Abstract
Synthetic cannabinoids (CBs) such as the JWH series have caused social problems concerning their abuse liability. Because the JWH series produces euphoric and hallucinogenic effects, they have been distributed illegally under street names such as “Spice” and “Smoke”. Many countries including Korea have started to schedule some of the JWH series compounds as controlled substances, but there are a number of JWH series chemicals that remain uncontrolled by law. In this study, three synthetic CBs with different binding affinities to the CB1 receptor (JWH-073, 081, and 210) and Δ9-tetrahydrocannabinol (Δ9-THC) were evaluated for their potential for psychological dependence. The conditioned place preference test (unbiased method) and self-administration test (fixed ratio of 1) using rodents were conducted. Ki values of the three synthetic cannabinoids were calculated as supplementary data using a receptor binding assay and overexpressed CB1 protein membranes to compare dependence potential with CB1 receptor binding affinity. All mice administered JWH-073, 081, or 210 showed significantly increased time spent at unpreferred space in a dose-dependence manner in the conditioned place preference test. In contrast, all tested substances except Δ9-THC showed aversion phenomenon at high doses in the conditioned place preference test. The order of affinity to the CB1 receptor in the receptor binding assay was JWH-210 > JWH-081 >> JWH-073, which was in agreement with the results from the conditioned place preference test. However, no change in self-administration was observed. These findings suggest the possibility to predict dependence potential of synthetic CBs through a receptor binding assay at the screening level.
Keywords: Synthetic cannabinoid, Δ9-Tetrahydrocannabinol (Δ9-THC), Psychological dependence, CB1 receptor, Binding affinity
INTRODUCTION
Synthetic cannabinoids (CBs) have been widely used worldwide because they have a euphoric effect similar to that of Δ9-tetrahydrocannabinol (Δ9-THC), the psychoactive constituent of Cannabis sativa (Gaoni and Mechoulam, 1964). These compounds are mixed in “herbal incense” preparations labeled “Spice” and “Smoke”, and are sold to those expecting the psychoactive effect of marijuana (Auwarter et al., 2009, Uchiyama et al., 2009a, Uchiyama et al., 2009b). Δ9-THC has been used to help elucidate CB receptors (Devane et al., 1988).
CB receptors have been divided into two groups of CB1 and CB2 receptors, based on functionality and distribution. CB1 receptors are found widely throughout the brain and perform a variety of modulatory functions, whereas CB1 receptors have generally been associated with peripheral and central regulation of the immune system (Van Sickle et al., 2005). The CB1 (nomenclature follows Alexander et al., 2008) receptor has been identified as the receptor that mediates the behavioral and psychoactive effects of Δ9-THC in animals and humans (Atwood et al., 2011). The CB1 receptor is predominately expressed in the central nervous system, particularly in areas such as the hippocampus, basal ganglia, cortex, amygdala, and cerebellum (Mackie, 2005).
