Abstract
Vitiligo is a pigmentary disorder induced by a loss of melanocytes. In addition to replacement of pure melanocytes, cocultures of melanocytes with keratinocytes have been used to improve the repigmentation outcome in vitiligo treatment. We previously identified by in vitro studies, that adipose-derived stem cells (ADSCs) could be a potential substitute for keratinocytes in cocultures with melanocytes. In this study, the efficacy of pigmentation including durability of grafted melanocytes and short-term safety was examined in the nude mouse and Sprague-Dawley rat after grafting of primary cultured human melanocytes, with or without different ratios of primary cultured human ADSCs. Simultaneous grafting of melanocytes and ADSCs, which were separately cultured and mixed on grafting at the ratios of 1:1, 1:2, or 1:3, showed better efficacy than that of pure melanocytes. Grafting of melanocytes cocultured with ADSCs resulted in a similar outcome as the grafting of cell mixtures. Skin pigmentation by melanocytes : ADSCs at the ratios of 1:1 and 1:2 was better than at 1:3. No significant difference was observed between the 1-week and 2-week durations in coculturing. Time-course microscopic examination showed that the grafted melanocytes remained a little longer than 6-week post-grafting. No inflammatory cell infiltration was observed in the grafted skin and no melanocytes were detectable in other organs. Collectively, grafting of melanocytes and ADSCs was equally safe and more effective than grafting of melanocytes alone. Despite the absence of significant differences in efficacy between the group of 1:1 and that of 1:2 ratio, 1:2 ratio for 1-week coculturing may be better for clinical use from the cost-benefit viewpoint.
Keywords: Melanocytes, Adipose-derived stem cells, Grafting, Animal, Efficacy, Short-term safety
INTRODUCTION
Vitiligo is a pigmentary disorder caused by uncertain mechanism, resulting in a loss of melanocytes. Repigmentation of vitiligo requires an increase in the number and migration of melanocytes to the depigmented epidermis (Wu et al., 2004). Because melanocytes undergo rare mitosis without help of growth factors or mitogenic factors (Jimbow et al., 1975; Pawelek, 1979), various ways of skin grafting or cell transplantation have been used for the treatment of vitiligo. Cell transplantation is one of the most fascinating and promising procedures to get size ratios greater than 1:10 to 1:60 between donor and recipient skin (Hong et al., 2011). Cultured or non-cultured cells have been used. However, long-term results of cultured melanocyte transplantation have been poorer than those by epidermal graft (Olsson and Juhlin, 2002).
Simultaneous transplantation of melanocytes and keratinocytes has been attempted to enhance engraftment rates and facilitate procedures (Falabella et al., 1989; Guerra et al., 2000; Quezada et al., 2011), since keratinocytes produce lots of growth factors for melanocyte proliferation and migration. In clinical trial, an easier way of obtaining autologous tissue is also an important consideration. However, culturing of keratinocytes requires additional skin specimens, which is an impetus to investigate a potential substitute for keratinocytes. Adipose-derived stem cells (ADSCs) are easily isolated and cultured from adipose tissue obtained by liposuction via a small incision. Therefore, we previously compared the effect of ADSCs vs. keratinocytes on proliferation and migration of cocultured melanocytes. Although the proliferation and migration induced by ADSCs was less than keratinocytes, it was significantly more than melanocyte monocultures (Kim et al., 2012). These in vitro results suggested that ADSCs could be a potential substitute for keratinocytes in cocultures with melanocytes.
Proven efficacy and safety of certain methods are required by animal study. The precise mechanisms triggering vitiligo are uncertain. Therefore, existing vitiligo animal models, which are generally based on the autoimmune etiology of vitiligo (Austin et al., 1992; Harris et al., 2012; Shi and Erf, 2012), are unable to provide a platform to evaluate therapeutic efficacy. On the other hand, immunodeficient animals including the nude mouse have been widely used for xenograft experiments, because they fail to reject grafted specimens (Manning et al., 1973). Grafting of cultured cells (Germain et al., 1995) or skin substitutes (Lam et al., 1999; Guerret et al., 2003) has been performed in the athymic nude mouse to examine the effect of wound healing. In addition, skin substitutes containing different number of melanocytes have been grafted in athymic mice for titration of appropriate cell number to repigment the burn-induced depigmented lesion (Swope et al., 2002).
