Abstract
Collagen pentapeptide (Lys-Thr-Thr-Lys-Ser, KTTKS) and its palmitoylated derivative (pal-KTTKS) have received a great deal of attention as cosmeceutical ingredients for their anti-wrinkle effects. The objective of this study was to evaluate stability and permeability of KTTKS and pal-KTTKS in hairless mouse skin. In this study, a liquid chromatography-tandem mass spectrometric method was developed for the quantification of pal-KTTKS, and used for stability and permeability studies. Stability studies were performed using skin extracts and homogenates. Both KTTKS and pal-KTTKS were rapidly degraded, but pal-KTTKS was more stable than KTTKS. When protease inhibitors were added, the stability of both compounds (KTTKS and pal-KTTKS) improved significantly. In the skin permeation study, neither KTTKS nor pal-KTTKS was detected in the receptor solution, which indicates that neither compound could permeate through the full-thickness hairless mouse skin in the experimental conditions of this study. While KTTKS was not detected in any of the skin layers (the stratum corneum, epidermis, and dermis), pal-KTTKS was observed in all skin layers: 4.2 ± 0.7 μg/cm2 in the stratum corneum, 2.8 ± 0.5 μg/cm2 in the epidermis, and 0.3 ± 0.1 μg/cm2 in the dermis. In conclusion, this study indicated that pal-KTTKS had greater stability and permeability than that of un-modified KTTKS, and may be a useful anti-wrinkle and anti-aging cosmeceutical agent.
Keywords: Collagen pentapeptide, KTTKS, Palmitoyl-KTTKS, Stability, Permeability
INTRODUCTION
Collagen pentapeptide (Lys-Thr-Thr-Lys-Ser, KTTKS) is a subfragment of the carboxyl-terminal propeptide of type I collagen (Katayama et al., 1991). It has been shown to promote extracellular matrix (ECM) production in subconfluent fibro-blasts (Katayama et al., 1993; Abu Samah and Heard, 2011). KTTKS has also been shown to promote the expression of type I collagen and stabilize mRNA associated with the up-regulation of transforming growth factor-β in rat Achilles tendon cells (Tsai et al., 2007). These properties made KTTKS to be widely studied and applied in the field of cosmeceuticals for its expected anti-wrinkle and anti-aging effects (Draelos, 2007; Lupo and Cole, 2007; Reszko et al., 2009). KTTKS belongs to a ‘signal peptide’, which can enhance dermal remodeling by triggering cellular processes such as inhibition of collagenase activities and promotion of ECM production (Gorouhi and Maibach, 2009).
When peptides are topically applied, their instability on or in the skin and poor permeability across the skin are challenges for successful dermal delivery. The conjugation of a fatty acid to a peptide is often an effective method to improve the stability and permeability of peptides in the skin (Benson and Namjoshi, 2008). Foldvari et al. reported that palmitoyl derivatives of interferon α increased penetration across human skin approximately five-fold over that of the native protein (Foldvari et al., 1998). In a similar manner, a palmitoyl derivative of KTTKS (pal-KTTKS) would be expected to improve stability and enhance permeability in the skin owing to increased lipophilicity drived from the palmitate residue (Lau et al., 2008; Pouillot et al., 2008). Several studies have shown the clinical benefits of pal-KTTKS. Topical formulations containing pal-KTTKS were shown to significantly reduce fine lines and wrinkles and improve skin texture. These effects may help to delay the aging process in the skin (Robinson et al., 2005; Abu Samah and Heard, 2011).
Although several studies have reported the clinical advantages of KTTKS and its derivatives, there are few in vitro studies supporting dermal delivery of KTTKS and pal-KTTKS (Pouillot et al., 2008; Gorouhi and Maibach, 2009; Abu Samah and Heard, 2011). The assessments of dermal stability and skin permeability of KTTKS and pal-KTTKS are important issues for successful dermal delivery. For these studies, appropriate analytical methods should be also developed. Recently, we reported a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method to monitor the stability of KTTKS in rat skin (Park et al., 2012). Chirita et al. reported the LC-MS/MS method for the quantification of pal-KTTKS in cosmetic preparations (Chirita et al., 2009).
In this study, the LC-MS/MS method for the quantification of pal-KTTKS in the skin was developed and the stability of KTTKS and pal-KTTKS in the skin of hairless mice was examined. Various types of proteolytic enzyme inhibitors were tested to prevent the degradation of KTTKS and pal-KTTKS in the skin. We also evaluated the skin permeation and retention of KTTKS and pal-KTTKS across whole hairless mouse skin.