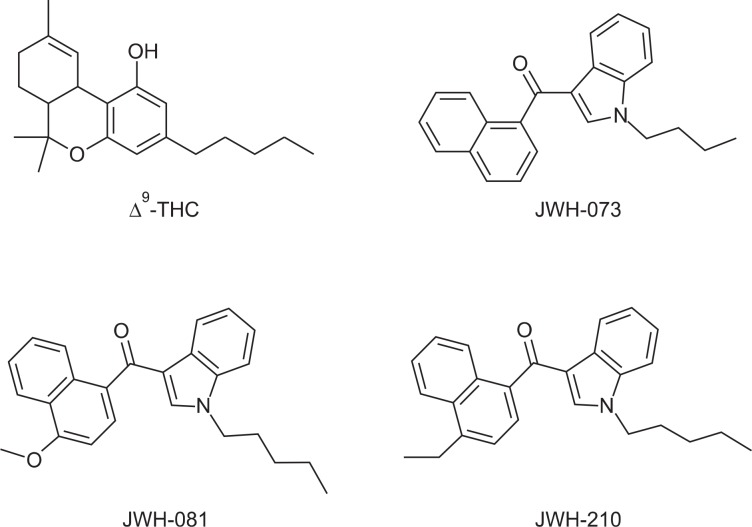
All synthetic CBs and Δ9-THC are CB1 receptor agonists, and many of them show high CB1 receptor binding affinity. JWH-018, one of the well characterized compounds among synthetic CBs, has a high binding affinity for the CB1 receptor in the low nanomolar ranges (∼9 nM) (Showalter et al., 1996, Aung et al., 2000). Although JWH-073, JWH-081, and JWH-210 show CB1 receptor binding affinity comparable to that of Δ9-THC, they have quite different chemical structures from Δ9-THC. The chemical structures are presented in Fig. 1. Additionally, the mechanism of action of CB1 receptor agonists has not been elucidated. However, it is known that activating the CB1 receptor reduces cellular excitability and the probability of neurotransmitter release. This allows both exogenous and endogenous CB agonists to modulate neuronal communication, and may cause their psychoactivity. Prolonged activation of CB1 receptors results in desensitization of the receptor and internalization (Hsieh et al., 1999, Jin et al., 1999, Roche et al., 1999). These processes are thought to be involved in tolerance to substances containing Δ9-THC (Wu et al., 2008).
Fig. 1.
Chemical structures of Δ9-THC, JWH-073, JWH-081, and JWH-210.
Many countries have started to schedule some of the synthetic CBs as controlled substances, but the controlling systems differ significantly from country to country. Only a few countries including the UK have applied a generic approach to synthetic CBs. Controlling synthetic CB analogs requires scientific evidence that the analog shows similar dependence potential with that of Cannabis. Because evaluating the dependence potential of every individual substance through animal behavioral tests is a time and labor consuming process, another way is needed to assess the abuse potential of synthetic CBs.
Thus, it is necessary to develop an in vitro method to evaluate dependence potential to predict the possibility for abuse of synthetic CBs in a short period of time, as animal behavioral evaluations take a long time, and it is nearly impossible to test all substances individually. Therefore, in the present study, to assess the relationship between dependence potential and receptor binding affinity, we evaluated psychological dependence and CB1 receptor binding of JWH-073, JWH-081, and JWH-210 among the aminoaltylindoles of synthetic CBs.
MATERIALS AND METHODS
Animals and substances
Sprague-Dawley rats (weight, 180–220 g) and ICR mice (weight, 15–20 g) were obtained from the Ministry of Food and Drug Safety (AAALAC member, Osong, Korea) and were housed in adequately sized groups in a temperature-controlled (23 ± 2°C) room with a 12 hr light/dark cycle (lights on 08:00 to 20:00). The animals received a solid diet and tap water ad libitum, and husbandry conformed to the Guide for the Care and Use of Laboratory Animals (NRC 1996). We performed all experiments between 09:00 and 18:00. The animal tests were approved by NIFDS/MFDS Animal Ethics Board (12101MFDS003). Δ9-THC, JWH-073, JWH-081 and JWH-210 were purchased from Cayman Chemical (Ann Arbor, MI, USA) and [3H]SR141716A, for the receptor binding affinity assay, was purchased from PerkinElmer (Waltham, MA, USA).
Apparatus
The conditioned place preference (CPP) test apparatus has three distinct compartments (white, black, and gray) separated by automatic guillotine doors. Infrared photo-beam detectors were added to automate data collection. The overall inside dimensions were 21×21×68 cm, and the unit’s base measured 86.4×25.4 cm. The manufacturer provided the mounting holes for the ENV-013 IR Infrared Sensor Package (Med Associates, St. Albans, VT, USA), which places six photo-beams across the white and black zones, 1.25 cm from each end wall, with 5 cm intervals between the beams.