In this study, the efficacy of pigmentation was compared in the nude mouse and Sprague-Dawley rat after grafting of primary cultured human melanocytes, with or without different ratios of primary cultured human ADSCs. Short-term safety was also examined. Grafting of cocultured cells in the ratio of melanocytes to ADSCs of 1:1 and 1:2 was better than that of monocultures. Skin inflammation and melanocytes in other organs were not detected by the grafting in animal skin.
MATERIALS AND METHODS
Normal human epidermal melanocyte culture
Adult skin specimens obtained from repeat Cesarean section deliveries and circumcisions were used for the cultures after obtaining approval from by the Institutional Review Board of the Dongguk University Ilsan Hospital (Approval number; 2012-69). The epidermis was separated from the dermis after treatment with 2.4 U/mL of dispase (Roche, Mannheim, Germany) for 1 hour. The epidermal sheets were treated with 0.05% trypsin for 10 minutes to produce a suspension of individual epidermal cells. The cells were suspended in Medium 254 (Cascade Biologics, Portland, OR, USA) supplemented with fetal bovine serum (FBS), bovine pituitary extract, bovine insulin, hydrocortisone, basic fibroblast growth factor (bFGF), bovine transferrin, heparin, and phorbol 12-myristate 13-acetate (Cascade Biologics). The concentration of FBS was 0.5%. When the cells reached 80% confluency, they were detached from the flask and seeded into other culture flasks either in preparation for an experimental procedure, or to maintain and propagate the cell culture. For the following experiments, the concentration of all supplements in the Medium 254 was reduced to 20% (supplement-starved Medium 254), with 0.1% FBS.
ADSCs preparation
Human ADSCs were kindly provided as a gift from Dr. Byungrok Do of Hurim BioCell Inc. (Seoul, South Korea). The cells were cultured in low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, Life Technology, NY, USA) supplemented with 10% FBS (Gibco/BRL), 100 U/mL of penicillin (Gibco/BRL), and 0.1 mg/mL of streptomycin (Gibco/BRL).
Coculture of ADSCs with melanocytes
For culture with melanocytes, ADSCs were dissociated with trypsin/EDTA solution and resuspended in low glucose DMEM (Invitrogen). The cells were counted by automatic cell counter, Countess® (Invitrogen) and were plated at 5×104 cells in 25 cm2 tissue culture flasks (NUNC, Roskilde, Denmark). Meanwhile, melanocytes were harvested by TrypLETM Express (Invitrogen) and then were added in tissue flasks containing ADSCs at a density of 2.5×104 cells. The co-culture cells were cul tured by standard medium of melanocytes, Medium 254 with 1X HMGS (Invitrogen), for two weeks and the medium was generally changed every two days.
Grafting of cultured cells to animal skin
8-week-old female BALB/c nude mice (Orient Bio Inc., Korea) and 4-week-old female Sprague-Dawley (SD) rats (Orient Bio Inc., Korea) were utilized in this study. The mice and rats were acclimatized for 1 week for stress relief and pre-feeding purposes. After acclimatization, the animals were anesthetized through intraperitoneal injection of Zoletyl and Lumpen. In case of SD rats, the hair on the dosal lateral back was shaved using an electric shaver. Then, the mice and rats were marked with an approximately 1×1 cm2 area, on the dorsal lateral back, using a pen. Dermabrasion was accomplished using a small felt wheel attached to a hand-piece motor (Saeshin Strong 204, Korea) and operated at 5,000 rpm. To improve the grafting efficiency, melanocytes alone, ADSCs alone or cocultures of these cells were treated with fibrinogen solution (Green cross, Korea; 501A13015; 10 mg/ml) and thrombin solution (Green cross; 501A13020; 50 IU/ml) prior to the application of the dermabraded area. After 5 minutes, the area was covered with a TegadermTM Film.
