Abstract
Mangostenone F (MF) is a natural xanthone isolated from Garcinia mangostana. However, little is known about the biological activities of MF. This study was designed to investigate the anti-inflammatory effect and underlying molecular mechanisms of MF in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. MF dose-dependently inhibited the production of NO, iNOS, and pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) in LPS-stimulated RAW264.7 macrophages. Moreover, MF decreased the NF-κB luciferase activity and NF-κB DNA binding capacity in LPS-stimulated RAW264.7 macrophages. Furthermore, MF suppressed the NF-κB activation by inhibiting the degradation of IκBα and nuclear translocation of p65 subunit of NF-κB. In addition, MF attenuated the AP-1 luciferase activity and phosphorylation of ERK, JNK, and p38 MAP kinases. Taken together, these results suggest that the anti-inflammatory effect of MF is associated with the suppression of NO production and iNOS expression through the down-regulation of NF-κB activation and MAPK signaling pathway in LPS-stimulated RAW264.7 macrophages.
Keywords: Mangostenone F, NO, iNOS, NF-κB, MAPK
INTRODUCTION
Inflammation is induced by physical or noxious chemical stimuli or microbiological toxins as the normal response of living tissue. It is well known that chronic inflammation can cause inflammatory diseases, such as arthritis, asthma, multiple sclerosis, inflammatory bowel disease, and atherosclerosis (Shin et al., 2010). Macrophages are activated by various factors such as pro-inflammatory cytokines, bacterial lipopolysaccharide (LPS), and phorbol esters. Activated macrophages produce many cytokines, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and other inflammatory mediators such as nitric oxide (NO) and prostaglandin E2 (PGE2) (Reddy and Reddanna, 2009). NO is a free radical produced by nitric oxide synthase (NOS), which exists as three NOS isoforms: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS). Macrophages after LPS stimulation produce NO by up-regulating iNOS expression through mitogen-activated protein kinases (MAPK) and NF-κB signaling pathways (Pansanit et al., 2013). In response to macrophage activation, LPS stimulates a Toll-like receptor 4 (TLR4)-mediated myeloid differentiation factor (MyD88)-dependent pathway, which in turn activates the transforming growth factor-β-activated protein kinase 1 (TAK1), which subsequently results in activation of nuclear factor-κB (NF-κB) and activating protein-1 (AP-1), and produces inflammatory cytokines including TNF-α, IL-6, and IL-1β (Kawai and Akira, 2006). Therefore, inhibition of these inflammatory mediators has been considered as an effective strategy for the development of anti-inflammatory drugs (Shin et al., 2010; Pansanit et al., 2013).
Mangosteen (Garcinia mangostana) is a tropical tree from Southeast Asia including Malaysia, India, Myanmar, Philippines, and Thailand. The seedcases of mangosteen-fruit have been traditionally used for treating skin infections and wounds for centuries by Southeast Asians. It is also used to treat inflammation, diarrhea, cholera, and dysentery in Ayurvedic medicine (Pedraza-Chaverri et al., 2008). Phytochemical studies have shown that G. mangostana contains a variety of secondary metabolites such as oxygenated and prenylated xanthones (Suksamrarn et al., 2002). These xanthone compounds have been reported to indicate various biological activities such as antioxidant, antitumor, anti-inflammatory, antiallergy, antibacterial, antifungal, antiviral, and antimalarial properties (Suksamrarn et al., 2002; Pedraza-Chaverri et al., 2008). A recent study documented that 12 xanthones including new xanthone mangostenone F (MF) isolated from the seedcases of G. mangostana indicate neuraminidase inhibitory activity (Ryu et al., 2010). It has also been reported that mangosteen’s xanthones including MF indicate α-glucosidase inhibition and antihyperglycemic activity (Ryu et al., 2011). In addition, it has been demonstrated that MF from the seedcases of G. mangostana inhibits melanin formation in B16F10 cells by down-regulating the tyrosinase expression (Ryu et al., 2012). However, the anti-inflammatory activity of MF has not yet been elucidated. Therefore, in the present study, we evaluated the anti-inflammatory effects of MF isolated from the seedcases of G. mangostana in LPS-stimulated RAW264.7 macrophages.
MATERIALS AND METHODS
Materials
Mangostenone F (MF, Fig. 1A) used in the present study was obtained from Dr. Hyung Won Ryu of Korea Research Institute of Bioscience and Biotechnology and dissolved in dimethyl sulfoxide. LPS, a Griess reagent, an NP40 cell lysis buffer, a protease inhibitor cocktail, and a NuCLEAR Extraction Kit were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies for β-Tubulin, IκBα, NF-κB p65, and lamin B were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibody for iNOS was purchased from BD Pharmingen (San Diego, CA, USA). Antibodies for β-actin, ERK, phosphor-ERK (T202/Y204), JNK, phosphor-JNK (T183/Y185), p38, and phosphor-p38 (Thr180/Tyr182) were purchased from Cell Signaling Technology (Danvers, MA, USA). Goat anti-mouse IgG HRP-conjugated antibody was from SouthernBiotech (Birmingham, AL, USA). Goat anti-rabbit IgG HRP-conjugated antibody, Opti-MEM I medium, and Lipofectamine 2000 were purchased from Invitrogen (Carlsbad, CA, USA). The pNF-κB-Luc and pAP-1-Luc reporter vectors were purchased from Stratagene (La Jolla, CA, USA) and Panomics (Fremont, CA, USA), respectively. The pRL-TK internal control vector was purchased from Promega (Madison, WI, USA). Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-6, and IL-1β were purchased from R&D Systems (Minneapolis, MN, USA).
