Abstract
Paeonol is a major phenolic micromolecular component of Moutan cortex Radicis, a traditional Chinese Medicine. It has shown antitumor effects in previous studies; however, the underlying mechanisms remain unknown. This study investigated the mechanism by giving treatments of placebo, cyclophosphamide, paeonol of 150 and 300 mg/kg to 4 groups of mice bearing EMT6 breast cancer. Apoptosis in tumor cells were confirmed by morphology analysis, including hematoxylin, eosin staining and TUNEL staining. The results showed that the weight of EMT6 breast tumor was significantly reduced in the groups treated with both 150 and 300 mg/kg of paeonol. Immunohistochemical and Western blot results showed that the expression of Bcl-2 was down-regulated while the expression of Bax, caspase 8 and caspase 3 was up-regulated respectively. These results suggest that paeonol exhibits antitumor effects and the mechanism of the inhibition is via induction of apoptosis, regulation of Bcl-2 and Bax expression, and activation of caspase 8 and caspase 3.
Keywords: Apoptosis, Pathway, Breast cancer, Paeonol
INTRODUCTION
Paeonol (2’-hydroxy-4’-methoxyacetophenone), a micro-molecular phenolic compound (Fig. 1), has been isolated from the root bark of the plant Paeonia moutan. Paeonol is traditionally used as a Chinese herbal medicine that has been widely used in sedation, hypnosis, antipyresis, analgesia, anti-oxidation, anti-inflammation, anti-bacterial and to activate the blood flow and remove blood stasis (Dai et al., 2000; Mimura and Baba, 2001; Chou, 2003; Zhang et al., 2011). Paeonol shows antitumor effects and inhibits the proliferation of different tumor cell lines, e.g. the K562, T6-17, Bel-7404, Hela and HT-29 etc. Certain mechanisms of paeonol antitumor induce tumor cell apoptosis and enhance the activity of various immune molecules. The induction of apoptosis was associated with a decrease of the ratio of Bcl-2/bax, an increase of fas/fasl and caspase 3 expression in HepA hepatoma mice (Pan et al., 2004; Sun et al., 2008) and human esophageal cancer cell lines (Sun et al., 2004; Liu et al., 2005; Wan et al., 2008). The previous study confirmed that paeonol inhibited the growth of tumor cells by reducing the expression of mutant p53, Bcl-2 and C-erbB-2 proteins in tumor tissues in mice bearing EMT6 breast cancer (Wang et al., 2010). No detailed report was carried out on the mechanism of paeonol against breast cancer through the induction of apoptosis. Therefore, the present study further investigated the possible mechanisms responsible for the antitumor activity of paeonol using EMT6 bearing mice. The purpose was to explore the therapeutic potential of paeonol for breast cancer and to provide a scientific explanation for the traditional application of this particular herbal medicine in breast cancer therapy.
Fig. 1.
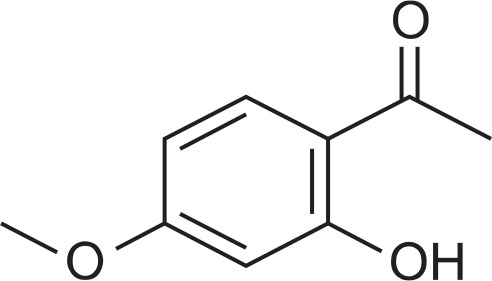
Molecular structure of paeonol.
MATERIALS AND METHODS
Materials
Paeonol was obtained from Shenyang Pharmaceutical University (Shenyang, China). Cyclophosphamide (CTX) was purchased from HengRui pharmacy Inc. (Jiangsu Province, China). The terminal deoxyribonucleotide transferase-mediated nick-end labeling assays (TUNEL) kit was purchased from KeyGEN Bio. Tech. Co. Ltd (Nanjing, China). Streptavidinbiotin Peroxidase immunohistochemical reagent kit was obtained from Beijing Zhongshan Biological Technology Co, Ltd. (Beijing, China). 3,3N-Diaminobenzidine Tertrahydrochlo-ride (DAB) substrate kit was from Beyotime Institute Biotechnology (Shanghai, China). Mouse anti-Bcl-2, Bax, caspase 8 and caspase 3 monoclonal antibodies were obtained from Santa Cruz Bio. Inc. (Santa Cruz, CA, USA). Horseradish pe roxidase-labeled rabbit antigoat IgG antibody and β-actin polyclonal antibody were purchased from Biosynthesis Bio. Co. (Beijing, China). The enhanced chemiluminescence kit was purchased from Amersham Pharmacia Biotech Co., Ltd (Sh anghai, China). Mouse breast cancer EMT6 cell line was ob tained from the Cancer Institute of the Chinese Academy of Medical Sciences. All other chemicals used were of analytical reagent grade.