Microscopic analysis
Mice were euthanized and the skin of the dermabraded area was removed and placed in 10% buffered formalin solution, and embedded in paraffin blocks. The paraffin-embedded skin samples were sectioned to 4 μm thickness, and then stained with hematoxylin and eosin (H&E). The cell density was expressed as the number of cells per 5 high-power fields (×400) for each section.
Statistical analysis
The statistical analysis of the experimental data was performed using the Student’s t-test. The results were expressed as the mean ± S.D. A p-value <0.05 was considered significant.
RESULTS
Simultaneous grafting of ADSCs increased the number of melanocytes in nude mice
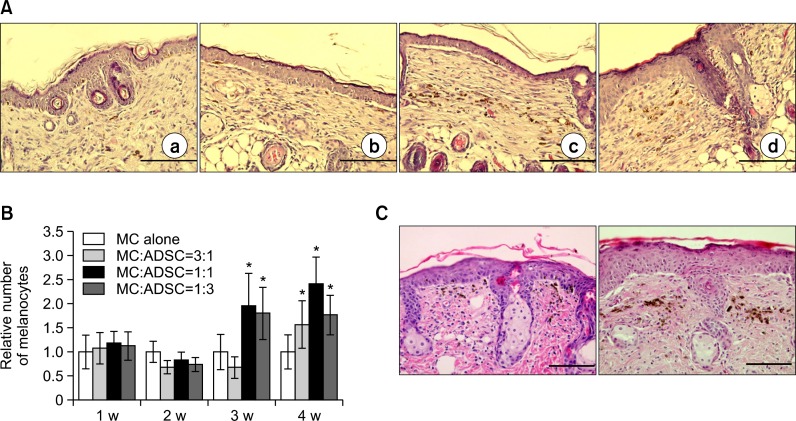
Based on the in vitro finding that 2.5×103 melanocytes/mL and 1.5×104 ADSCs/mL (mitomycin C-treated) were required to be seeded for coculturing (Kim et al., 2012), 1:1 and 1:10 cell mixtures of melanocytes to ADSCs were preliminary examined for grafting in nude mice. Because the result was sig nificantly better with the 1:1 cell mixture (data not shown), tra nsplantation of cultured cell mixture to nude mice was performed using ratios of melanocytes to ADSCs as 1:3, 1:1, and 3:1. A group of melanocyte monocultures and that of ADSCs were used as positive and negative controls, respectively. The number of melanocytes was examined in each group composed of 4 mice weekly for 4 weeks. Light microscopic examination in skin specimens stained with H&E suggested that the number of melanocytes was not increased in all groups, until 3-week post-grafting. After 3 weeks, the melanocyte number was increased, particularly in the groups of 1:1 and 1:3 mixtures (Fig. 1A, B). Although cultured cells were grafted on the skin after removal of epidermis by dermabrasion alone, melanocytes were detected in epidermis, upper dermis, and middermis at 1-week and 2-week post-transplantation, respectively (Fig. 1C). No inflammatory cells infiltrated the epidermis and dermis (Fig.1A, C).
Fig. 1.
Simultaneous grafting of melanocytes, with or without ADSCs in nude mice. (A) Light microscopy of skin specimens stained with H&E (×400). Skin was grafted with indicated cells, such as melanocytes alone (a, 3.3×104 cells), melanocytes to ADSCs ratio as 3:1 (b), 1:1 (c) and 1:3 (d) for 2 weeks. Scale bar=200 μm (B) Relative number of melanocytes, which was determined by calculating the ratio of melanocyte number cultured in each condition to that cultured melanocyte alone for 1 week, for 4 weeks after grafting of melanocytes, with or without ADSCs as indicated ratios. The number of melanin-containing melanocytes was counted in 5 random microscopic fields (high power, ×400) for statistical analysis. The data represent the mean ± SD of 5 nude mice. *p<0.05 vs. melanocyte monoculture (C) H&E staining of skin specimens 1 week (left) and 2 weeks (right) after grafting of melanocytes alone to compare their residing location. ×400, Scale bar=200 μm.