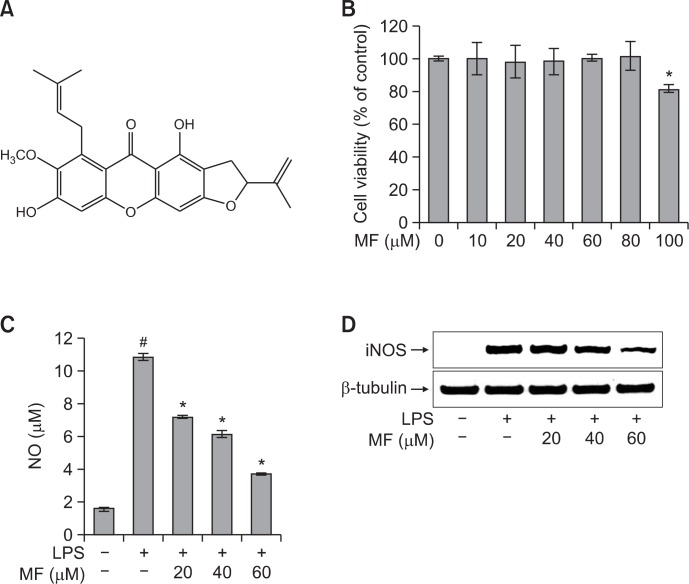
Fig. 1.
Effect of MF on cell viability (B), NO production (C), and iNOS expression levels (D) in LPS-stimulated RAW264.7 macrophages. (A) Chemical structure of MF. (B) RAW264.7 cells were treated with MF (0, 10, 20, 40, 60, 80, and 100 μM) for 24 h, and the relative cell viability was assessed by WST-1 assay. (C) RAW264.7 cells were pretreated with MF (20, 40, and 60 μM) for 1 h and then incubated for 16 h with LPS (1 μg/ml). The culture supernatant was subjected to a nitrite assay. Error bars represent the mean ± SD. #p<0.001 vs. control, *p<0.001 vs. LPS. (D) The iNOS expression levels were determined by western blot analysis.
Cell culture
RAW264.7 macrophage cells were purchased from American Type Culture Collection (Manassas, VA, USA). The cells were grown in DMEM supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA), 100 units/ml of penicillin, and 100 μg/ml of streptomycin (Invitrogen, Carlsbad, CA, USA) in a humidified atmosphere at 37oC with 5% CO2.
Cytotoxicity assay
Cell viability was measured using an EZ-Cytox cell viability assay kit (DAEIL lab, Seoul, Korea) according to the manufacturer’s instruction. RAW264.7 cells were cultured in a 96-well plate at a density of 2×105 cells/ml for 20 h. The cells were subsequently treated with different concentrations of MF (0, 10, 20, 40, 60, 80, and 100 μM). After incubation for 24 h, 10 μl of the kit solution was added to each well and further incubated for 4 h at 37°C and 5% CO2. The index of the cell viability was determined by measuring the formazan production with an ELISA reader (Benchmark Plus, Bio-Rad, Hercules, CA, USA) at an absorbance of 480 nm. The reference wavelength was 650 nm. Cell viability was determined relative to the untreated control cells.
Determination of NO, TNF-α, IL-6, and IL-1β production
RAW264.7 cells were cultured in a 96-well plate at a density of 2×105 cells/ml for 24 h. After incubation, the cells were pretreated with different concentrations of MF (20, 40, and 60 μM) for 2 h and were treated with 1 μg/ml of LPS for an additional 18 h. The culture media were collected at the end of the culture period for the NO, TNF-α, IL-6, and IL-1β assays.
For a nitrite assay, the culture media (100 μl) was mixed with an equal volume of Griess reagent (Sigma-Aldrich) in a 96-well plate and then incubated for 15 min at room temperature. The absorbance at 540 nm was measured, and the concentration of nitrite was calculated using a calibration standard curve constructed using sodium nitrite dissolved in DMEM.
The concentrations of TNF-α, IL-6, and IL-1β in culture media were measured using an ELISA kit (R&D Systems) according to the manufacturer’s instructions. The results are presented as the mean ± SD of three replicates from one representative experiment.
