella et al., 1996; Kaminaga et al., 1996; Giner et al., 2000; Li et al., 2004; Gapter et al., 2005). The ultimate goal of the present study was to determine the hypnotic effects of PA on pentobarbital-induced sleeping behaviors, and also to elucidate the possible underlying mechanism of sleep, possibly via GABAA-ergic transmission and Cl− channel activation. Both in vivo and in vitro studies were conducted to evaluate hypnotic effects of PA. First of all, the locomotor activity was performed in mice. Experimental animals showed that the oral administration of PA 5 mg/kg) significantly inhibited locomotor activity in mice (Fig. 2). Moreover, diazepam (2 mg/kg) also produced significant inhibition of locomotor activity in tested animals. Thus, this evidence supports the hypothesis that locomotor activity was suppressed due to an interaction with the benzodiazepine site on the GABAA receptors. Earlier reports suggest that the increase and decrease of pentobarbital-induced sleep time can be a useful tool for examining the stimulatory or inhibitory effects on CNS, especially for investigating influences on GABAA-ergic systems in CNS (de Sousa et al., 2005; Ma et al., 2007). Therefore, in an effort to explore the hypnotic effects of PA, we investigated the effects of different doses of PA and muscimol in mice with pentobarbital treatment. We observed that that both PA and muscimol (GABAA receptors agonist) potentiated the sedative-hypnotic effects of pentobarbital in mice by decreasing sleep latency and increasing sleep duration. Experimental data showed that PA decreased the latency of sleep and increased total sleep time when given with hypnotic dose of pentobarbital (40 mg/kg). PA produced a dose-dependent prolongation of pentobarbital-induced sleep time. Notably, PA (5 mg/kg) only showed significant reduction in the latency of sleep. Muscimol (a GABAA agonist), also produced an increase in the sleeping time and a decrease in the latency of sleep (Fig. 3). Additionally, PA also increased the rate of sleep onset and the duration of sleep time induced by a sub hypnotic dose of pentobarbital (28 mg/kg, Table 1). Thus, it is important to state that PA prolonged pentobarbital-induced sleep behaviors and interact with pentobarbital on the CNS via GABAA-ergic mechanisms in CNS.
Benzodiazepine drugs have been used extensively for the treatment of insomnia, in addition to other central nervous system disorders. These drugs act through potentiating of the effects of the inhibitory neurotransmitter of GABA, by binding to a specific site on the GABAA receptors to produce allosteric enhancement of anion flux through this ligand-gated chloride channel (McKernan and Whiting, 1996; Barnard et al., 1998; Mohler et al., 2002). To confirm the effects of PA on producing increased sleep activity due to an interaction with the benzodiazepine site on the GABAA receptors, we focused to know whether PA acted to initiate intracellular Cl− channel opening of GABAA receptors. We observed that PA (1.0 and 10 μM) produced a significant increase in intracellular chloride ion influx dose-dependently in hypothalamus culture model. Reports suggest that muscimol and other GABAA receptors agonists cause potentiating of Cl− influx when administered with pentobarbital or other agonists (Chistina Grobin et al., 1999). Moreover, Pentobarbital (10 μM) also showed significant increase in the influx of Cl− in primary cultured hypothalamic neurons (Fig. 4). Thus, we hypothesize that this might have resulted through the activation of the GABAA receptors and hyperpolarization of the neuronal membrane (Imamura and Prasad, 1998).
The classical view of neuronal activity relies on the balance between two modes of transmission, excitation mediated by glutamate and inhibition mediated by GABA. Glutamic acid de carboxylase (GAD) is the rate-limiting enzyme in GABA bio-synthesis which also plays an important role in maintaining GABA levels in the brain (Tillakaratne et al., 1995). Therefore, alteration of expression levels of this enzyme may change GABA transmission in the brain. In order to confirm the PA affects pentobarbital-induced sleeping behaviors through the synthesis of GABA, we investigated the expression levels of GAD65/67 in 8 day in vitro cultured neuronal cells, which is responsible for GABA production. PA treatment at 50 μM significantly increased the protein content of GAD65/67 (Fig. 5).
It is noteworthy to mention that the analysis of subunit expression is an important tool to understand the underlying basic pharmacology and functional significance of GABAA receptors subunits in various brain regions. The most abundant GABAA receptors subunits composition, α1β2γ2, is pre sent in most brain areas, including the hypothalamus and these subunits are related to the hypnotic/sedative effects of GABAA receptors agonists (Rudolph and Mohler, 2006). There is a wide diversity of GABAA receptor subtypes, and those most highly expressed in the CNS include the α1βγ2, α2βγ2, α3βγ2 and α5βγ2 isoforms. In vitro work examining the expression of GABAA receptors subunits by hypothalamic ne u ronal cells has indicated that the developmental change in subunits from α1β2γ2 in young neuroanl cells to α1α6β2γ2 in mature cells alters substantially the pharmacodynamics of GABAA receptors expressed by these cells as they develop (Mathews et al., 1994). We examined expression patterns of 3α-subunits (α3, α4 and α5) 2β-subunits (β1 and β2) and γ-subunit (γ3) in GABAA receptors in 8 day in vitro primary cultured neuronal cells, to assess the regulation of GABAA receptors expression. Treatment of cells with PA produced over-expressed protein levels for α- and β-subunits in GABAA receptors in this culture model. However, PA treatment with cell decreased γ-subunits in GABAA receptors in rats. Pentobarbital (positive control) increased expression for α4, α5, β1, β2 and γ3-subunits, but decreased α3-subunits in GABAA receptor (Fig. 6). This complex and varied expression supports the hypothesis that GABA may play a role in cellular and synaptic differentiation. Thus, we hypothesize that these results suggest the stimulation of receptors affects their expression.