Grafting of melanocytes cocultured with ADSCs increased melanocyte number in nude mice compared to melanocyte monocultures
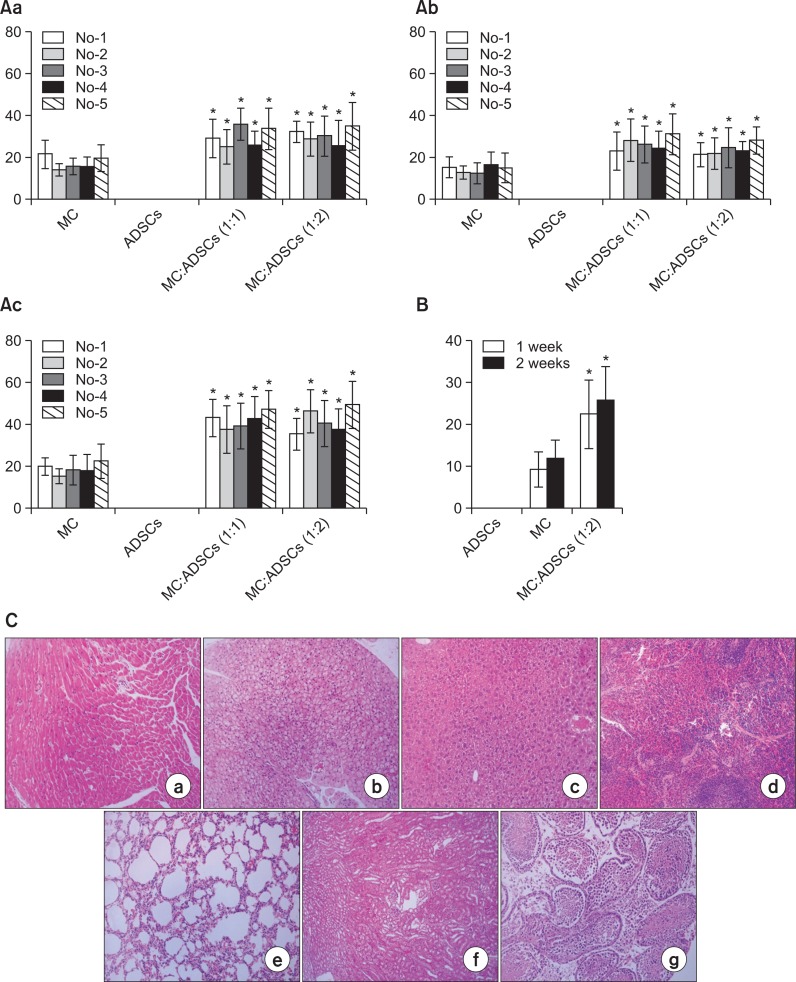
It was uncertain whether transplantation of cocultured melanocytes and ADSCs could result in an outcome similar to the transplantation of each of the cultured cell (Fig.1A, B). However, grafting of melanocytes and ADSCs cocultured for 2 weeks at ratios of 1:1, 1:2, and 1:3 showed more melanocytes at the ratios of 1:1 and 1:2 (data not shown). Therefore, the number of melanocytes was examined on grafting the cocultured cells at the ratios of 1:1 and 1:2 in nude mice for 3 weeks. A group of melanocyte monocultures and that of ADSCs were used as positive and negative controls, respectively. The number of mice in each group was 5. The number of melanocytes was significantly increased by grafting of cocultured cells from 1-week to 3-week post-examination (Fig. 2A). No significant differences in melanocyte number were detected between the cocultures at 1:1 and 1:2 (Fig. 2A).
Fig. 2.