Animals
Fifty female 6-week-old Kunming mice were purchased from the Laboratory Animal Center of the Academy of Military Me dical Sciences. The mice were randomly divided into five groups (n=10 per group). One group was used for the preparation of EMT6 tumor cells. The other four groups were used for different administrations (see details in the section: Animal model and drug treatment). Animals were housed in plastic cages with free access to food and water and maintained on a regulated environment (20 ± 2°C). All animal experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No.80-23; revised 1978 and the number approved by Administrated-Committee of Laboratory Animals was 062310).
Preparation of EMT6 tumor cells
The EMT6 breast cancer cell line (107 cell/ml) was injected into the right forelimb in one group of mice (0.2 ml/mouse). When each tumor had grown to 1 cm in size, it was removed and suspended in normal saline to a concentration of 5×106 cell/ml under aseptic conditions.
Animal model and drug treatment
Four groups of mice were all injected with 0.2 ml/mouse of 5×106 cell/ml EMT6 breast cancer cells in the skin under the right forelimb. After 24h inoculation, one group was administered with vehicle alone (distilled water, p.o.) as the control group. One group was given the standard antitumor reference drug cyclophosphamide (CTX, 25 mg/kg body weight, i.p.), which was designated as the positive control group, the CTX group. The other two groups were administrated paeonol, low dosage (150 mg/kg body weight, p.o.) and high dosage (300 mg/kg body weight, p.o.). After 15 days, all mice were weighed and killed; each tumor was removed and weighed. According to the mean weight of the tumor, the rate of tumor inhibition was calculated as follows: the rate of inhibition (%)=[(mean tumor weight of Control group–mean tumor weight of Treated group)÷mean tumor weight of Control group]×100.
Morphological analysis of tumor tissues
Tumor specimens which were collected from the control group, CTX group and Paeonol group (300 mg/kg) were fixed in 10% (v/v) neutral formalin solution, dehydrated through a graded ethanol series and embedded in paraffin. Tissue sections (4 μm) were stained with hematoxylin and eosin and examined under the light microscope.
TUNEL assay
Apoptotic cells in sections of mice tumor tissues from the four groups were detected by using an in situ apoptosis detection kit according to the manufacturer’s instructions. Tissue sections were treated by dewaxing and hydration, firstly, according to a conventional method, and then processed with 10 mg/ml protease K for 30 minutes at room temperature. The slides were immersed in a 2% H2O2 solution to block endogeneous peroxidase activity after rinsing 3 times in PBS. Terminal Deoxynucleotidyl Transferase (TdT) was used to ca talyze the addition of Biotin-conjugated dUTP to the 3’-OH ends of DNA fragments. 50 μl TdT enzyme reaction solution was added to each sample except for the control group and covered for 60 minutes at 37°C. After washing 3 times in PBS, 50 μl Streptavidin-HRP solution was added and reacted for 30 minutes at 37°C. Finally, DAB staining was carried out and apoptotic cells were detected under a light microscope.
Immunohistochemical analysis of Bcl-2, Bax and caspase-8 in tumor tissues
Tumor specimens which were collected from the four groups were fixed in 10% (v/v) neutral formalin solution and embedded in paraffin. Tumor sections were prepared and used to examine the expression of Bcl-2, Bax and caspase-8 proteins. The tumor sections were stained by the standard immunohistochemical streptavidin peroxidase method which was described in the program of streptavidin peroxidasereagents kit. Previously known positive tumor tissues were used as a positive control for Bcl-2, Bax and caspase-8. The cells which stained brown suggest positive cells while cells stained blue indicate negative cells under the microscope. The immunohistochemical results were quantitatively analyzed using a biological image analysis system and biological image analysis software (Leica Microsystems Ltd. Germany). The average positive rate was analyzed by microscopy using six randomly selected optical fields (200 cells per field).