MATERIALS AND METHODS
Materials
KTTKS, GHK (Gly-His-Lys) and pal-KTTKS were obtained from Peptron (Daejon, Korea). Ethylenediaminetetraacetic acid (EDTA), DL-thiorphan (TP), thimerosal (TM), phenylmeth anesulfonylfluoride (PMSF), 1,10-phenanthroline (PNT), bovine serum albumin (BSA) and ascorbic acid 6-palmitate (pal-AA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pentafluoropropionic acid (PFPA) was obtained from TCI (Tokyo, Japan). All other chemicals were of analytical reagent grade.
Experimental animals
Hairless mice were obtained from Orient Bio Inc. (Gyeonggi, Korea). Male CrlOri:SKH1-hr strain hairless mice, weighing 25 ± 3 g, were used throughout this experiment. Skin preparation process was carried out immediately when the mice were delivered. The animal experiments were approved by Kyungsung University Animal Care and Use Committee, and all procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institute of Health.
Preparation of epidermal and dermal skin extracts
Hairless mice were sacrificed by cervical dislocation and dorsal skin was removed from the nape of the neck and back. The adherent fat and subcutaneous tissue were removed. Hairless mouse skin without the stratum corneum was prepared by a tape stripping method (Benson et al., 2003; Kikwai et al., 2005). The prepared mouse skin without the stratum corneum was mounted on a side-by-side diffusion cell with the epidermal layer facing the donor compartment. The diffusion cell had approximately 0.8 cm2 effective diffusion surface area. The donor and receptor compartments were filled with 2.5 ml of HEPES buffer (10 mM, pH 7.4) and kept at 37°C. The receptor and donor solutions were collected at 6, 10, and 24 h. After collection, the compartments were immediately refilled with 2.5 ml of fresh HEPES buffer (10 mM, pH 7.4). The total volume of skin extracts from the donor and receptor compartments for each diffusion cell study was 7.5 ml. The receptor solution extracted from the dermal side of the skin was used as the dermal skin extract, and the donor solution extracted from the epidermis without the stratum corneum was used as the epidermal skin extract for the stability tests. These extracts were filtered using Minisart RC 15 (pore size: 0.2 μm, Sartorius, Germany) and stored at 4°C prior to use.
Preparation of a whole skin homogenate
Hairless mouse whole skin (0.8 cm2) was cut into small pieces. A skin homogenate was prepared using 1 ml of ice-cold sterilized distilled water for 15 min by using a microhomogenizer (High Intensity Ultrasonic Liquid Processors, Sonics & Materials, CT, USA). The skin homogenate was centrifuged at 10,000 rpm for 30 min at 4°C. The supernatant was collected and filtered with Minisart RC 15 (pore size: 0.2 μm, Sartorius, Germany). The filtrate was diluted to the final volume of 7.5 ml with the HEPES buffer solution (10 mM, pH 7.4).
Stability test of KTTKS and pal-KTTKS
A portion (200 μl) of KTTKS or pal-KTTKS (40 μg/ml in 10 mM HEPES buffer, pH 7.4, as peptide concentration) was incubated with 200 μl of the epidermal skin extract, dermal skin extract, or skin homogenates at 37°C for 120 min. The protein concentrations of epidermal skin extract, dermal skin extract, and skin homogenates determined by Lowry method using BSA as standard (Lowry et al., 1951) were 2, 15, and 100 μg/ml, respectively. At predetermined times, samples were taken from the incubation mixture. The amount of KTTKS or pal-KTTKS in the samples was analyzed using LC-MS/MS. The effects of various proteolytic enzyme inhibitors were also investigated. Five kinds of protease inhibitors, TM (0.5 mM), TP (0.25 mM), EDTA (0.5 mM), PMSF (5 mM), and PNT (1 mM), were added to KTTKS or pal-KTTKS in skin extracts or homogenates at 37°C for 120 min. In addition, the combination of PMSF (5 mM) and PNT (1 mM) was added to KTTKS or pal-KTTKS in skin extracts or homogenates.
In vitro skin permeation study of KTTKS and pal-KTTKS
Intact hairless mouse skin was mounted on Franz diffusion cells with the epidermal side facing the donor compartment. The receptor compartment was filled with HEPES buffer (10 mM, pH 7.4) mixed with 15% ethanol containing PMSF and PNT (final concentrations of 5 mM and 1 mM, respectively) as proteolytic enzyme inhibitors. The donor compartment was loaded with 1 ml of KTTKS or pal-KTTKS (100 μg/ml in 15% ethanol) solution. The receptor solution was taken out periodically and replaced with fresh buffer solution over a period of 48 h. The collected samples were centrifuged and the super-natants were analyzed by LC-MS/MS.