(A) Number of melanocytes at 1 week (Aa), 2 weeks (Ab), and 3 weeks (Ac) after grafting of melanocytes-ADSCs cocultures vs. melanocyte monocultures in nude mice. The data represented the mean ± SD of 5 nude mice. *p<0.05 vs. melanocyte monoculture (B) Melanocyte number in nude mice skin at 2-week post-grafting of melanocytes alone (a, 1.0×105 cells), fixed ratio of melanocytes to ADSCs (b, 1:2), and ADSCs alone (c). The cells were cultured in vitro for 1 or 2 weeks. The data represent the mean ± SD of 5 nude mice. *p<0.05 vs. melanocyte monoculture (C) Light microscopy of different internal organs, such as lymph nodes (a), lung (b), liver (c), spleen (d), kidney (e), heart (f), and ovary (g) 12 weeks after melanocyte grafting.
To examine an appropriate duration of cocultures, melanocytes and ADSCs were cocultured at the fixed ratio of 1:2, for 1 and 2 weeks. The cells were then transplanted to nude mice. A group of melanocyte monocultures and that of ADSCs were used as positive and negative controls, respectively. The number of mice in each group was 5. The number of melanocytes, determined by microscopy, at 2-week post-transplantation, was significantly increased in the group grafted with cocultured cells. No significant difference was observed between the 1-week and 2-week durations, although the melanocyte number increased a little more in 2-week cocultures (Fig. 2B). No melanocytes were detected in other tissues or organs (Fig. 2C).
Grafting of cocultured cells compared to melanocyte monocultures also increased melanocyte number in Sprague-Dawley rat
No significant effect of the culture duration was observed on the melanocyte number in nude mice (Fig. 2B). Therefore, melanocytes and ADSCs cocultured at the fixed ratio of 1:2 for 2 weeks were grafted in SD rats, and the melanocyte number was examined at 1-week and 2-week post-grafting. A group of melanocyte monocultures and that of ADSCs were used as positive and negative controls, respectively. The number of rats in each group was 5. Similar to the result in nude mice, the number of melanocytes was significantly increased in the group grafted with cocultured cells at 1-week and 2-week post-grafting (Fig. 3).
Fig. 3.
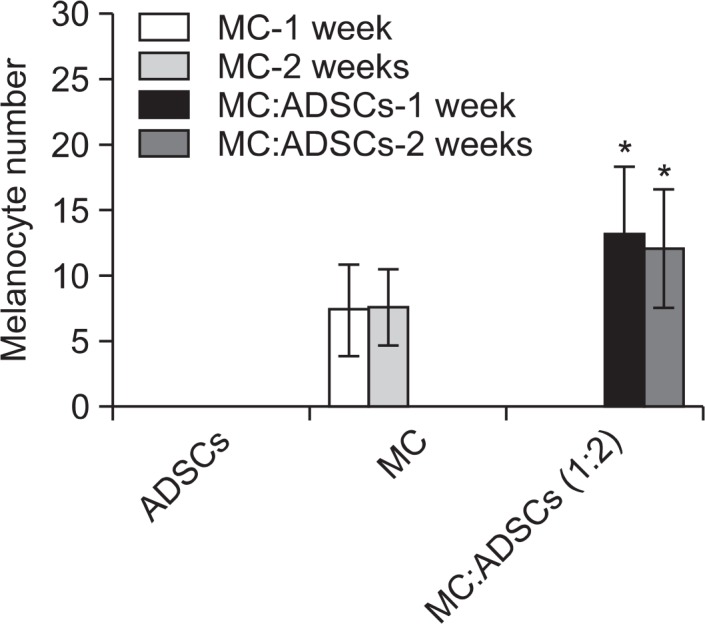
Number of melanocytes at 1 week and 2 weeks after grafting of melanocytes-ADSCs cocultures at a fixed ratio (1:2) vs. melanocyte monocultures in SD rats. The cells were cultured in vitro for 2 weeks. The data represent the mean ± SD of 5 SD rats. *p<0.05 vs. melanocyte monoculture.