Western blot analysis for Bcl-2, Bax, caspase 8 and caspase 3
Tumor tissues from the four groups were minced and lysed in 500 μl cell lysis buffer for 30 minutes, and centrifuged at 12,000 g for 15 minutes at 4°C. The supernatant was collected and protein concentrations were determined according to the Bradford method. Samples were subjected to 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) after which they were boiled for 5 minutes, and the resolved proteins were electrophoretically transferred to polyvinylidene difluoride membranes by a semi-dry transfer method. The membranes were blocked with 5% non-fat dried milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 hour at room temperature, washed three times with TBST, and incubated with TBST containing 5% of dried skim milk and primary antibody (Bcl-2, Bax, caspase 8 or caspase 3) for 2 hours at room temperature. After washing three times with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Proteins were visualized by using an enhanced chemiluminescence kit and exposed to X-ray film. At the same time, actin was used as an internal control for all Western blots. The intensity of protein bands was quantified by using LabWork 3.0 UVP software (UVP, Upland, CA, USA).
Statistical analysis
All values are expressed as mean ± S.D. One-way analysis of variance and Duncan’s multiple range tests were used for determining differences among groups, and p<0.05 was regarded as statistically significant.
RESULTS
Cytotoxicity of paeonol on mice breast cancer
After tumor-bearing mice were treated with two dosages of paeonol 150 or 300 mg/kgbody weight and CTX for 15 days, there was no significant influence on the body weight of mice in both CTX group and Paeonol group. However, comparing with the control group to the groups administered with paeonol of 150 mg/kg and 300 mg/kg the tumor weight significantly decreased and the tumor inhibition rate was 41.25% and 51.05%, respectively. CTX, which is the standard chemotherapeutic, produced an inhibition rate of 53.84% (Table 1).
Table 1.
The inhibitory effect of paeonol on EMT6 solid tumors (x ± s)
| Groups | n | Treatment (mg/kg) | Body weight (g) | Mean weight of tumor (g) | Inhibition rate (%) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Start | End | |||||
| Control | 10 | Vehicle | 20.23 ± 2.02 | 21.79 ± 2.23 | 2.86 ± 0.53 | 0 |
| CTX | 10 | 25 | 21.48 ± 1.89 | 19.85 ± 1.34 | 1.32 ± 0.21a | 53.84 |
| Paeonol | 10 | 150 | 20.69 ± 2.04 | 22.26 ± 2.85 | 1.68 ± 0.26a | 41.25 |
| 10 | 300 | 21.38 ± 1.91 | 23.17 ± 2.34 | 1.40 ± 0.24a | 51.05 | |
p<0.05 as compared with control group, values are mean ± S.D
Morphological analysis of cell apoptosis
Tumor cells in the control group were arranged closely in different shapes and sizes, the cells displayed a small cytoblastema and a larger nucleus which was thickly stained and obvious heteromorphism, and hyperplasia. As shown in Fig. 2, the number of tumor cells in the Paeonol treatment groups decreased markedly, and tumor cell chromatin accumulated at the side of the nucleic membranes. The nucleic shapes were irregular and the surface of the nucleic membrane was rough. The nucleus was broken but it was encapsulated by intact membrane, containing intact organelles and apoptotic bodies. Comparing it with the Control group, there were more apoptotic cells in the Paeonol group under a light microscopy (Fig. 2). TUNEL staining results depicted that the apoptotic index reached 19.72% in the Paeonol group (300 mg/kg body weight) (Table 2, Fig. 3), this demonstrated that paeonol treatment produced markedly more apoptotic cells.
Fig. 2.
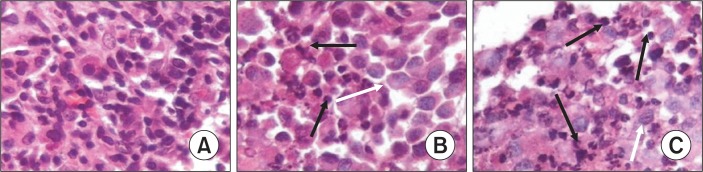
Morphological changes of tumors in the EMT6 model treated with paeonol (H&E stain, ×400). (A) The Control group. (B) CTX group. (C) Paeonol group (300 mg/kg body weight). White arrows indicate necrosis tumor cells and black arrows indicate apoptotic cells. The data was generated from six fields per slide, with four slides analyzed from each tumor, and 10 tumors examined from each group.
Table 2.