The self-administration test chamber was purchased from Med Associates and measured 29×21×24 cm. The chambers contained two holes; an active hole that delivered a substance through the catheter connected to a jugular vein, and an inactive hole that was not connected to the animal. Infusion pumps were placed outside the chamber and connected to a 10 ml syringe. We connected the apparatus to a computer to record test data and control the experimental processes.
Methods
CPP test: The mice were acclimated to the experimental apparatus and handled for 6 days before starting the experiment. The procedure was similar to that described previously in an unbiased manner (Bozarth, 1987, Narita et al., 2004).
Each experiment consisted of three phases, as follows. Pre-conditioning: The mice were allowed free access to both compartments of the apparatus for 20 min each day for 2 days (days 1 and 2). On the third day, the time spent by the mice in each compartment was recorded and served as the baseline.
Conditioning: The mice were conditioned for 10 days (days 3–12) during one session per day. On the third day, one group of selected mice was treated with substances (one of five doses of 9-THC or JWH-073, 081, 210: 0, 0.05, 0.1, 0.5, and 1 mg/kg, intraperitoneally), and placed in the non-preferred compartment for 45 min. The other group of mice was treated with saline, and placed in the preferred compartment for 45 min. The groups were switched everyday, and the same procedure was performed.
Post-conditioning: The mice were allowed to move freely between compartments of the apparatus for 20 min on day 13. The time spent by the mice in each compartment was recorded, and these were considered the test line.
Self-administration test: The rats were anesthetized with pentobarbital sodium (Entobar®, Hanlim Pharmaceuticals, Seoul, South Korea) for surgery. The surgical procedures adhered to aseptic conditions described previously (Mucha et al., 1982). Briefly, a catheter was inserted into each rat’s right jugular vein. The catheter exited at the rat’s shoulder. The rats received heparin everyday during the experimental period. After surgery, each rat recovered for at least 7 days in a controlled cage, and received a solid diet and tap water ad libitum.
The testing procedures were as follows. The rats self-administered the substances at a dose that showed the highest value in the CPP test or a negative control substance (vehicle, 0.1 ml/infusion) for 5 sec during a 1 hr session on a fixed-ratio 1 reinforcement schedule. The time-out period was 10 sec. When rats inserted their nose into the active lever, they received a test substance dose injected through the catheter. The self-administration chamber contained two holes linked to a computer program that recorded the data. The test was carried out for more than 8 days.
Receptor binding assay: Chemiscreen membrane preparation recombinant human CB1 cannabinoid receptor (EMD Millipore Corp., Milford, MA, USA) was purchased and stored at −70°C until use. A saturation binding assay was conducted with CP-55940 and [3H]SR141716A based on a previous report [18]. Briefly, binding buffer (50 mM Hepes, 5 mM MgCl2, 1 mM CaCl2, 0.2% BSA) and washing buffer (50 mM Hepes, 500 mM NaCl, 0.1% BSA) were prepared and stored at 4°C until use. The binding buffer, DMSO, and radioactive ligand were mixed and incubated for 1 hr at 30°C for the membrane binding reaction. After washing, cpm values from each binding group were counted using a beta-scintillation counter. Bmax and Kd values were calculated from the specific binding cpm values using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA).
The competition receptor binding assay was performed with a similar protocol as the saturation binding assay described above. The amount of radiolabel specifically bound in the absence of competing compounds was calculated by subtracting non-specific from total binding. The percentage of specific binding was calculated for the amount of radiolabel bound in the presence of various concentrations of each competing compound. The data were analyzed with GraphPad Prism software, and IC50 and Ki values were calculated.
Statistics: All data are presented as means ± standard errors. CPP data were analyzed by one-way analysis of variance (ANOVA) followed by the Newman-Keuls multiple comparison test, and the self-administration data were analyzed by repeated two-way ANOVA followed by the Bonferroni post hoc test. A p-value<0.05 was considered significant.