Lasting duration of grafted cells in nude mice skin
To examine the lasting duration of grafted cells in nude mice skin, melanocytes and ADSCs cocultured for 1 week at the 1:2 ratio were grafted in nude mice, and melanocyte number was subsequently examined at a 2-week interval, up to 12 weeks. A group of melanocyte monocultures and that of ADSCs were used as positive and negative controls, respectively. The number of mice in each group was 5. The number of melanocytes was increased up to 4 weeks after the grafting of either cocultured cells or monocultured melanocytes, despite more cells in the group of cocultures. The melanocyte number at 6-week post-grafting was not so low as compared to that at 2-week and 4-week post-grafting, however, it was suddenly decreased thereafter, without remaining detectable melanocytes at 8-week of grafting, or later (Fig. 4).
Fig. 4.
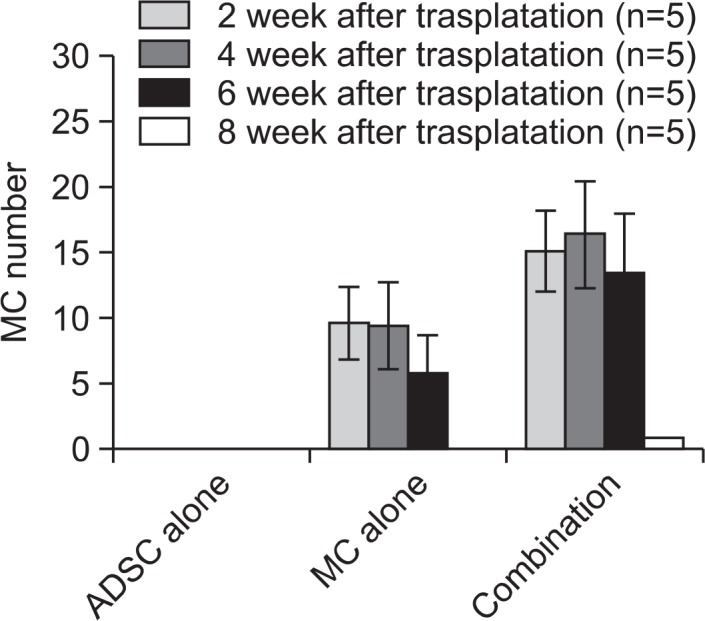
Durability of grafted cells in nude mice. The data represent the mean ± SD of 5 nude mice. *p<0.05 vs. melanocyte monoculture.
DISCUSSION
Vitiligo results from a loss of melanocytes. Although various ways have been developed to replace melanocytes, their efficacy remains to be improved. We previously identified that ADSCs could be a potential substitute for keratinocytes in co-cultures with melanocytes in vitro (Kim et al., 2012), hence we accordingly determined the in vivo effectiveness in this study.
Separate cultures of melanocytes and ADSCs were easier than coculturing these cells. Therefore, grafts using a mixture of separately cultured melanocytes and ADSCs were performed for screening the efficacy and proper conditions for coculturing. A higher number of melanocytes remained in the skin, whenever the cell mixture was grafted (Fig.1A, B). A better outcome of the cell mixture compared to pure melanocytes corroborated the earlier in vitro result (Kim et al., 2012). The result obtained from grafting of cocultured melanocytes and ADSCs in nude mice (Fig. 2A) also supported that from mixed cell grafting. In addition, a similar result was obtained in the other animal model of SD rat (Fig. 3). Therefore, grafting of melanocytes-ADSCs mixtures/cocultures could be a better way to improve pigmentation efficiency compared to grafting of pure melanocytes.