To assess the amount of the peptides retained in the skin layers, the skin was removed from the diffusion cell after 24 h of sample loading. Donor solution remaining on the surface of the skin was removed by washing with 1 ml of distilled water (4 times repeatedly). Lint-free absorbent wipes were used to dry the skin. The stratum corneum layer was carefully collected by raking with a blunt spatula. The epidermis was separated from the dermis by using a blunt knife. Each separated skin layer (stratum corneum, epidermis, and dermis) was minced using scissors. KTTKS or pal-KTTKS distributed in each skin layer was extracted using 1 ml of methanol for 24 h with continuous shaking. After extraction for 24 h, the samples were centrifuged and the supernatants were analyzed by performing LC-MS/MS.
LC-MS/MS
LC-MS/MS was performed using an Agilent Series 1200 LC instrument with 6410 Triple Quad LC-MS/MS system (Agilent Technologies, Palo Alto, CA, USA). Analysis of KTTKS was performed using Acclaim 300 C18 column (2.1×150 mm, 3 μm, Dionex, Sunnyvale, CA, USA) by isocratic elution using a mobile phase consisting of 5 mM PFPA aqueous solution and acetonitrile (87:13, v/v) as described previously (Park et al., 2012). Pal-KTTKS was analyzed using Capcell Pak C8 column (2.0×100 mm, 5 μm, Shisheido, Tokyo, Japan) by isocratic elution using a mobile phase consisting of 20 mM ammonium acetate solution and acetonitrile (30:70, v/v) containing 0.05% acetic acid. The flow rate was 0.2 ml/min. The GHK and the pal-AA were used as internal standards (IS) for KTTKS and pal-KTTKS, respectively. Data from mass spectrometry were collected and processed using Qualitative Analysis software version B.04.00 from Mass Hunter Workstation.
Validation of LC-MS/MS method
A standard stock solution of pal-KTTKS at a concentration of 1 mg/ml was prepared by dissolving pal-KTTKS in methanol. Then, dilutions were made to obtain calibration standards at concentrations of 0.5, 1, 2, 5, 10, and 20 μg/ml. The concentration of pal-AA used as IS was 10 μg/ml. The calibration curve was plotted logarithmically using peak-area ratios of pal-KTTKS to IS versus concentrations of pal-KTTKS. Intraand inter-day precision and accuracy of the LC-MS/MS method were determined by analyzing quality control (QC) samples of pal-KTTKS at concentrations of 0.5, 2, 5, and 20 μg/ml. Intra-day precision was determined by repeating the analysis of each QC sample three times a day. Inter-day precision and accuracy were determined by repeating this analysis on three consecutive days. Precision (expressed as the relative standard deviation) should be <15%, and accuracy should be within ± 15%. The lower limit of quantitation (LLOQ) is acceptable with precision <20% and accuracy within ±20%.
RESULTS
LC-MS/MS of pal-KTTKS
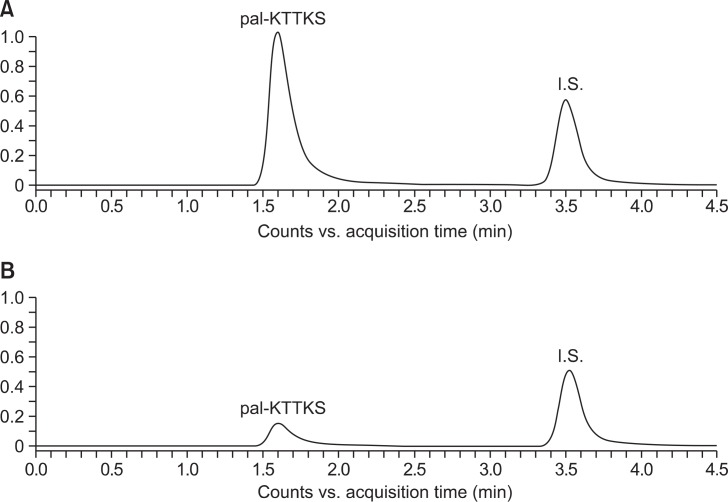
The LC-MS/MS method for the quantification of pal-KTTKS was developed by simultaneously monitoring the selected MS/MS transitions for pal-KTTKS (m/z 802.3→129.0) and pal-AA as IS (m/z 415.3→239.1). Fig. 1 shows the LC-MS/MS chromatograms of pal-KTTKS and IS (pal-AA) spiked with the dermal skin extract and after incubation in the dermal skin extract for 60 min at 37°C. The retention times of pal-KTTKS and IS were 1.6 and 3.5 min, respectively. LC-MS/MS of KTTKS was performed using the previously developed methods (Park et al., 2012).
Fig. 1.