Effects of paeonol on the expression of Bcl-2, Bax, caspase 8 and apoptotic cells in tumor tissue (x ± s, %)
| Groups | Dose (mg/kg) | Bcl-2 | Bax | Caspase-8 | Apoptosis cells |
|---|---|---|---|---|---|
| Control | Vehicle | 69.38 ± 9.76 | 29.45 ± 5.47 | 36.29 ± 7.65 | 1.93 ± 1.62 |
| CTX | 25 | 46.22 ± 7.69a | 49.26 ± 8.32a | 54.37 ± 9.28a | 6.73 ± 3.23a |
| Paeonol | 150 | 37.54 ± 7.26a | 57.62 ± 8.79a | 61.23 ± 10.76a | 10.78 ± 4.69a |
| 300 | 27.63 ± 6.82a | 63.77 ± 9.74a | 78.65 ± 10.73a | 19.72 ± 6.55a |
p<0.05 as compared with control group, values are mean ± S.D
Fig. 3.
Morphological changes of apoptosis in vivo to EMT6 breast cancer model treated with paeonol. (A) Control group. (B) Paeonol group (300 mg/kg body weight). Black arrows indicate apoptotic cells. The data was generated from six fields per slide, with four slides analyzed from each tumor, and 10 tumors examined from each group (TUNEL stain, ×200).
Effects of paeonol on the expression of Bcl-2, Bax and Caspase-8
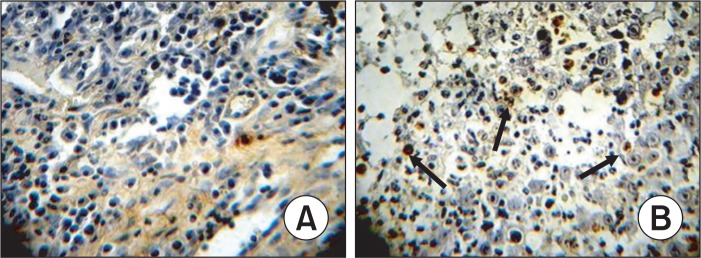
The S-P immunohistochemical method was used to examine the expression of Bcl-2, Bax and Caspase-8. The results demonstrated that treatment with CTX and paeonol (300 mg/kg body weight, administered orally) reduced the expression of Bcl-2, whereas increased the expression of Bax, and Caspase-8. The percentage of Bcl-2 positive cells was 69.38% in the control group, whereas treatment with CTX and paeonol (300 mg/kg body weight, administered orally), the percentage of positive cells for Bcl-2 decreased to 46.22% in the CTX group and 27.63% in the paeonol group (Table 2, Fig. 4). The number of cells positive for Bax was 29.45 % in the control group, but the percentage of cells positive for Bax in the groups treated with CTX and paeonol increased significantly to 49.26% and 63.77 %, respectively (Table 2, Fig. 5). The per centage of cells positive for Caspase-8 was 36.29 % in the control group. However, treatment with CTX and paeonol si gnificantly increased the number of Caspase-8 positive cells to 54.37% in the CTX group and 78.65% in Paeonol group (Table 2, Fig. 6).
Fig. 4.
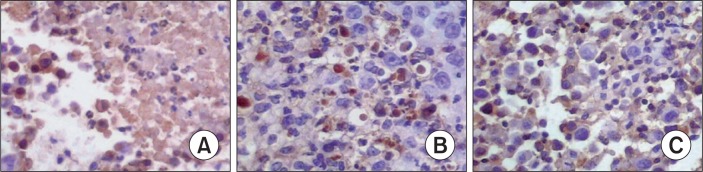
Effect of paeonol on the expression of Bcl-2 in EMT6 tumor tissues (S-P, ×400). (A) Control group, (B) CTX group, (C) Paeonol group (300 mg/kg). The data was generated from six fields per slide, with four slides analyzed from each tumor, and 10 tumors examined from each group.
Fig. 5.
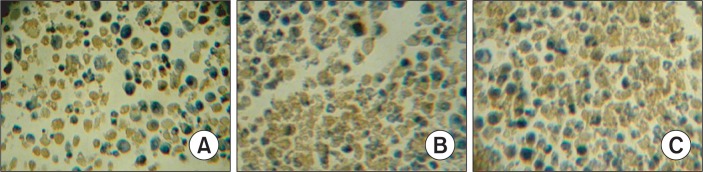
Effect of paeonol on the expression of Bax protein in EMT6 tumor tissues (S-P, ×400). (A) Control group; (B) CTX group; (C) Paeonol group (300 mg/kg). The data was generated from six fields per slide, with four slides analyzed from each tumor, and 10 tumors examined from each group.