RESULTS
CPP test
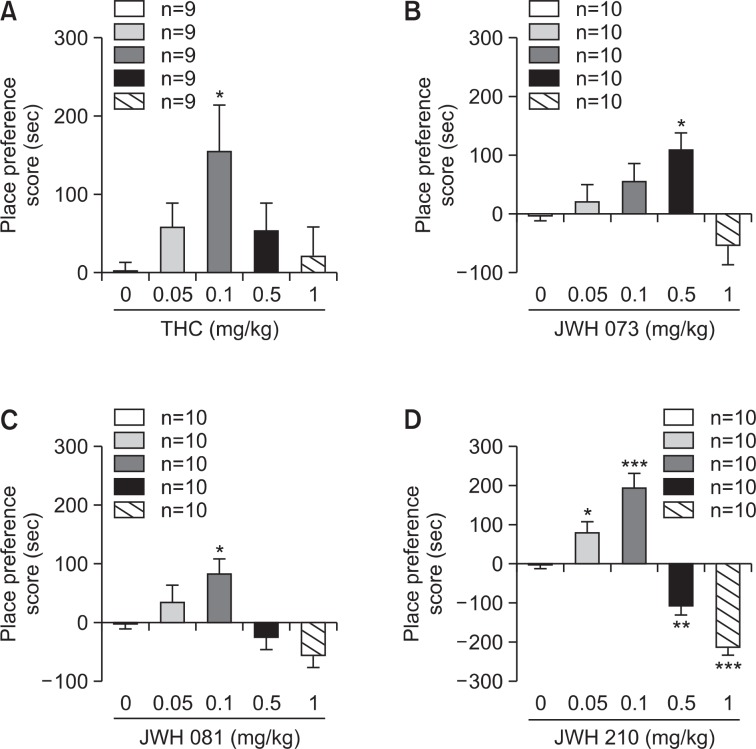
The CPP test was conducted using an unbiased method. The animal’s place preference clearly changed in every group during the 10 day conditioning period. In contrast with the mice treated with saline, the entire group treated with test substances (Δ9-THC, JWH-073, 081 and 210) spent more time in the undesirable room after the conditioning period. When comparing the differences between the negative control and substance-treated groups, the animals receiving substances showed a dose-dependent place preference to a certain dose, then the preference decreased thereafter. An aversion phenomenon appeared in all animals administered the JWH series. All substance-treated mice except the JWH-073-treated animals (0.5 mg/kg) showed the highest place preference at a 0.1 mg/kg dose. Based on the CPP results, JWH-210 and JWH-081 showed significantly higher preference scores than those of JWH-073. JWH-210 also showed a slightly higher preference score than that of JWH-081 (Fig. 2).
Fig. 2.
Conditioned place preference test results for Δ9-THC, JWH-073, JWH-081, and JWH-210. The mice were pre-tested without substances on day 3 of the experiment. The substance was administered to the mice at five different doses (0.05, 0.1, 0.5 and 1 mg/kg, intraperitoneally) for 10 days. Results were measured on day 11 of the experiment. Data are mean ± standard error (n=9 or 10). *p<0.05, **p<0.01, ***p<0.001 substance-treated group vs. vehicle-treated group.