LC-MS/MS chromatograms of pal-KTTKS (m/z 802.3→129.0) and pal-AA as IS (m/z 415.3→239.1) spiked with the dermal skin extract of hairless mouse before (A) and after (B) incubation in the dermal skin extract for 60 min at 37°C.
The LC-MS/MS method for analysis of pal-KTTKS was validated (Table 1). In terms of specificity, no interfering peaks were found with the same retention times as pal-KTTKS and IS in the skin extracts and homogenates. The calibration curve showed good linearity in the concentration range of 0.5–20 μg/ml with a slope of 1.5265, an intercept of −1.844, and r2 value of 0.9982. Intra- and inter-day precision and accuracy were determined by analyzing three replicates at each of 0.5 (LLOQ), 2, 5, and 20 μg/ml of pal-KTTKS. At LLOQ, intra- and inter-day accuracies were 97.8% and 116.4%, respectively, and intraand inter-day precisions were 14.3% and 10.3%, respectively. Overall, the intra- and inter-day accuracies were 97.8–101.4% and 98.0–116.4%, respectively, and intra- and inter-day precisions were 1.7–14.3% and 1.2–10.3%, respectively.
Table 1.
Validation of LC-MS/MS method for pal-KTTKS
| pal-KTTKS added (μg/ml) | Intra-day | Inter-day | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Amount measured (μg/ml)a | Accuracy (%) | Precision (%) | Amount measured (μg/ml)a | Accuracy (%) | Precision (%) | |
| 0.5 | 0.49 ± 0.16 | 97.8 | 14.3 | 0.58 ± 0.06 | 116.4 | 10.3 |
| 2 | 2.00 ± 0.18 | 100.1 | 9.0 | 2.03 ± 0.09 | 101.3 | 4.3 |
| 5 | 5.07 ± 0.25 | 101.4 | 5.0 | 4.90 ± 0.50 | 98.0 | 10.1 |
| 20 | 19.97 ± 0.33 | 99.9 | 1.7 | 19.93 ± 0.24 | 99.7 | 1.2 |
The values represent mean ± standard deviation.
Stability of KTTKS and pal-KTTKS in the skin
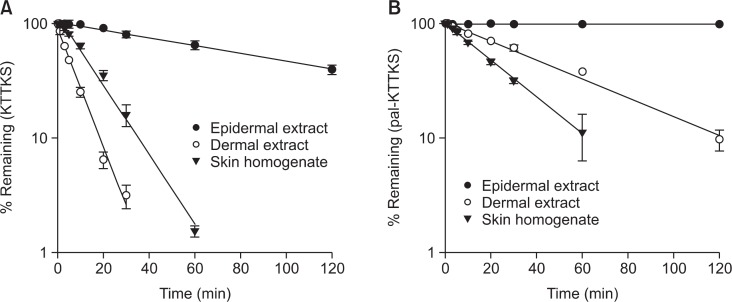
Stability of KTTKS and pal-KTTKS in skin extracts and homogenates was measured using LC-MS/MS. Fig. 2 shows the remaining amounts of KTTKS and pal-KTTKS in dermal skin extract, epidermal skin extract, and skin homogenates over 120 min at 37°C. Prior to the incubation in skin extracts and homogenates, both KTTKS and pal-KTTKS were found to be stable in the HEPES buffer (10 mM, pH 7.4) used as the incubation medium in stability and permeation studies. KTTKS was rapidly degraded in skin extracts and homogenates, as shown in Fig. 2(A). In the dermal skin extract and skin homogenate, KTTKS was almost degraded within 30 min, with 3.2% remaining in the dermal skin extract and at 60 min in skin homogenate with 1.5% remaining. The degradation of KTTKS in the epidermal skin extract was slower than that seen in dermal skin extract and skin homogenate. This may be attributed to lower amounts of proteolytic enzymes in the epidermal skin extract than in the dermal skin extract and the skin homogenate. Determination of protein concentration by the Lowry method showed that the epidermal skin extract contained 7.5-fold less protein than the dermal skin extract and 50-fold less protein than skin homogenate. As shown in Fig. 2(B), pal-KTTKS was more stable than KTTKS. In the epidermal skin extract, the concentration of pal-KTTKS throughout the 120-min incubation period was almost similar to its initial concentration. In dermal skin extract, 9.7% of pal-KTTKS remained after 120 min and 11.2% of pal-KTTKS remained at 60 min in the skin homogenate.
Fig. 2.
Degradation kinetics of KTTKS (A) and pal-KTTKS (B) in epidermal and dermal skin extracts, and skin homogenates (n=3).