Total proteins extraction
RAW264.7 cells were cultured in a 100 mm dish at a density of 2×105 cells/ml for 24 h. After incubation, the cells were pretreated with different concentrations of MF (20, 40, and 60 μM) for 2 h and were treated with 1 μg/ml of LPS for the indicated times. The cells were harvested and lysed with the NP40 cell lysis buffer in the presence of a protease inhibitor cocktail (Sigma-Aldrich) and PMSF (Sigma-Aldrich). The samples were incubated for 30 min on ice and then centrifuged at 12,000 rpm for 15 min at 4°C. The cell extracts were collected and stored at −80°C until used for further studies. The protein contents were determined by a Bio-Rad Protein Assay (Bio-Rad).
Nuclear proteins extraction
RAW264.7 cells were cultured in a 100 mm dish at a density of 2×105 cells/ml for 24 h. After incubation, the cells were pretreated with different concentrations of MF (20, 40, and 60 μM) for 2 h and were treated with 1 μg/ml of LPS for 30 min. The cells were harvested and nuclear and cytosolic fractions were prepared using a NuCLEAR Extraction Kit (Sigma-Aldrich) according to the manufacturer’s instruction.
Western blotting
After quantification of the protein contents, equal amounts of protein were resolved on SDS-polyacrylamide gels and then transferred to nitrocellulose membranes (Hybond ECL Nitro-cellulose; Amersham Biosciences, Bucks, UK). The membranes were blocked with 5% nonfat dried milk and incubated with the primary antibodies in a 10 ml buffer (Tris-buffered saline and 0.1% Tween 20 with 5% nonfat dried milk) with gentle shaking at 4°C overnight. After incubation, the membranes were washed, incubated with HRP conjugated secondary antibody for 2 h at room temperature, and then washed again. The blotted proteins were detected using an enhanced chemiluminescence detection system (GE Healthcare, Bucks, UK).
Luciferase assay
RAW264.7 cells were cultured in a 6-well plate at a density of 4×105 cells/ml for 24 h. The cells were transfected with a pNF-κB-Luc, a pAP-1-Luc reporter vector, and a pRL-TK internal control vector using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After transfection, the cells were pretreated with different concentrations of MF (20, 40, and 60 μM) for 2 h and were treated with 1 μg/ml of LPS. After 24 h of stimulation, the cells were then collected, lysed, and luciferase activity was measured using the Dual-luciferase Reporter Assay System (Promega) and a luminometer (Berthold Technologies, Bad Wildbad, Germany).
Electrophoretic mobility shift assay (EMSA)
After nuclear extraction, NF-κB DNA binding capacity was determined using an NF-κB EMSA kit (Panomics) according to the manufacturer’s instructions. Briefly, nuclear extracts (6 μg) were mixed with a biotin labeled NF-κB probe and incubated at 37°C for 30 min. DNA-protein complexes were separated in a 6% non-denaturing polyacrylamide gel in a 0.5x TBE buffer at 120 V for 55 min and then transferred to a Biodyne B nylon membrane (Pierce, Rockford, IL, USA) at 300 mA for 30 min. The membrane was fixed using a UV crosslinker, blocked with a blocking buffer and incubated with a streptavidin-HRP mixture for 15 min at room temperature. After incubation, the membrane was washed and detected using an imaging system (Princeton Instruments, NJ, USA).
Statistical analysis
All data are presented as the mean ± SD. The significance of the differences between the means of the treated and untreated groups was determined through a Student’s t test. A p value <0.05 was considered to be significant.
RESULTS
Effect of MF on NO production and iNOS expression levels in LPS-stimulated RAW264.7 cells
First, we determined the cell cytotoxicity of MF against RAW264.7 cells. The cells were treated with different concentrations of MF (0, 10, 20, 40, 60, 80, and 100 μM) for 24 h, and the cell cytotoxicity was determined using the EZ-Cytox cell viability assay kit. As shown in Fig. 1B, MF did not exhibit cytotoxicity to RAW264.7 cells up to 80 μM but did inhibit cell viability at doses of 100 μM. To investigate whether MF possess potential anti-inflammatory effects in LPS-stimulated RAW264.7 cells, we investigated the inhibitory effects of MF on NO production (Fig. 1C). The levels of NO in the culture media were determined using the Griess reagent. LPS treatment significantly increased the production of NO by approximately 7-fold when compared with the untreated cells (p<0.001). However, the LPS-induced NO production was significantly reduced in the cells pretreated with MF (20, 40, and 60 μM) in a dose-dependent manner. To further examine the cause of reduced NO production by MF, iNOS expression was measured by western blot analysis. As expected, LPS treatment significantly increased the expression of iNOS in RAW264.7 cells. However, the LPS-induced expression of iNOS was reduced by pretreating cells with MF (20, 40, and 60 μM) in a dose-dependent manner (Fig. 1D). These results suggest that MF decreased the NO production by inhibiting the expression of iNOS.