Fig. 6.
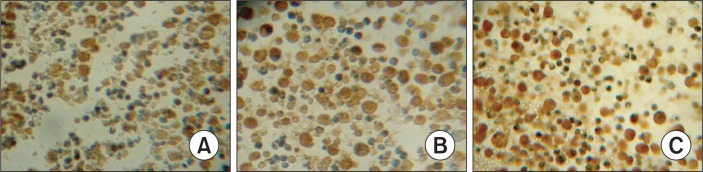
Effect of paeonol on the expression of caspase 8 in EMT6 tumor tissues. (S-P, ×400). (A) Control group, (B) CTX group, (C) Paeonol group (300 mg/kg). The data was generated from six fields per slide, with four slides analyzed from each tumor, and 10 tumors examined from each group.
Effects of paeonol on the expression of Bcl-2, Bax, caspase 8 and caspase 3 proteins by Western Blot
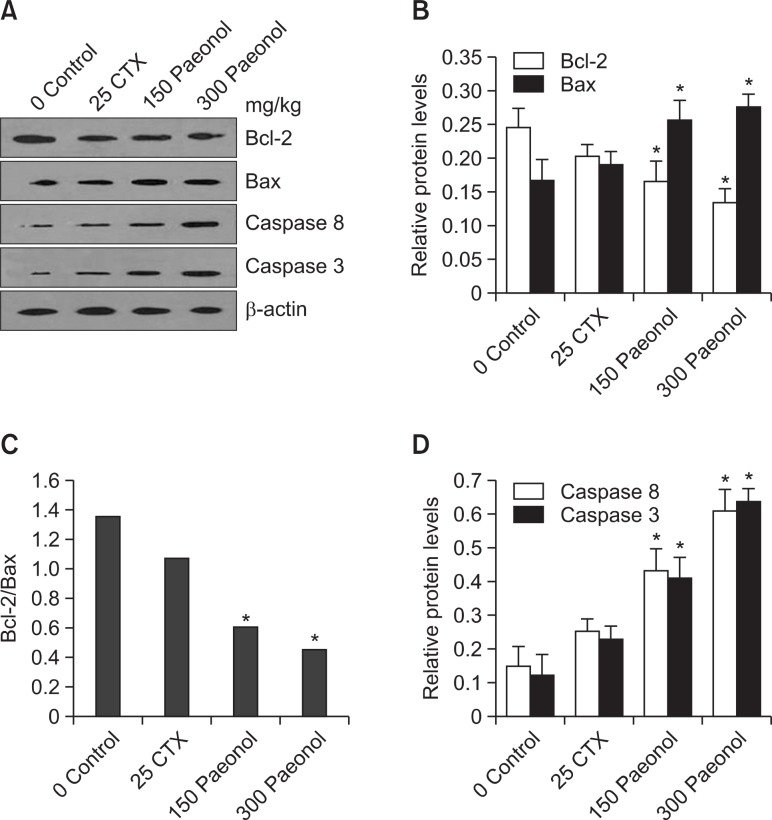
To further define the effects of paeonol on tumor cells associated with the apoptotic pathway, protein levels of Bcl-2, Bax, caspase 8 and caspase 3 were evaluated by Western Blot. As shown in Fig. 7, the expression of Bcl-2 decreased no ticeably, whereas the protein expression of Bax, caspase 8 and caspase 3 increased in the CTX and paeonol groups.
Fig. 7.
Western blot analysis for Bcl-2, Bax, caspase 8 and caspase-3 in tumor tissue. (A) Bcl-2, Bax, caspase 8 and caspase-3 expression by Western Blot method. β-actin was used as a control; data shown is representative of three independent experiments. (B) The intensity of Bcl-2 and Bax bands was quantified and was shown as relative expression level after it was normalized by β-actin (n=3, mean ± S.D.); *p<0.05, vs. Control group. (C) the ratio of Bcl-2/Bax was shown. *p<0.05 vs. Control group. (D) The intensity of caspase 8 and caspase 3 was quantified and was shown as relative expression level after it was normalized by β-actin (n=3, mean ± S.D.). *p<0.05, vs. Control group.