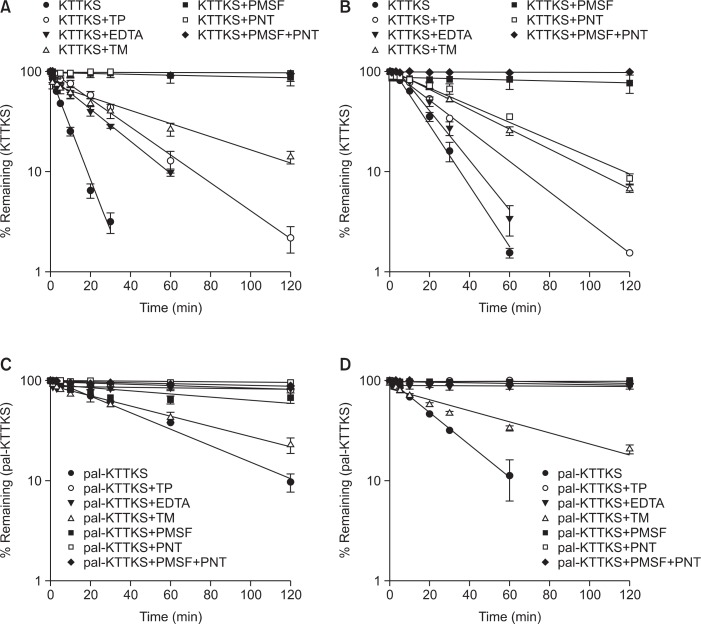
Fig. 3 shows the effects of various proteolytic enzyme inhibitors on the stability of KTTKS and pal-KTTKS in the dermal skin extract and the skin homogenates. PMSF and PNT showed strong protective effects against the degradation of KTTKS in the dermal skin extract and the skin homogenates. This indicates that KTTKS is susceptible to enzymatic degradation by serine proteases such as trypsin and chymotrypsin, and metalloenzymes. The combination of PMSF and PNT completely prevented the degradation of KTTKS in skin, as shown in Fig. 3A, B. The stability of pal-KTTKS was also significantly improved by protease inhibitors. While TM did not show a substantial protective effect, the other proteolytic inhibitors strongly inhibited the degradation of pal-KTTKS as shown in Fig. 3C, D.
Fig. 3.
Effects of proteolytic enzyme inhibitors on the stability of KTTKS and pal-KTTKS in dermal skin extract and skin homogenates (n=3). (A) KTTKS in dermal skin extract, (B) KTTKS in skin homogenates, (C) pal-KTTKS in dermal skin extract, and (D) pal-KTTKS in skin homogenates.
Skin permeation study of KTTKS and pal-KTTKS
The skin permeability of KTTKS and pal-KTTKS was studied using intact skin from a hairless mouse mounted on Franz diffusion cells with the epidermal layer facing the donor compartment. In the skin permeation experiments, no detectable levels of KTTKS and pal-KTTKS were observed in the receptor solution over a period of 48 h. Although a trace of pal-KTTKS appeared in the receptor solutions after 24 h by LC-MS/MS analysis, it was below the LOQ (<0.5 μg/ml). Therefore, it was concluded that neither KTTKS nor pal-KTTKS could permeate through full-thickness hairless mouse skin over the time period used in these experiments.
Fig. 4 presents the retention profiles of pal-KTTKS in the di fferent skin layers (stratum corneum, epidermis and dermis) over a period of 24 h. KTTKS was not detected in any skin layer. On the other hand, pal-KTTKS was observed in every skin layer: 4.2 ± 0.7 μg/cm2 in the stratum corneum, 2.8 ± 0.5 μg/cm2 in the epidermis, and 0.3 ± 0.1 μg/cm2 in the dermis. Totally, 14.6% of applied pal-KTTKS was retained in the skin: 8.3% in the stratum corneum, 5.6% in the epidermis, and 0.6% in the dermis.
Fig. 4.
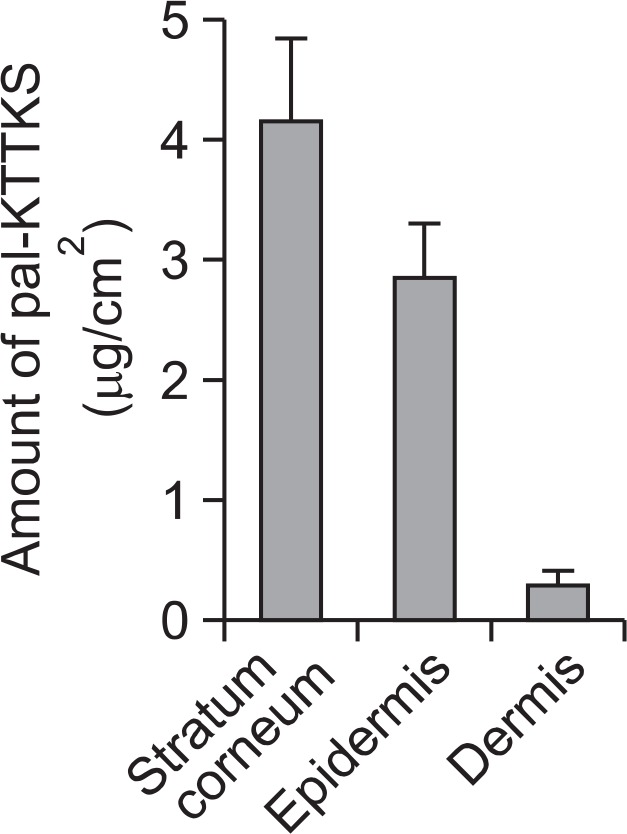
Amounts of pal-KTTKS found in the stratum corneum, epidermis, and dermis of hairless mouse skin (n=3). The loading amount of pal-KTTKS on the skin was 50 μg/cm2.