For clinical application, shorter duration of coculturing using fewer melanocytes may be useful to reduce the cost. The more effective ratios between melanocytes and ADSCs were 1:1 to 1:2 in nude mice in our study (Fig. 1A, B), which differed from the in vitro ratio of 1:6 (Kim et al., 2012). However, ADSCs in vitro were treated with mitomycin C to inhibit the cell growth, whereas the cells for the graft in vivo were not. Without mitomycin C treatment under the melanocyte culture medium, the ratio of 1:2 in seeding was changed into that of 3:7 to 1:3 in harvesting. Proliferation and migration could be different in each cell type in vivo after the grafting, which may also influence the ratios. No significant difference between the co-cultures at the ratios of 1:1 and 1:2 (Fig. 2A) and between the 1-week and 2-week culture durations (Fig. 2B) may provide a clue to cut down the melanocyte number and culture duration. In fact, the reproducible result that grafting of a 1:2 fixed ratio of melanocytes to ADSCs induced significantly higher number of melanocytes and more repigmentation in both nude mice and SD rat skin compared to pure melanocytes (Fig. 2B, 3), may support be favorable from a clinical application point of view.
Microscopic examination revealed that the location of grafted melanocytes in nude mice was mainly the dermis, although some cells were detected in the lower epidermis as early as 1-week post-grafting (Fig. 1C). Similar findings were examined in SD rats (data not shown). Because solely the epidermis was removed in experimental animals by dermabrasion, melanocytes were expected to reside in the lower portion of the epidermis. In humans, melanocytes reside on the basement membrane after grafting, by the same method (Brysk et al., 1989). Because the time course of microscopic examination showed that melanocytes remained in the epidermis and upper dermis at 1 week, but in the mid-dermis at 2 weeks after the grafting (Fig. 1C), a migration of melanocytes may be involved in the melanocyte location. Although ADSCs stimulated melanocyte migration in the in vitro Boyden Chamber assay (Kim et al., 2012), the difference in residing location of grafted melanocytes, in animals remains to be clarified. In addition, residing location of grafted melanocytes should be carefully examined in vitiligo patients if melanocytes coculured ADSCs were grafted for the treatment.
The nude mouse is an immunodeficient animal, and it has been considered not to reject grafted specimens (Manning et al., 1973). The grafted cells grew longer than 6 weeks in nude mice (Fig. 4), as in culture medium. However, the cells did not last longer than 8 weeks (Fig. 4). Because there were no clinical and microscopic findings of inflammation in grafted mice skin, the disappearance of grafted cells was likely not due to the rejection processes. No melanocytes, as well as inflammatory cells were observed in other organs, such as lymph nodes, lung, liver, spleen, kidney, heart, and ovary up to 12 weeks after the grafting (Fig. 2C). This result also suggested that the grafting of melanocytes, with or without ADSCs could be safe, although long-term safety remains to be clarified.
Collectively, better efficacy of melanocytes-ADSCs grafting was identified in two kinds of animal models, i.e. nude mice and SD rats. The safety was also confirmed. Therefore, grafting of melanocytes with ADSCs could be a treatment modality for vitiligo.
Acknowledgments
This study was supported by a grant of the Korea Health-care technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. : A120302) and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) 2011-0028962.
REFERENCES
- Austin LM, Boissy RE, Jacobson BS, Smyth JR., Jr The detection of melanocyte autoantibodies in the Smyth chicken model for vitiligo. Clin Immunol Immunopathol. 1992;64:112–120. doi: 10.1016/0090-1229(92)90188-t. [DOI] [PubMed] [Google Scholar]