Effect of MF on TNF-α, IL-6, and IL-1β production in LPS-stimulated RAW264.7 cells
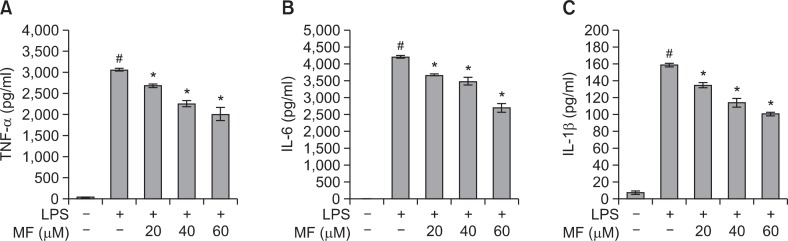
To determine the effects of MF on the production of proinflammatory cytokines TNF-α, IL-6, and IL-1β, RAW264.7 cells were pretreated with different concentrations of MF (20, 40, and 60 μM) and stimulated with 1 μg/ml of LPS for 16 h. The pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) in the culture media were measured using ELISA kits. As shown in Fig. 2, the levels of TNF-α (p<0.001), IL-6 (p<0.001), and IL-1β (p<0.001) were markedly increased in the LPS-stimulated RAW264.7 cells when compared with the untreated cells. However, the treatment of MF before 2 h LPS treatment markedly decreased the levels of TNF-α (p<0.001), IL-6 (p<0.001), and IL-1β (p<0.001) in a dose-dependent manner.
Fig. 2.
Effect of MF on TNF-α (A), IL-6 (B), and IL-1β (C) production in LPS-stimulated RAW264.7 macrophages. RAW264.7 cells were pretreated with MF (20, 40, and 60 μM) for 1 h and then incubated for 16 h with LPS (1 μg/ml). The culture supernatant was subjected to ELISA. Error bars represent the mean ± SD. #p<0.001 vs. control, *p<0.001 vs. LPS.
Effect of MF on NF-κB transcriptional and DNA binding activity in LPS-stimulated RAW264.7 cells
To address the mechanism in which MF reduces LPS-induced iNOS expression, we analyzed the NF-κB activation using a luciferase reporter gene assay and an EMSA assay. To investigate the effect of MF on the NF-κB transcriptional activity, RAW264.7 cells were transiently transfected with the pNF-κB-Luc plasmid (containing repeats of NF-κB recognition sequences) and pRL-TK plasmid (containing cDNA encoding Renilla luciferase). As shown in Fig. 3A, the NF-κB reporter activity was significantly increased in the LPS-treated cells when compared with the untreated cells (p<0.001). However, NF-κB reporter activity was significantly decreased in the cells pretreated with MF in a dose-dependent manner. Moreover, NF-κB DNA binding activity increased in the LPS-treated cells as shown by EMSA. However, MF treatment in the LPS-treated cells dose-dependently decreased the NF-κB DNA binding activity (Fig. 3B). These results indicated that MF suppressed the production of NO and pro-inflammatory cytokines through the attenuation of NF-κB DNA binding activity.
Fig. 3.
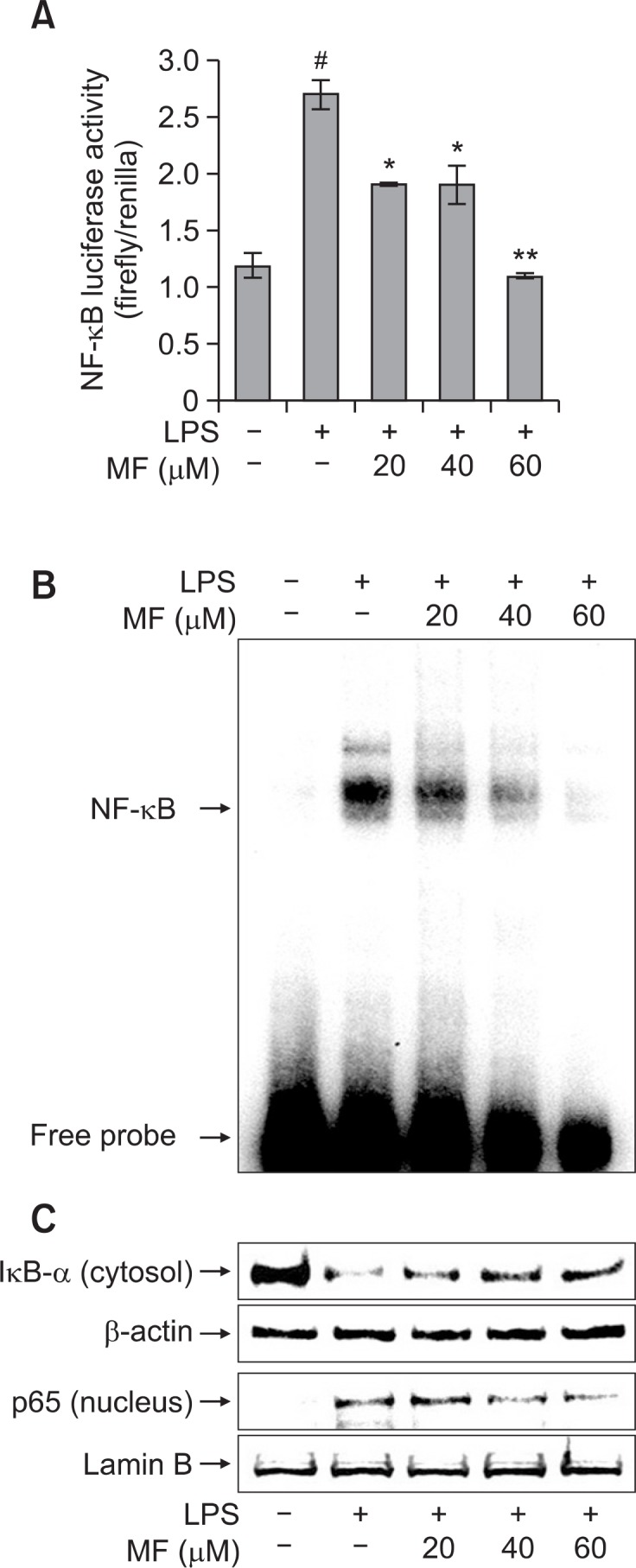