Self-administration test
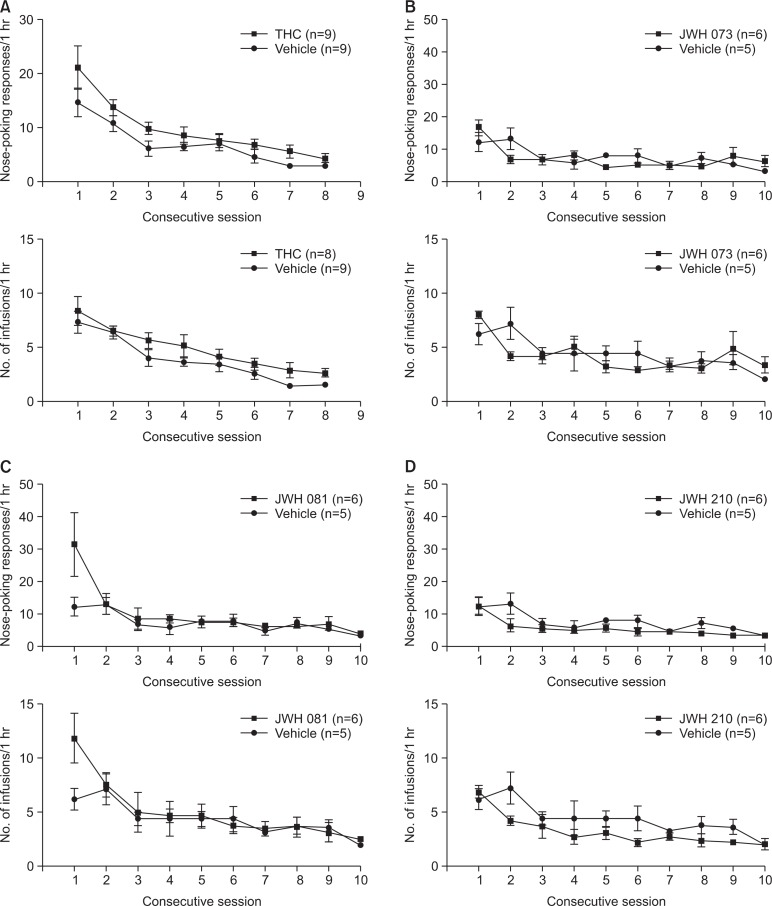
The self-administration test was maintained on a fixed ratio 1 schedule for more than 8 days, and responses on the active hole were checked daily. The negative control (saline-treated) group and substance-treated group did not show active responses. The self-administration test results are depicted in Fig. 3.
Fig. 3.
Self-administration test result for Δ9-THC, JWH-073, JWH-081, and JWH-210. The rats had surgery at the jugular vein and recovered for 7 days. Doses of the tested substances were determined by the results of the conditioned place preference test (the highest preferred dose). The experiment was performed for at least 8 days. Data are mean ± standard error (n=5–9).
Receptor binding assay
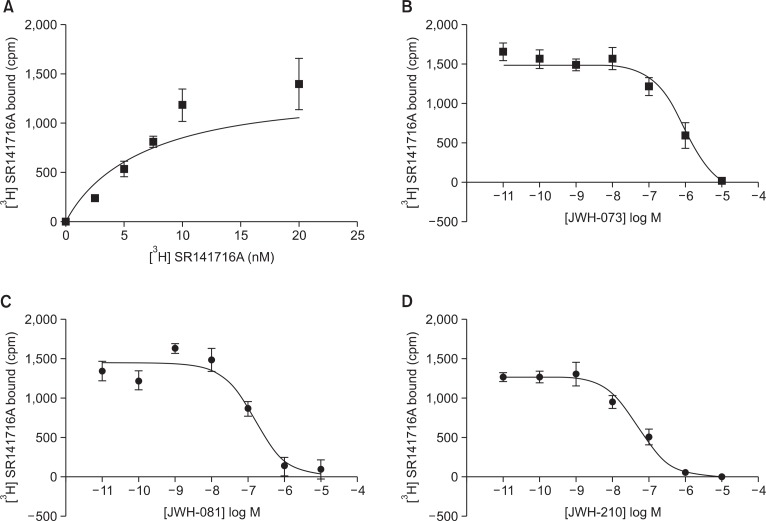
The CB1 receptor binding assay was performed as a saturation binding assay and a competitive binding assay using over-expressed protein membrane with JWH-073, 081, and 210. In the saturation binding assay with [3H]SR141716A, the Bmax was 1398 cpm and the Kd value was 6.6 nM (Fig. 4). The IC50 and Ki values of the JWH series were calculated through the competitive binding assay and the calculated Bmax and Kd values. The Ki values of JWH-073, 081 and 210 were 2.8×10−7, 7.5×10−8 and 2.7×10−8, respectively. The affinity of the JWH series to CB1 was JWH-210 > JWH-081 >> JWH-073. The saturation and competitive binding curves are shown in Fig. 3.