DISCUSSION
The collagen pentapeptides (KTTKS and pal-KTTKS), which have been widely used in the cosmeceutical field for anti-wrinkle and anti-aging effects, are known to enhance dermal remodeling by triggering cellular process such as inhibition of collagenase activities and promotion of ECM production (Gorouhi and Maibach, 2009). Although several studies have shown the clinical benefits of KTTKS and pal-KTTKS as anti-wrinkle agents, there are few in vitro studies supporting dermal delivery of KTTKS and pal-KTTKS (Pouillot et al., 2008; Gorouhi and Maibach, 2009; Abu Samah and Heard, 2011).
Recently, we reported the development of a LC-MS/MS me thod for monitoring the stability of KTTKS in rat skin (Park et al., 2012). In this study, a LC-MS/MS method was developed for the quantification of pal-KTTKS via simultaneous mo nitoring of the selected MS/MS transitions for pal-KTTKS and pal-AA, the IS. As pal-AA is physico-chemically similar to pal-KTTKS and easily available, it was adopted as the IS. The method was appropriately validated and showed good linearity in a range useful for performing stability and permeability studies (Table 1). Previously, Chirita et al. reported a LC-MS/MS method for pal-KTTKS in cosmetic preparations (Chirita et al., 2009). However, this method was focused only on the measurement of pal-KTTKS content in anti-wrinkle cosmetics. To the best of our knowledge, thus far, a LC-MS/MS method for stability and permeability studies of pal-KTTKS has not been reported. The LC-MS/MS method developed in this study was successfully applied to assess the stability and permeability of pal-KTTKS.
The bioconjugation of various polymers or fatty acids to peptide drug is a widely used technique in the pharmaceutical field for improving stability, increasing solubility, and extending half-life in vivo (Na et al., 2004; Park et al., 2010; Zhang and Bulaj, 2012; Park and Na, 2014). Recently, Choi et al. reported the enhanced stability of an ascorbyl conjugate of KTTKS, known as stabilized ascorbyl pentapeptide (SAP), in the rat skin homogenate (Choi et al., 2009). In this study, pal-KTTKS was found to be more stable than KTTKS in skin extracts and homogenate (Fig. 2). In a previous study, the major degradation products of KTTKS were found to be TTKS and TKS, which indicates KTTKS was mostly degraded by skin amino-peptidases in a stepwise manner (Park et al., 2012). Therefore, the enhanced stability of pal-KTTKS might be attributed to the blocking of the N-terminus of KTTKS by the conjugation with palmitic acid.
When proteolytic enzymes inhibitors were added, the stability of KTTKS and pal-KTTKS in skin extracts and homogenates significantly improved (Fig. 3). Protease inhibitors with different mechanisms (PMSF, PNT, EDTA, TM, and TP) were selected for these studies. PMSF is an inhibitor of serine pro-teases including trypsin and chymotrypsin. PNT is an inhibitor of metalloenzymes including aminopeptidases and endopeptidases. EDTA is a chelating agent. TM inhibits aminopeptidases and dipeptidylaminopeptidases. TP is an inhibitor of dipeptidylaminopeptidases, which include enkephalin dipeptidyl carboxypeptidase and angiotensin converting enzyme (Aoki et al., 1984; Lee et al., 1994; Ogiso et al., 2000). Among the protease inhibitors, PMSF and PNT were most effective in protecting KTTKS and pal-KTTKS from the enzymatic degradation in dermal skin extracts and skin homogenates. Therefore, the combination of PMSF and PNT was expected to be effective for stabilizing KTTKS and pal-KTTKS in the skin. As shown in Fig. 3C, D, the combination of PMSF and PNT completely protected KTTKS and pal-KTTKS from enzymatic degradation in skin extracts and the skin homogenate for the duration of the incubation period.