DISCUSSION
This study is further investigation into the effects and mechanisms of paeonol on mice harboring breast cancer cells. The results demonstrated that the paeonol plays an important antitumor role. Compared with the control group, paeonol administration can significantly decrease the tumor weight and increase the tumor inhibition rates; induce morphological changes indicative of apoptosis; increase tumor cell apoptosis; and regulate the expression of Bcl-2 and Bax, leading to a decrease of the ratio of Bcl-2/Bax; increase the activity of caspase 8 and caspase 3. The findings are consistent with previous studies using paeonol which showed Bcl-2 down-regulation, Bax and caspase-3 activation in mouse HepA-hepatoma and human colon cancer cell lines (Sun et al., 2008; Li et al., 2010; Xing et al., 2010).
Apoptosis, also called programmed cell death, is an evolutionarily conserved genetic program of cellular characteristics. It is well recognized that an alteration of the cellular homeostasis occurs in cancer, which disrupts the balance between cellular proliferation and cell death (apoptosis). Apoptosis is also a form of cell death which is characterized morphologically by extreme chromatin condensation and formation of apoptotic bodies (Zhang et al., 2008; Wang et al., 2012). Many anti-cancer drugs induce tumor cell apoptosis which is an obvious strategy for cancer therapy.
The mechanisms of apoptosis induced by drugs are complex due to the differences in cell types and drugs (Li et al., 2011). However, mitochondrial and cell-surface death receptor-mediated apoptosis are the two principal pathways leading to programmed cell death. The mitochondrial pathway is thought to play a major role in response to cancer treatments and is mediated by the Bcl-2 family proteins, which are always over-expressed in many tumor cells (Reed, 2000; Sjöström et al., 2002; Ohtsuka et al., 2003) and they act as repressors of apoptosis by blocking the release of cytochrome-c, whereas proapoptotic members, e.g., Bax, act as promoters. These effects are more dependent on the balance between Bcl-2 and Bax than on Bcl-2 quantity alone.
In the current study, the treatment with paeonol decreased the expression of Bcl-2 and increased the expression of Bax. The up-regulation of Bax expression and the reduction of Bcl-2 expression in the treated groups leads to a decrease in the ratio of Bcl-2 to Bax, which might be responsible for the drug-induced apoptotic processes and which might be associated with better prognosis.
Caspase 8, an initiator of apoptosis, could be activated under the stimulation of external signals. Activated caspase 8 continues to cleave procaspase 3 into active caspase 3 to achieve apoptosis. Caspase 3 is an executioner caspase of the apoptosis pathway (Mlejnek, 2001; Cheung et al., 2002). In the present study, after administration with paeonol there was a considerable increase in caspase 8 and caspase 3 expressions, which indicated that paeonol promoted caspase 8 and caspase 3 activation.
The results of the study demonstrate that paeonol has the effect of inhibiting the growth of tumor cells. The mechanism of inhibition by inducing apoptosis could possibly occur through the triggering of the mitochondrial-dependent pathway and caspase activation. Paeonol, a traditional natural plant compound, may be a novel chemotherapy against breast cancer.
Acknowledgments
Authors thank Mrs Sakina Ayub Ali and Miss Halima Ayub for their contribution and comments.
REFERENCES
- Cheung TH, Chung TK, Lo KW, Yu MY, Krajewski S, Reed JC, Wong YF. Apotosis-related proteins in cervical intraepithelial neoplasia and squamous cell carcinoma of the cervix. Gynecol Oncol. 2002;86:14–18. doi: 10.1006/gyno.2002.6655. [DOI] [PubMed] [Google Scholar]
- Chou TC. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br J Pharmacol. 2003;139:1146–1152. doi: 10.1038/sj.bjp.0705360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Liu QY, Gu CG, Zhang HY. Inhibitory effect of paeonol on lipid peroxidational reaction and oxidational decorate of low density lipoprotein. Zhongguo Zhong Yao Za Zhi. 2000;25:625–627. [PubMed] [Google Scholar]