- Brysk MM, Newton RC, Rajaraman S, Plott T, Barlow E, Bell T, Penn P, Smith EB. Repigmentation of vitiliginous skin by cultured cells. Pigment Cell Res. 1989;2:202–207. doi: 10.1111/j.1600-0749.1989.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Falabella R, Escobar C, Borrero I. Transplantation of in vitro-cultured epidermis bearing melanocytes for repigmenting vitiligo. J Am Acad Dermatol. 1989;21:257–264. doi: 10.1016/s0190-9622(89)70170-5. [DOI] [PubMed] [Google Scholar]
- Germain L, Guignard R, Rouabhia M, Auger FA. Early basement membrane formation following the grafting of cultured epidermal sheets detached with thermolysin or Dispase. Burns. 1995;21:175–180. doi: 10.1016/0305-4179(95)80004-8. [DOI] [PubMed] [Google Scholar]
- Guerra L, Capurro S, Melchi F, Primavera G, Bondanza S, Cancedda R, Luci A, De Luca M, Pellegrini G. Treatment of “stable” vitiligo by Timedsurgery and transplantation of cultured epidermal autografts. Arch Dermatol. 2000;136:1380–1389. doi: 10.1001/archderm.136.11.1380. [DOI] [PubMed] [Google Scholar]
- Guerret S, Govignon E, Hartmann DJ, Ronfard V. Long-term remodeling of a bilayered living human skin equivalent (Apligraf) grafted onto nude mice: immunolocalization of human cells and characterization of extracellular matrix. Wound Repair Regen. 2003;11:35–45. doi: 10.1046/j.1524-475x.2003.11107.x. [DOI] [PubMed] [Google Scholar]
- Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869–1876. doi: 10.1038/jid.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong WS, Hu DN, Qian GP, McCormick SA, Xu AE. Ratio of size of recipient and donor areas in treatment of vitiligo by autologous cultured melanocytes transplantation. Br J Dermatol. 2011;165:520–525. doi: 10.1111/j.1365-2133.2011.10398.x. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Roth SI, Fitzpatrick TB, Szabo G. Mitotic activity in non-neoplastic melanocytes in vivo as determined by histochemical, autoradiographic, and electron microscope studies. J Cell Biol. 1975;66:663–670. doi: 10.1083/jcb.66.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Park CD, Lee JH, Lee CH, Do BR, Lee AY. Co-culture of melanocytes with adipose-derived stem cells as a potential substitute for co-culture with keratinocytes. Acta Derm Venereol. 2012;92:16–23. doi: 10.2340/00015555-1174. [DOI] [PubMed] [Google Scholar]
- Lam PK, Chan ES, Liew CT, Lau CH, Yen SC, King WW. The efficacy of collagen dermis membrane and fibrin on cultured epidermal graft using an athymic mouse model. Ann Plast Surg. 1999;43:523–528. doi: 10.1097/00000637-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Manning DD, Reed ND, Shaffer CF. Maintenance of skin xenografts of widely divergent phylogenetic origin of congenitally athymic (nude) mice. J Exp Med. 1973;138:488–494. doi: 10.1084/jem.138.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson MJ, Juhlin L. Long-term follow-up of leucoderma patients treated with transplants of autologous cultured melanocytes, ultrathin epidermal sheets and basal cell layer suspension. Br J Dermatol. 2002;147:893–904. doi: 10.1046/j.1365-2133.2002.04837.x. [DOI] [PubMed] [Google Scholar]
- Pawelek JM. Evidence suggesting that a cyclic AMP-dependent protein kinase is a positive regulator of proliferation in Cloud-man S91 melanoma cells. J Cell Physiol. 1979;98:619–625. doi: 10.1002/jcp.1040980320. [DOI] [PubMed] [Google Scholar]
- Quezada N, Machado Filho CA, De La Sotta P, Uribe P. Melanocytes and keratinocytes transfer using sandpaper technique combined with dermabrasion for stable vitiligo. Dermatol Surg. 2011;37:192–198. doi: 10.1111/j.1524-4725.2010.01856.x. [DOI] [PubMed] [Google Scholar]
- Shi F, Erf GF. IFN-γ, IL-21, and IL-10 co-expression in evolving autoimmune vitiligo lesions of Smyth line chickens. J Invest Dermatol. 2012;132:642–649. doi: 10.1038/jid.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope VB, Supp AP, Boyce ST. Regulation of cutaneous pigmentation by titration of human melanocytes in cultured skin substitutes grafted to athymic mice. Wound Repair Regen. 2002;10:378–386. doi: 10.1046/j.1524-475x.2002.10607.x. [DOI] [PubMed] [Google Scholar]
- Wu CS, Yu CL, Wu CS, Lan CC, Yu HS. Narrow-band ultraviolet-B stimulates proliferation and migration of cultured melanocytes. Exp Dermatol. 2004;13:755–763. doi: 10.1111/j.0906-6705.2004.00221.x. [DOI] [PubMed] [Google Scholar]