In the skin permeability study of KTTKS and pal-KTTKS con ducted using hairless mouse skin mounted on Franz diffusion cells, neither KTTKS nor pal-KTTKS was found in the receptor solution over an incubation period of 48 h. Therefore, it was concluded that neither KTTKS nor pal-KTTKS could permeate through full-thickness hairless mouse skin during the incubation period of these experiments. In general, peptides are known to have difficulty in permeating into or through the skin. For example, an article by Babu et al. (2004) reported on the percutaneous absorption of spantide II through hairless rat skin. Spantide II binds to neurokinin-1 receptor and blocks proinflammatory activities associated with substance P. In the permeation experiments with or without cysteine hydrochloride as a penetration enhancer, no detectable level of spantide II permeated across the skin.
The amount of pal-KTTKS retained in different skin layers (the stratum corneum, epidermis, and dermis) was assessed by LC-MS/MS (Fig. 4). KTTKS was not detected in any skin layer. Since KTTKS is hydrophilic, it was unable to penetrate into the skin. On the other hand, pal-KTTKS was observed in all skin layers: 4.2 ± 0.7 μg/cm2 in the stratum corneum, 2.8 ± 0.5 μg/cm2 in the epidermis, and 0.3 ± 0.1 μg/cm2 in the dermis. The skin retention of pal-KTTKS may be attributed to its increased lipophilicity compared to that of KTTKS. The predicted log p value of KTTKS is −3.27, whereas that of pal-KTTKS is 3.32 (Abu Samah and Heard, 2011). The retained pal-KTTKS in the epidermis and dermis could be expected to contribute to the clinical effects of KTTKS such as anti-wrinkle effects and improvement in photoaged skin (Robinson et al., 2005; Draelos, 2007; Gorouhi and Maibach, 2009).
In conclusion, this study indicates that compared to native KTTKS, pal-KTTKS has increased stability and permeability in the skin. The pal-KTTKS formulated with PMSF and PNT as protease inhibitors may be useful as an anti-wrinkle and anti-aging agent in cosmeceutical products.
Acknowledgments
This research was supported by Basic Science Research Programs through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2010-0025345) and the Ministry of Science, ICT and future Planning (NRF-2013R1A2A2A01068858).
REFERENCES
- Abu Samah NH, Heard CM. Topically applied KTTKS: a review. Int J Cosmetic Sci. 2011;33:483–490. doi: 10.1111/j.1468-2494.2011.00657.x. [DOI] [PubMed] [Google Scholar]
- Aoki K, Kajiwara M, Oka T. The role of bestatin-sensitive aminopeptidase, angiotensin converting enzyme and thiorphan-sensitive “enkephalinase” in the potency of enkephalins in the guinea-pig ileum. Jpn J Pharmacol. 1984;36:59–65. doi: 10.1254/jjp.36.59. [DOI] [PubMed] [Google Scholar]
- Babu RJ, Kikwai L, Jaiani LT, Kanikkannan N, Armstrong CA, Ansel JC, Singh M. Percutaneous absorption and anti-inflammatory effect of a substance P receptor antagonist: spantide II. Pharm Res. 2004;21:108–113. doi: 10.1023/b:pham.0000012157.80716.73. [DOI] [PubMed] [Google Scholar]
- Benson HAE, Caccetta R, Chen Y, Kearns P, Toth I. Transdermal delivery of a tetrapeptide: evaluation of passive diffusion. Lett Peptide Sci. 2003;10:615–620. [Google Scholar]
- Benson HAE, Namjoshi S. Proteins and peptides: strategies for delivery to and across the skin. J Pharm Sci. 2008;97:3591–3610. doi: 10.1002/jps.21277. [DOI] [PubMed] [Google Scholar]
- Chirita R, Chaimbault P, Archambault J, Robert I, Elfakir C. Development of a LC-MS/MS method to monitor palmitoyl peptides content in anti-wrinkle cosmetics. Anal. Chim. Acta. 2009;641:95–100. doi: 10.1016/j.aca.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Choi H, Kim H, Park J, Shin E, Kim D, Kim S. Design and efficient synthesis of novel ascorbyl conjugated peptide with high collagen biosynthesis stimulating effects. Bioorg Med Chem Lett. 2009;19:2079–2082. doi: 10.1016/j.bmcl.2008.10.112. [DOI] [PubMed] [Google Scholar]
- Draelos ZD. The latest cosmeceutical approaches for anti-aging. J Cosmetic Dermatol. 2007;6:2–6. doi: 10.1111/j.1473-2165.2007.00309.x. [DOI] [PubMed] [Google Scholar]