Most sedative-hypnotics used in the treatment of insomnia target the GABAA receptors. It is revealed that anxiolytic, anticonvulsant, myorelaxant, as well as amnestic, sedative and hypnotic actions of these drugs are due to their “general” inhibitory effect on neuronal activity (Rudolph and Mohler, 2004). There is growing fact that the search for novel plant-derived pharmacotherapies for psychiatric illness has progressed significantly in the past decade. With time a considerable number of herbal constituents whose behavioral effects and pharmacological actions have been well characterized may be good candidates for further investigations that may ultimately lead to clinical use of these constituents. The potential benefits of herbal remedies such as St. John’s wort and Kava-kava in psychiatric practice have been addressed previously (Zhang, 2004). Both in vivo and in vitro studies were performed to evaluate PA as potential sleep therapeutic agent. In the present investigation, PA alone showed effect in chloride influx and expression of GAD in primary neuronal model. Some drugs such as Honokiol showed enhanced sleeping behaviors with pentobarbital induction (Ma et al., 2008). Moreover, specific sleep studies such as EEG (Electroencephalogram) provide the drugs alone effect (Qu et al., 2012). The present study was to determine the sedative-hypnotic effects of PA on pentobarbital-induced sleeping behaviors. Of a note, we tested PA in pentobarbital- treated mice. In addition, we defined the mediation of γ-aminobutyric acid (GABA)-ergic systems to understand the possible mechanisms. Thereby, we suggest that PA held promise for a new class of compounds as potential agents in the treatment of sleep disorders, such as insomnia.
In conclusion, our study provided evidence that PA enhanced hypnotic effects in pentobarbital-treated mice. Additionally, this invention illustrates the enhancement may result from Cl− channel activation and GABAA-ergic transmission. Thus, PA deserves further investigation to understand the underlying pharmacological characteristics. The present findings provide compelling evidence to support a critical role of this compound an attractive candidate as an improved treatment for insomnia, a concept which is currently being tested in multiple clinical studies. We are going to continue to further experiments whether PA induces sleep in animals, using EEG sleep analysis method.
Acknowledgments
This work is financially supported by the Ministry of Trade, Industry & Energy (MOTIE, 1415126993) through the fostering project of Osong Academy-Industry Convergence (BAIO).
REFERENCES
- Abourashed EA, Koetter U, Brattstrom A. In vitro binding experiments with a Valerian, hops and their fixed combination extract (Ze91019) to selected central nervous system receptors. Phytomedicine. 2004;11:633–638. doi: 10.1016/j.phymed.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Chen FP, Jong MS, Chen YC, Kung YY, Chen TJ, Chen FJ, Hwang SJ. Prescriptions of Chinese Herbal Medicines for Insomnia in Taiwan during 2002. Evid Based Complement Alternat Med. 2011;2011:236341. doi: 10.1093/ecam/nep018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistina Grobin A, Inglefield JR, Schwartz-Bloom RD, Devaud LL, Morrow AL. Fluorescence imaging of GABAA receptor-mediated intracellular [Cl−] in P19-N cells reveals unique pharmacological properties. Brain Res. 1999;827:1–11. doi: 10.1016/s0006-8993(99)01223-8. [DOI] [PubMed] [Google Scholar]
- Cuella MJ, Giner RM, Recio MC, Just MJ, Manez S, Rios JL. Two fungal lanostane derivatives as phospholipase A2 inhibitors. J Nat Prod. 1996;59:977–979. doi: 10.1021/np9604339. [DOI] [PubMed] [Google Scholar]
- Darias V, Abdala S, Martin-Herrera D, Tello ML, Vega S. CNS effects of a series of 1,2,4-triazolyl heterocarboxylic derivatives. Pharmazie. 1998;53:477–481. [PubMed] [Google Scholar]
- de Sousa FC, Pereira BA, Lima VT, Lacerda CD, Melo CT, Barbosa-Filho JM, Vasconcelos SM, Viana GS. Central nervous system activity of yangambin from Ocotea duckei Vattimo (Lauraceae) in mice. Phytother Res. 2005;19:282–286. doi: 10.1002/ptr.1499. [DOI] [PubMed] [Google Scholar]