Fig. 4.
Competitive binding curves for JWH-073, JWH-081 and JWH-210 in CB1 over-expressed cell membranes. The Ki values of the tested substances were 2.8×10−7 M (R2=0.9150), 7.5×10−8 M (R2=0.8840) and 2.7×10−8 M (R2=0.9420), respectively. Data are mean ± standard error.
DISCUSSION
Synthetic CBs have been emerging as drugs of abuse because of their marijuana-like effects such as euphoria. They are classified by their chemical structures, including classical CBs, nonclassical CBs, hybrid CBs, aminoalkylindoles, eicosanoids, and others (ACMD). Among them, aminoalkylindoles may be the most problematic because of their structural diversity. Our test substances, JWH-073, 081, and 210 have the common mother naphthoylindole structure, one of the aminoalkylindoles. JWH-073 has the shortest alkyl chain (butanyl) at the indole moiety, whereas both JWH-081 and 210 have a pentanyl chain. Furthermore, the difference between JWH-081 and 210 are methoxy and ethyl functional groups on the naphthalene moiety.
A relationship was observed between chemical structure and receptor binding affinity based on the results of CB1 receptor binding affinity test. We assume that in the case of the naphthoylindoles, the pentanyl chain on the indole moiety binds better to the CB1 receptor than that of a shorter chain, and a hydrophobic functional group on the naphthalene moiety binds better than that of a hydrophilic group. This observation agrees with a previous report (Elsohly et al., 2014).
The psychological dependence potential of the JWH series was evaluated using CPP and self-administration tests. Significant dose-dependent increases in place preference were observed on the CPP test for the test substance (Δ9-THC, JWH-073, JWH-081, and JWH-210) administered groups.
The animals showed decreased preference or aversion with higher doses of the JWH-073, JWH-081, and JWH-210. This aversion phenomenon has been reported previously in studies with CB1 receptor agonists (Corcoran and Amit, 1974, Leite and Carlini, 1974, Alexander et al., 2008). The major factor affecting the rewarding properties of CB1 agonists in the place conditioning paradigm could be the consequences of the possible dysphoric effects induced by first exposure to the substance (Valjent and Maldonado, 2000). However, the aversion mechanism has not been elucidated clearly; thus, further studies on the precise reward pathways in the brain are needed.
Some reports discussed that some of CB1 receptor agonists such as Δ9-THC, WIN-55,212-2, and CP-55,940 fail to induce a positive self-administration effect (Chaperon and Thiebot, 1999, Carlezon and Chartoff, 2007). Our findings are also in agreement with the observation that experimental rodents do not show any effect on a self-administration test with CB1 receptor agonists through the venous route. However, it has been reported that animals show a self-administration effect when the catheter is inserted directly into the ventral tegmental area (Zangen et al., 2006).
Based on the results here and those in previous reports on animal behavioral changes induced by synthetic cannabinoids, we conclude that JWH-073, 081, and 210 have psychological dependence potential.
It is difficult that evaluating abuse potential of every JWH series chemical with animal experiments because it consumes too much time and labor. Additionally, scheduling synthetic cannabinoids generically requires scientific evidence to prove similar dependence potential compared with that of known CB1 receptor agonists, particularly those with similar chemical structures. Thus, we employed an in vitro assay to demonstrate the relationship between CPP and CB1 receptor binding affinity. The CB1 receptor is responsible for behavioral changes and drug dependence potential. Our findings about the proportional relationship between CPP and receptor binding affinity with Δ9-THC, JWH-073, 081, and 210 suggest that higher affinity to the CB1 receptor leads to greater psychological dependence.