Effect of MF on LPS-induced NF-κB activation in RAW-264.7 ma crophages. (A) RAW264.7 cells were transiently transfected with pNF-κB-Luc and pRL-TK vector. The cells were pre-treated with MF (20, 40, and 60 μM) for 1 h and then incubated for 24 h with LPS (1 μg/ml). The NF-κB luciferase activity was determined using a dual-luciferase reporter assay. Error bars represent the mean ± SD. #p<0.001 vs. control, *p<0.01 vs. LPS, **p<0.001 vs. LPS. (B and C) RAW 264.7 cells were pretreated with MF (20, 40, and 60 μM) for 1 h and then incubated for 30 min with LPS (1 μg/ml). The nuclear and cytosolic extracts were prepared and the NF-κB DNA binding activity was analyzed using an EMSA assay (B). The IκB-α degradation and p65 nuclear translocation levels were determined using western blot analysis (C).
Effect of MF on degradation of IκB-α and nuclear translocation of p65 in LPS-stimulated RAW264.7 cells
To further investigate the molecular mechanisms of MF-mediated inhibition of iNOS expression, we investigated nuclear translocation of p65 subunit of NF-κB and degradation of IκB-α by western blot. As shown in Fig. 3C, LPS treatment significantly increased the nuclear translocation of p65 when compared with the untreated cells. However, MF pretreatment dose-dependently inhibited the nuclear translocation of p65. In addition, LPS treatment markedly induced the degradation of cytosolic IκB-α in RAW264.7 cells. However, this effect was inhibited by MF treatment in a dose-dependent manner. These results strongly suggested that MF prevented the nuclear translocation of NF-κB p65 by blocking the degradation of IκB-α.
Effect of MF on AP-1 transcriptional activity and MAPK phosphorylation in LPS-stimulated RAW264.7 cells
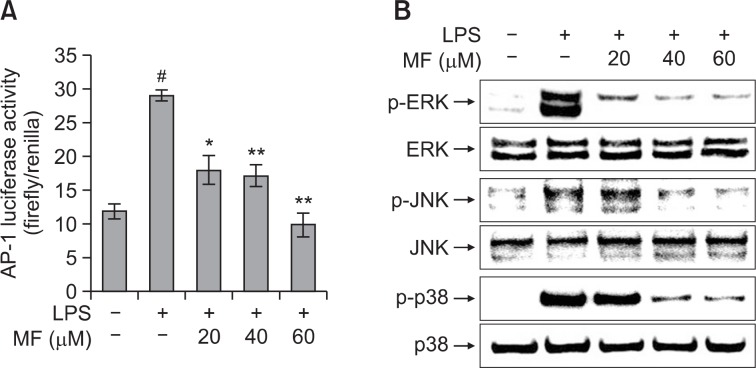
To further identify the mechanism through which MF exhibits anti-inflammatory effects in LPS-stimulated RAW264.7 cells, we assessed the activation of AP-1 by a luciferase reporter gene assay. RAW264.7 cells were transiently transfected with the pAP-1-Luc plasmid and pRL-TK plasmid. As shown in Fig. 4A, LPS treatment significantly increased the AP-1 reporter activity when compared with the untreated cells (p<0.001). However, MF treatment significantly decreased the LPS-induced AP-1 reporter activity in a dose-dependent manner. To further clarify the molecular mechanism of the anti-inflammatory effects of MF, we examined the phosphorylation of ERK, JNK, and p38 MAPK using a western blot analysis. LPS treatment significantly increased the phosphorylation of ERK, JNK, p38. However, MF treatment reduced phosphorylated ERK, JNK, p38 levels in LPS-stimulated RAW264.7 cells in a dose-dependent manner (Fig. 4B). These results suggest that the inhibitory effect of MF on LPS-induced inflammation might be involved in MAPK signaling pathway.