- Li K, Li QW, Zhang T, Han ZS, Li J, Liu ZW, Zheng FL. Procyanidins from Pinus koraiensis bark inhibits HeLa cell growth by inducing apoptosis and reducing survivin protein expression. Afr J Biotechnol. 2011;40:7766–7771. [Google Scholar]
- Li N, Fan LL, Sun GP, Wan XA, Wang ZG, Wu Q, Wang H. Paeonol inhibits tumor growth in gastric cancer in vitro and in vivo. World J Gastroenterol. 2010;16:4483–4490. doi: 10.3748/wjg.v16.i35.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CQ, Tan SY, Ji CY, Luo HS, Yu JP. The effects of paeonol on inhibiting the proliferation of human colorectal cancer cell lines HT-29 and its molecular mechanism. Chin Pharm Bull. 2005;21:1251–1254. [Google Scholar]
- Mimura K, Baba S. Determination of paeonol metabolites in man by the use of stable isotopes. Chem Pharm Bull. 2001;29:2043–2050. doi: 10.1248/cpb.29.2043. [DOI] [PubMed] [Google Scholar]
- Mlejnek P. Caspase inhibition and N6-benzyladenosine-induced apoptosis in HL-60 cells. J Cell Biochem. 2001;83:678–689. doi: 10.1002/jcb.1262. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Buchsbaum D, Oliver P, Makhija S, Kimberly R, Zhou T. Synergistic induction of tumor cell apoptosis by death receptor antibody and chemotherapy agent through JNK/p38 and mitochondrial death pathway. Oncogene. 2003;22:2034–2044. doi: 10.1038/sj.onc.1206290. [DOI] [PubMed] [Google Scholar]
- Pan XD, Fei QZ, Zhu CG, Liang B. Synthesis of five paeonol esters and their activity in vitro and in vivo anti-tumor. Anhui Medical and Pharmaceutical Journal. 2004;8:16–18. [Google Scholar]
- Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J, Blomqvist C, von Boguslawski K, Bengtsson NO, Mjaaland I, Malmström P, Ostenstadt B, Wist E, Valvere V, Takayama S, Reed JC, Saksela E. The predictive value of Bcl-2, Bax, Bcl-xL, bag-1, fas and fasL for chemotherapy response in advanced breast cancer. Clin Cancer Res. 2002;8:811–816. [PubMed] [Google Scholar]
- Sun GP, Wan X, Xu SP, Wang H, Liu SH, Wang ZG. Antiproliferation and apoptosis induction of paeonol in human esophageal cancer cell lines. Dis. Esophagus. 2008;21:723–729. doi: 10.1111/j.1442-2050.2008.00840.x. [DOI] [PubMed] [Google Scholar]
- Sun GP, Wang H, Xu SP, Wu Q, Chen ZD, Wei W. Anti-tumor effects of paeonol in a HepA- hepatoma bearing mouse model via induction of tumor cell apoptosis and stimulation of IL-2 and TNF-α production. Eur J Pharmacol. 2008;584:246–252. doi: 10.1016/j.ejphar.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Sun YC, Shen YX, Sun GP. Advances in the studies of major pharmacological activity of paeonol. Chin Chin Tradit Pat Med. 2004;26:579–582. [Google Scholar]
- Xing G, Zhang Z, Liu J, Hu H, Sugiura N. Antitumor effect of extracts from moutan cortex on DLD-1 human colon cancer cells in vitro. Mol Med Report. 2010;3:57–61. doi: 10.3892/mmr_00000218. [DOI] [PubMed] [Google Scholar]
- Wan XA, Sun GP, Wang H, Xu SP, Wang ZG, Liu SH. Synergistic effect of paeonol and cisplatin on oesophageal cancer cell lines. Dig Liver Dis. 2008;40:531–539. doi: 10.1016/j.dld.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Li QW, Ou YT, Li CJ, Sun XX, Tian YZ. Antitumor effects of paeonol on mice bearing EMT6 breast infiltrating ductal carcinoma. Lat Am J Pharm. 2010;5:369–375. [Google Scholar]
- Wang PJ, Li QW, Li K, Zhang XB, Han ZS, Wang JJ, Gao DW, Li J. Betulinic acid exerts immunoregulation and anti-tumor effect on cervical carcinoma (U14) tumor-bearing mice. Pharmazie. 2012;67:733–739. [PubMed] [Google Scholar]
- Zhang H, Gao M, Yang X. Electrochemical oxidation and detection of paeonol on modified electrode with acetylene black nanoparticles. Colloids Surf B Biointerfaces. 2011;87:378–381. doi: 10.1016/j.colsurfb.2011.05.045. [DOI] [PubMed] [Google Scholar]
- Zhang T, Li QW, Li K, Li YR, Li J, Wang GJ, Zhou SB. Antitumor effects of saponin extract from Patrinia villosa juss on mice bearing U14 cervical cancer. Phytother Res. 2008;22:640–645. doi: 10.1002/ptr.2354. [DOI] [PubMed] [Google Scholar]