- Gapter L, Wang Z, Glinski J, Ng KY. Induction of apoptosis in prostate cancer cells by pachymic acid from Poria cocos. Biochem Biophys Res Commun. 2005;332:1153–1161. doi: 10.1016/j.bbrc.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Giner EM, Manez S, Recio MC, Giner RM, Cerda-Nicolas M, Rios JL. In vivo studies on the anti-inflammatory activity of pachymic and dehydrotumulosic acids. Planta Med. 2000;66:221–227. doi: 10.1055/s-2000-8563. [DOI] [PubMed] [Google Scholar]
- Imamura M, Prasad C. Increased GABA-gated chloride ion influx in the hypothalamus of low-anxiety rats. Physiol Behav. 1998;64:415–417. doi: 10.1016/s0031-9384(98)00105-x. [DOI] [PubMed] [Google Scholar]
- Kaminaga T, Yasukawa K, Kanno H, Tai T, Nunoura Y, Takido M. Inhibitory effects of lanostane-type triterpene acids, the components of Poria cocos, on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology. 1996;53:382–385. doi: 10.1159/000227592. [DOI] [PubMed] [Google Scholar]
- Lee SM, Lee YJ, Yoon JJ, Kang DG, Lee HS. Effect of Poria cocos on hypertonic stress-induced water channel expression and apoptosis in renal collecting duct cells. J Ethnopharmacol. 2012;141:368–376. doi: 10.1016/j.jep.2012.02.048. [DOI] [PubMed] [Google Scholar]
- Li G, Xu ML, Lee CS, Woo MH, Chang HW, Son JK. Cytotoxicity and DNA topoisomerases inhibitory activity of constituents from the sclerotium of Poria cocos. Arch Pharm Res. 2004;27:829–833. doi: 10.1007/BF02980174. [DOI] [PubMed] [Google Scholar]
- Ma Y, Han H, Eun JS, Kim HC, Hong JT, Oh KW. Sanjoinine A isolated from Zizyphi Spinosi Semen augments pentobarbital-induced sleeping behaviors through the modification of GABA-ergic systems. Biomol Ther. 2007;30:1748–1753. doi: 10.1248/bpb.30.1748. [DOI] [PubMed] [Google Scholar]
- Ma Y, Jo YJ, Woo SS, Hong JT, Oh KW. Honokiol potentiates pentobarbital-induced sleeping behaviors through GABA (A) receptor CI-channel activation. J Applied Pharmacol. 2008;16:328–335. [Google Scholar]
- Mathews GC, Bolos-Sy AM, Holland KD, Isenberg KE, Covey DF, Ferrendelli JA, Rothman SM. Developmental alteration in GABAA receptor structure and physiological properties in cultured cerebellar granule neurons. Neuron. 1994;13:149–158. doi: 10.1016/0896-6273(94)90465-0. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Kaiyala KJ, Fisher JD, Ogimoto K, Schwartz MW, Wisse BE. Identification of a physiological role for leptin in the regulation of ambulatory activity and wheel running in mice. Am J Physiol Endocrinol Metab. 2011;300:E392–401. doi: 10.1152/ajpendo.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Cha HY, Seo JJ, Hong JT, Han K, Oh KW. Anxiolytic-like effects of ginseng in the elevated plus-maze model: comparison of red ginseng and sun ginseng. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:895–900. doi: 10.1016/j.pnpbp.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Qu WM, Yue XF, Sun Y, Fan K, Chen CR, Hou YP, Urade Y, Huang ZL. Honokiol promotes non-rapid eye movement sleep via the benzodiazepine site of the GABA(A) receptor in mice. Br J Pharmacol. 2012;167:587–598. doi: 10.1111/j.1476-5381.2012.02010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann. Clin. Psychiatry. 2006;18:49–56. doi: 10.1080/10401230500464711. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev. 2006;11:128–150. [PubMed] [Google Scholar]
- Tai T, Akita Y, Kinoshita K, Koyama K, Takahashi K, Watanabe K. Anti-emetic principles of Poria cocos. Planta Med. 1995;61:527–530. doi: 10.1055/s-2006-959363. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJ, Medina-Kauwe L, Gibson KM. gamma-Aminobutyric acid (GABA) metabolism in mammalian neural and nonneural tissues. Comp Biochem Physiol A Physiol. 1995;112:247–263. doi: 10.1016/0300-9629(95)00099-2. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Ebert B. Emerging anti-insomnia drugs: tackling sleeplessness and the quality of wake time. Nat Rev Drug Discov. 2008;7:530–540. doi: 10.1038/nrd2464. [DOI] [PubMed] [Google Scholar]
- West MR, Molloy CR. A microplate assay measuring chloride ion channel activity. Anal Biochem. 1996;241:51–58. doi: 10.1006/abio.1996.0377. [DOI] [PubMed] [Google Scholar]
- Wolfman C, Viola H, Marder M, Wasowski C, Ardenghi P, Izquierdo I, Paladini AC, Medina JH. Anxioselective properties of 6,3’-dinitroflavone, a high-affinity benzodiazepine receptor ligand. Eur J Pharmacol. 1996;318:23–30. doi: 10.1016/s0014-2999(96)00784-4. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ. Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci. 2004;75:1659–1699. doi: 10.1016/j.lfs.2004.04.014. [DOI] [PubMed] [Google Scholar]