Fig. 4.
Effect of MF on LPS-induced AP-1 activity (A) and phosphorylation of MAPK (B) in RAW264.7 macrophages. (A) After transfection with pAP-1-Luc and pRL-TK, RAW264.7 cells were pretreated with MF (20, 40, and 60 μM) for 1 h and then incubated for 24 h with LPS (1 μg/ml). The AP-1 luciferase activity was determined using a dual-luciferase reporter assay. Error bars represent the mean ± SD. #p<0.001 vs. control, *p<0.01 vs. LPS, **p<0.001 vs. LPS. (B) RAW264.7 cells were pretreated with MF (20, 40, and 60 μM) for 1 h and then incubated for 30 min with LPS (1 μg/ml). The cell extracts were prepared and the phosphorylation of MAPK was determined using western blot analysis.
DISCUSSION
The objective of the present study was to evaluate the anti-inflammatory effects of MF isolated from the seedcases of G. mangostana in LPS-stimulated RAW264.7 macrophages. In this study, we showed that MF inhibited MAP kinases, AP-1 and NF-κB activation, and the subsequent induction of proinflammatory mediators such as NO, iNOS, TNF-α, IL-6, and IL-1β.
In response to LPS stimulation, macrophages produce proinflammatory mediators such as NO and several cytokines such as TNF-α, IL-6, and IL-1β (Reddy and Reddanna, 2009). LPS-induced NO production is mediated by iNOS expre ssion. Excessive cytokines and iNOS-mediated NO production has been linked with many pathophysiological conditions such as inflammation and tumorigenesis (Pan et al., 2009). A previous report revealed that 1,5-anhydro-D-fructose attenuates LPS-induced iNOS expression and NO production in RAW264.7 cells (Meng et al., 2009). Consistent with this previous report, our results demonstrated that MF inhibited the LPS-induced NO production and decreased the iNOS expression in RAW264.7 cells. TNF-α and IL-6 have been implicated in autoimmune diseases including rheumatoid arthritis. It is also known that IL-1β is associated with the nociceptive activity of certain stimulating factors such as zymosan and acetic acid (Wang et al., 2012). Many researches have shown that phyto-chemicals such as schisantherin A (Ci et al., 2010), stevioside (Fengyang et al., 2012), and sophoraflavanone G (Wun et al., 2013) suppress pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β in LPS-stimulated RAW264.7 cells. In addition, Lee et al. (2012) documented that 3,4,5-trihydroxycinnamic acid inhibits LPS-induced production of NO, TNF-α, and IL-1β in BV2 microglial cells. Consistent with previous reports, we showed that MF suppresses the LPS-induced production of TNF-α, IL-6, and IL-1β in RAW264.7 cells. Therefore, our findings suggest that MF has an anti-inflammatory effect that may inhibit iNOS-mediated NO production, as well as TNF-α, IL-6, and IL-1β production.
It is known that NF-κB plays an important role in various pathological conditions as a transcription factor for pro-inflammatory mediators such as iNOS, TNF-α, and IL-1β (Lee et al., 2012). In response to LPS stimulation, NF-κB is translocated into a nucleus through IκB degradation by activating TLR4-mediated MyD88-dependent signaling pathway and in turn induces pro-inflammatory mediators (Kawai and Akira, 2006). Many studies have reported that the suppression of NF-κB activation by phytochemicals inhibits the increase of pro-inflammatory mediators such as iNOS, TNF-α, IL-6, and IL-1β in LPS-stimulated RAW264.7 cells (Gao et al., 2012; Han et al., 2012; Park et al., 2012). In the present study, we showed that MF significantly attenuated LPS-induced IκB degradation and nuclear translocation of p65 in LPS-stimulated RAW264.7 cells. Moreover, MF significantly decreased the NF-κB lucifer-ase and DNA binding activities in LPS-stimulated RAW264.7 cells. These findings suggest that MF may indicate an anti-inflammatory activity by inhibiting NF-κB activation.
In addition to NF-κB, MAPK including p38, ERK, and JNK plays an important role in cytokines production as well as cell growth and differentiation (Han et al., 2010). Upon stimulation with LPS, the phosphorylation of MAPK is involved in the activation of transcription factors including AP-1, CREB, STAT1, and NF-κB, and subsequently produces cytokines (Han et al., 2010; Choi et al., 2012; Wang et al., 2012). Previous reports have revealed that pro-inflammatory mediators are regulated by the down-regulation of MAPK, NF-κB, and AP-1 in LPS-treated RAW264.7 cells (Lee et al., 2010; Su et al., 2011). In agreement with previous reports, the present study showed that MF diminished the phosphorylation of p38, ERK, and JNK, and reduced the activation of AP-1 and NF-κB in LPS-stimulated RAW264.7 cells. To the best of our knowledge, MF is the anti-inflammatory natural compound that down-regulates LPS-induced MAPK, NF-κB, and AP-1 signaling pathways.