- Foldvari M, Attah-Poku S, Hu J, Li Q, Hughes H, Babiuk LA, Kruger S. Palmitoyl derivatives of interferon α: potential for cutaneous delivery. J Pharm Sci. 1998;87:1203–1208. doi: 10.1021/js980146k. [DOI] [PubMed] [Google Scholar]
- Gorouhi F, Maibach HI. Role of topical peptides in preventing or treating aged skin. Int J Cosmetic Sci. 2009;31:327–345. doi: 10.1111/j.1468-2494.2009.00490.x. [DOI] [PubMed] [Google Scholar]
- Katayama K, Seyer JM, Raghow R, Kang AH. Regulation of extracellular matrix production by chemically synthesized subfragments of type I collagen carboxy propeptide. Biochemistry. 1991;30:7097–7104. doi: 10.1021/bi00243a009. [DOI] [PubMed] [Google Scholar]
- Katayama K, Armendariz-Borunda J, Raghow R, Kang AH, Seyer JM. A pentapeptide from type I procollagen promotes extracellular matrix production. J Biol Chem. 1993;268:9941–9944. [PubMed] [Google Scholar]
- Kikwai L, Babu RJ, Prado R, Kolot A, Armstrong CA, Ansel JC, Singh M. In vitro and in vivo evaluation of topical formulations of Spantide II. AAPS PharmSciTech. 2005;6:E565–572. doi: 10.1208/pt060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WM, White AW, Gallagher SJ, Donaldson M, McNaughton G, Heard CM. Scope and limitations of the co-drug approach to topical drug delivery. Curr Pharm Des. 2008;14:794–802. doi: 10.2174/138161208784007653. [DOI] [PubMed] [Google Scholar]
- Lee CH, Lee KJ, Chun IK, Sung YG, Shin YH. Degradation and stabilization of methionine enkephalin and [DAla2]-methionine enkephalinamide in the corneal extracts of rabbits. J Kor Pharm Sci. 1994;24:1–9. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lupo MP, Cole AL. Cosmeceutical peptides. Dermatol Ther. 2007;20:343–349. doi: 10.1111/j.1529-8019.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- Na DH, Youn YS, Park EJ, Lee JM, Cho OR, Lee KR, Lee SD, Yoo SD, DeLuca PP, Lee KC. Stability of PEGylated salmon calcitonin in nasal mucosa. J Pharm Sci. 2004;93:256–261. doi: 10.1002/jps.10537. [DOI] [PubMed] [Google Scholar]
- Ogiso T, Iwaki M, Tanino T, Yono A, Ito A. In vitro skin penetration and degradation of peptides and their analysis using a kinetic model. Biol Pharm Bull. 2000;23:1346–1351. doi: 10.1248/bpb.23.1346. [DOI] [PubMed] [Google Scholar]
- Park EJ, Tak TH, Na DH, Lee KC. Effect of PEGylation on stability of peptide in poly(lactide-co-glycolide) micro-spheres. Arch Pharm Res. 2010;33:1111–1116. doi: 10.1007/s12272-010-0718-z. [DOI] [PubMed] [Google Scholar]
- Park EJ, Kim MS, Choi YL, Shin YH, Lee HS, Na DH. Liquid chromatography-tandem mass spectrometry to determine the stability of collagen pentapeptide (KTTKS) in rat skin. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;905:113–117. doi: 10.1016/j.jchromb.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Park EJ, Na DH. Palmitoylation of octreotide for incorporation into poly(amidoamine) dendrimers. J Pharm Invest. 2014;44:141–145. [Google Scholar]
- Pouillot A, Dayan N, Polla AS, Polla LL, Polla BS. The stratum corneum: a double paradox. J Cosmetic Dermatol. 2008;7:143–148. doi: 10.1111/j.1473-2165.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- Reszko AE, Berson D, Lupo MP. Cosmeceuticals: practical applications. Dermatol Clin. 2009;27:401–416. doi: 10.1016/j.det.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Robinson LR, Fitzgerald NC, Doughty DG, Dawes NC, Berge CA, Bissett DL. Topical palmitoyl pentapep-tide provides improvement in photoaged human facial skin. Int J Cosmetic Sci. 2005;27:155–160. doi: 10.1111/j.1467-2494.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Tsai WC, Hsu CC, Chung CY, Lin MS, Li SL, Pang JH. The pentapeptide KTTKS promoting the expressions of type I collagen and transforming growth factor-β of tendon cells. J Orthop Res. 2007;25:1629–1634. doi: 10.1002/jor.20455. [DOI] [PubMed] [Google Scholar]
- Zhang L, Bulaj G. Converting peptides into drug leads by lipidation. Curr Med Chem. 2012;19:1602–1618. doi: 10.2174/092986712799945003. [DOI] [PubMed] [Google Scholar]