Taken together, the CB1 receptor binging assay could be used to determine dependence potential as a predictive model at the screening level. Our findings provide valuable evidence for scheduling synthetic cannabinoids using a generic approach.
Acknowledgments
This study was supported by the National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety (12181MFDS660).
REFERENCES
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Pharmacol. 2008;153:S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lee D, Straiker A, Widlanski TS, Mackie K. CP47, 497-C8 and JWH073, commonly found in ‘Spice’ herbal blends, are potent and efficacious CB1 cannabinoid receptor agonists. Eur J Pharmacol. 2011;659:139–145. doi: 10.1016/j.ejphar.2011.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MM, Griffin G, Huffman JW, Wu MJ, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend. 2000;60:133–140. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- Auwarter V, Dresen S, Weinmann W, Muller M, Puts M, Ferreiros N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009;44:832–837. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- Bozarth MA. Conditioned place preference: A parametric analysis using systemic heroin injections. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag; New York: 1987. pp. 241–273. [Google Scholar]
- Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Corcoran ME, Amit Z. Reluctance of rats to drink hashish suspensions: free-choice and forced consumption, and the effects of hypothalamic stimulation. Psychopharmacologia. 1974;35:129–147. doi: 10.1007/BF00429580. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- ElSohly MA, Gul W, Wanas AS, Radwan MM. Synthetic Cannabinoids: Analysis and Metabolites. Life Sci. 2014;97:78–90. doi: 10.1016/j.lfs.2013.12.212. [DOI] [PubMed] [Google Scholar]
- Hsieh C, Brown S, Derleth C, Mackie K. Internalization and recycling of the CB1 cannabinoid receptor. J Neurochem. 1999;73:493–501. doi: 10.1046/j.1471-4159.1999.0730493.x. [DOI] [PubMed] [Google Scholar]
- Jin W, Brown S, Roche JP, Hsieh C, Celver JP, Kovoor A, Chavkin C, Mackie K. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J Neurosci. 1999;19:3773–3780. doi: 10.1523/JNEUROSCI.19-10-03773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite JR, Carlini EA. Failure to obtain ‘cannabis-directed behavior’ and abstinence syndrome in rats chronically treated with Cannabis sativa extracts. Psychopharmacologia. 1974;36:133–145. doi: 10.1007/BF00421785. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005;168:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Van Der Kooy D, O’Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- Narita M, Akai H, Nagumo Y, Sunagawa N, Hasebe K, Nagase H, Kita T, Hara C, Suzuki T. Implications of protein kinase C in the nucleus accumbens in the development of sensitization to methamphetamine in rats. Neuroscience. 2004;127:941–948. doi: 10.1016/j.neuroscience.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Roche JP, Bounds S, Brown S, Mackie K. A mutation in the second transmembrane region of the CB1 receptor selectively disrupts G protein signaling and prevents receptor internalization. Mol Pharmacol. 1999;56:611–618. doi: 10.1124/mol.56.3.611. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y. Identification of a cannabimimetic indole as a designer drug in a herbal product. Forensic Toxicol. 2009a;27:61–66. [Google Scholar]
- Uchiyama N, Kikura-Hanajiri R, Kawahara N, Haishima Y, Goda Y. Identification of a cannabinoid analog as a new type of designer drug in a herbal product. Chem Pharm Bull. 2009b;57:439–441. doi: 10.1248/cpb.57.439. [DOI] [PubMed] [Google Scholar]
- Valjent E, Maldonado R. A behavioural model to reveal place preference to delta9-tetrahydrocannabinol in mice. Psycho-pharmacology. 2000;147:436–438. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Wu DF, Yang LQ, Goschke A, Stumm R, Brandenburg LS, Liang YJ, Hollt V, Koch T. Role of receptor internalization in the agonist-induced desensitization of cannabinoid type 1 receptors. J Neurochem. 2008;104:1132–1143. doi: 10.1111/j.1471-4159.2007.05063.x. [DOI] [PubMed] [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26:4901–4907. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]