In conclusion, the present study demonstrates that MF isolated from G. mangostana suppresses the production of NO, iNOS, and pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) in LPS-stimulated RAW264.7 macrophages. Furthermore, our results show that these effects of MF are related to the inhibition of MAPK, NF-κB, and AP-1 activation in LPS-stimulated RAW264.7 macrophages. This report indicates for the first time that MF exhibits the anti-inflammatory effect, suggesting that MF should be considered as a candidate potential anti-inflammatory agent for the treatment of inflammatory diseases. Therefore, further studies are necessary to clearly elucidate the exact mechanisms and clinical therapeutic potential of MF.
Fig. 5.
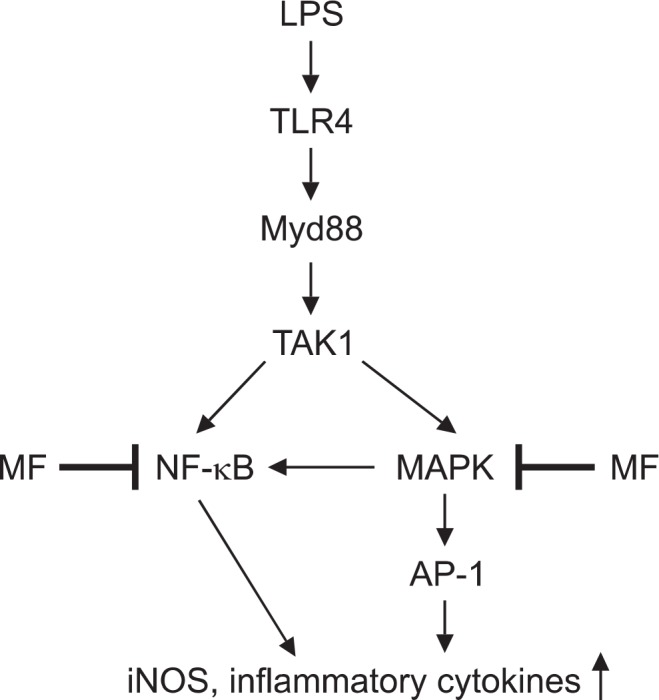
Possible model for anti-inflammatory effect by MF in LPS-stimulated RAW264.7 macrophages.
Acknowledgments
This research was supported by the Ministry of Science, ICT & Future Planning (MSIFP), Republic of Korea.
REFERENCES
- Choi WS, Shin PG, Lee JH, Kim GD. The regulatory effect of veratric acid on NO production in LPS-stimulated RAW264.7 macrophage cells. Cell Immunol. 2012;280:164–170. doi: 10.1016/j.cellimm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Ci X, Ren R, Xu K, Li H, Yu Q, Song Y, Wang D, Li R, Deng X. Schisantherin A exhibits anti-inflammatory properties by down-regulating NF-κB and MAPK signaling pathways in lipopolysaccharide-treated RAW 264.7 cells. Inflammation. 2010;33:126–136. doi: 10.1007/s10753-009-9166-7. [DOI] [PubMed] [Google Scholar]
- Fengyang L, Yunhe F, Bo L, Zhicheng L, Depeng L, Dejie L, Wen Z, Yongguo C, Naisheng Z, Xichen Z, Zhengtao Y. Stevioside suppressed inflammatory cytokine secretion by downregulation of NF-κB and MAPK signaling pathways in LPS-stimulated RAW264.7 cells. Inflammation. 2012;35:1669–1675. doi: 10.1007/s10753-012-9483-0. [DOI] [PubMed] [Google Scholar]
- Gao Y, Jiang W, Dong C, Li C, Fu X, Min L, Tian J, Jin H, Shen J. Anti-inflammatory effects of sophocarpine in LPS-induced RAW 264.7 cells via NF-κB and MAPKs signaling pathways. Toxicol. In Vitro. 2012;26:1–6. doi: 10.1016/j.tiv.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Han JM, Jin YY, Kim HY, Park KH, Lee WS, Jeong TS. Lavandulyl flavonoids from Sophora flavescens suppress lipopolysaccharide-induced activation of nuclear factor-κB and mitogen-activated protein kinases in RAW264.7 cells. Biol Pharm Bull. 2010;33:1019–1023. doi: 10.1248/bpb.33.1019. [DOI] [PubMed] [Google Scholar]
- Han Y, Jung HW, Lee JY, Kim JS, Kang SS, Kim YS, Park YK. 2,5-Dihydroxyacetophenone isolated from Rehmanniae radix preparata inhibits inflammatory responses in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Med. Food. 2012;15:505–510. doi: 10.1089/jmf.2011.1940. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Lee J, Tae N, Lee JJ, Kim T, Lee JH. Eupatolide inhibits lipopolysaccharide-induced COX-2 and iNOS expression in RAW264.7 cells by inducing proteasomal degradation of TRAF6. Eur J Pharmacol. 2010;636:173–180. doi: 10.1016/j.ejphar.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Lee JW, Bae CJ, Choi YJ, Kim SI, Kim NH, Lee HJ, Kim SS, Kwon YS, Chun W. 3,4,5-Trihydroxycinnamic acid inhibits LPS-induced iNOS expression by suppressing NF-κB activation in BV2 microglial cells. Korean J Physiol Pharmacol. 2012;16:107–112. doi: 10.4196/kjpp.2012.16.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Kawahara K, Matsushita K, Nawa Y, Shrestha B, Kikuchi K, Sameshima H, Hashiguchi T, Maruyama I. Attenuation of LPS-induced iNOS expression by 1,5-anhydro-D-fructose. Biochem Biophys Res Commun. 2009;387:42–46. doi: 10.1016/j.bbrc.2009.06.108. [DOI] [PubMed] [Google Scholar]
- Pan MH, Yang JR, Tsai ML, Sang S, Ho CT. Anti-inflammatory effect of Momordica grosvenori Swingle extract through suppressed LPS-induced upregulation of iNOS and COX-2 in murine macrophages. J. Funct. Foods. 2009;1:145–152. [Google Scholar]
- Pansanit A, Park EJ, Kondratyuk TP, Pezzuto JM, Lirdprapamongkol K, Kittakoop P. Vermelhotin, an anti-inflammatory agent, suppresses nitric oxide production in RAW 264.7 cells via p38 inhibition. J Nat Prod. 2013;76:1824–1827. doi: 10.1021/np400565e. [DOI] [PubMed] [Google Scholar]
- Park HY, Kim GY, Hyun JW, Hwang HJ, Kim ND, Kim BW, Choi YH. 7,8-Dihydroxyflavone exhibits anti-inflammatory properties by downregulating the NF-κB and MAPK signaling pathways in lipopolysaccharide-treated RAW264.7 cells. Int J Mol Med. 2012;29:1146–1152. doi: 10.3892/ijmm.2012.935. [DOI] [PubMed] [Google Scholar]
- Pedraza-Chaverri J, Cardenas-Rodriguez N, Orozco-Ibarra M, Perez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food chem Toxicol. 2008;46:3227–3239. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Reddy DB, Reddanna P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW 264.7 macrophages. Biochem Biophys Res Commun. 2009;381:112–117. doi: 10.1016/j.bbrc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Ryu HW, Cho JK, Curtis-Long MJ, Yuk HJ, Kim YS, Jung S, Kim YS, Lee BW, Park KH. α-Glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochemistry. 2011;72:2148–2154. doi: 10.1016/j.phytochem.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Ryu HW, Curtis-Long MJ, Jung S, Jin YM, Cho JK, Ryu YB, Lee WS, Park KH. Xanthones with neuraminidase inhibitory activity from the seedcases of Garcinia mangostana. Bioorg Med Chem. 2010;18:6258–6264. doi: 10.1016/j.bmc.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Ryu HW, Jeong SH, Curtis-Long MJ, Jung S, Lee JW, Woo HS, Cho JK, Park KH. Inhibition effects of mangosenone F from Garcinia mangostana on melanin formation in B16F10 cells. J Agric Food Chem. 2012;60:8372–8378. doi: 10.1021/jf3015987. [DOI] [PubMed] [Google Scholar]
- Shin JS, Park YM, Choi JH, Park HJ, Shin MC, Lee YS, Lee KT. Sulfuretin isolated from heartwood of Rhus verniciflua inhibits LPS-induced inducible nitric oxide synthase, cyclooxygenase-2, and pro-inflammatory cytokines expression via the down-regulation of NF-κB in RAW 264.7 murine macrophage cells. Int Immunopharmacol. 2010;10:943–950. doi: 10.1016/j.intimp.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Su YW, Chiou WF, Chao SH, Lee MH, Chen CC, Tsai YC. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int Immunopharmacol. 2011;11:1166–1172. doi: 10.1016/j.intimp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Suksamrarn S, Suwannapoch N, Ratananukul P, Aroonlerk N, Suksamrarn A. Xanthones from the green fruit hulls of Garcinia mangostana. J Nat Prod. 2002;65:761–763. doi: 10.1021/np010566g. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jiang W, Zhang Z, Qian M, Du B. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J Ethnopharmacol. 2012;144:145–150. doi: 10.1016/j.jep.2012.08.041. [DOI] [PubMed] [Google Scholar]
- Wun ZY, Lin CF, Huang WC, Huang YL, Xu PY, Chang WT, Wu SJ, Liou CJ. Anti-inflammatory effect of sophoraflavanone G isolated from Sophora flavescens in lipopolysaccharide-stimulated mouse macrophages. Food Chem Toxicol. 2013;62:255–261. doi: 10.1016/j.fct.2013.08.072. [DOI] [PubMed] [Google Scholar]