Abstract
We have developed a fully automated high throughput drug screening (HTDS) system based on the microfluidic cell culture array to perform combinational chemotherapy. This system has 64 individually addressable cell culture chambers where the sequential combinatorial concentrations of two different drugs can be generated by two microfluidic diffusive mixers. Each diffusive mixer has two integrated micropumps connected to the media and the drug reservoirs respectively for generating the desired combination without the need for any extra equipment to perfuse the solution such as syringe pumps. The cell array is periodically exposed to the drug combination with the programmed LabVIEW system during a couple of days without extra handling after seeding the cells into the microfluidic device and also, this device does not require the continuous generation of solutions compared to the previous systems. Therefore, the total amount of drug being consumed per experiment is less than a few hundred micro liters in each reservoir. The utility of this system is demonstrated through investigating the viability of the prostate cancer PC3 cell line with the combinational treatments of curcumin and tumor necrosis factor-alpha related apoptosis inducing ligand (TRAIL). Our results suggest that the system can be used for screening and optimizing drug combination with a small amount of reagent for combinatorial chemotherapy against cancer cells.
Keywords: Microfluidic system, HTDS, Combinational chemotherapy, PC-3, TRAIL, Curcumin
INTRODUCTION
It is widely accepted that combination chemotherapy has a benefit to increase efficacies at lower dose and less side-effects compared to single chemotherapy with high dose. Previous researches have shown that combination chemotherapies are even effective for some cancer cell lines that are resistant to anticancer drugs. Combination chemotherapy has demonstrated high survival benefits especially in treating prostate cancer and particularly in the cases of hormone refractory and metastatic disease state (Beltran et al., 2011). Recently, it has been reported that induction of apoptosis through administration of TRAIL can be effective in treating prostate cancer cells both in-vivo and in-vitro (Simons et al., 1999; Shankar et al., 2005). TRAIL is a member of the TNF (tumor necrosis factor) super-family of cytokines that induces apoptosis in a variety of cancer cell lines via death receptors (DR4, DR5) on the cell surface (Zhang and Fang, 2005). TRAIL has attracted significant attention due to its ability to selectively induce apoptosis in human tumor cells, while having minimal toxicity effects to normal cells (Sheikh and Fornace, 2000; Kelley and Ashkenazi, 2004; Bouralexis et al., 2005). Despite of the attractive advantage of TRAIL as anticancer drug, some cancer cell lines are inherently resistant to TRAIL-mediated apoptosis. The toxicity could increase in normal cells when the concentration of TRAIL is escalated to treat the resistant cancer cells. To enhance cancer cell sensitivity to the TRAIL, therapeutic strategies involving DNA-damaging genotoxins (Ohtsuka et al., 2003), radiotherapy (Shankar et al., 2004), and peptides (Barua et al., 2010) have been investigated. Diverse agents including anticancer drugs and many natural compounds were treated with TRAIL as a sensitizer to enhance cancer cell sensitivity. Among them, combination treatment of TRAIL with naturally occurring phytochemical compounds have been known as the effective method due to its inexpensiveness and nontoxic property to normal cells (Shankar et al., 2007; Siddiqui et al., 2008; Amin et al., 2009). Curcumin, called as the Indian solid gold, which is the main component of curry powder, is one of the natural components used to enhance the cancer cell sensitivity to TRAIL for treating cancer cells at lower concentrations, thereby minimizing the damage to normal cells. In India and Asia, turmeric is used for treating many health conditions and is reported to have anti-inflammatory, antioxidant, and perhaps even anticancer properties (Aggarwal et al., 2003; Wilken et al., 2011). In order to identify an appropriate sensitizer and find effective drug combinations that induce apoptosis of cancer cell lines resistant to anticancer drug, a large number of experimental platforms to screen variety of concentrations/combinations are required. Therefore, a high throughput drug screening (HTDS) system is the perfect candidate. The HTDS has become the most important and integrated part in drug discovery in most pharmaceutical and many biotechnology industries worldwide, and is now entrenched in the drug discovery processes (Hung et al., 2005a; Hung et al., 2005b). Compared to microfluidic system based HTDS systems, the conventional cell based HTDS systems require cumbersome processes such as cell loading and drug treatment using expensive robotic equipment. To overcome this limitation, many research groups have applied microfluidic technologies to develop HTDS systems. Although previously introduced microfluidic based HTDS were very successful, they required multiple tubing connections and syringe pumps for operation that results in large dead volume in tubing and connection parts (Wang et al., 2007; Kim et al., 2010a; Kim et al., 2010b). Here, we report a fully automated microfluidic system based HTDS with the integrated micropump system which does not require any external syringe pump or tubing connections for combinatorial screening of drugs. The integrated micropump system is composed of array of valves operating individually. Two sets of micropumps, one for anticancer drug and the other for sensitizer, operate automatically and independent from each other. Anticancer drugs and sensitizer are introduced into each of gradient mixer and generate 8 different concentrations for each and delivered into cell culture chambers, resulting in 64 different combinations in 64 chambers. Therefore, this tubingless microfluidic system based HTDS to perform efficient and facile combinational chemotherapy could be used as the methodological technique in pharmaceutical and many biotechnology industries. We demonstrate that this fully automated and integrated platform could be optimized for the combinational condition of curcumin concentration as sensitizer to TRAIL against prostate cancer PC3 cell line.
MATERIALS AND METHODS
Cells and materials
Human prostate cancer PC3 cells (Korean Cell line Bank, Seoul, Korea) were cultured and propagated in RPMI cell culture medium with 10% fetal bovine serum (WelGENE, Seoul, Korea) and 100 U/ml penicillin and 100 μg/μl streptomycin at 37°C under 5% CO2 and 95% atmospheric air in 95% humidity incubator under 85% cell confluence. The use of Cell from passage 4 is described as follows. Curcumin (C1386) and TRAIL (Recombinant human, 375-TEC) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and R&D systems (Minneapolis, MN, USA), and stored at a stock concentration of 40 mM and 1 μg/ml in starvation media respectively. The Live/Dead assay kit (L-3224) was purchased from Invitrogen (Carlsbad, CA, USA).
Microfluidic device design and fabrication
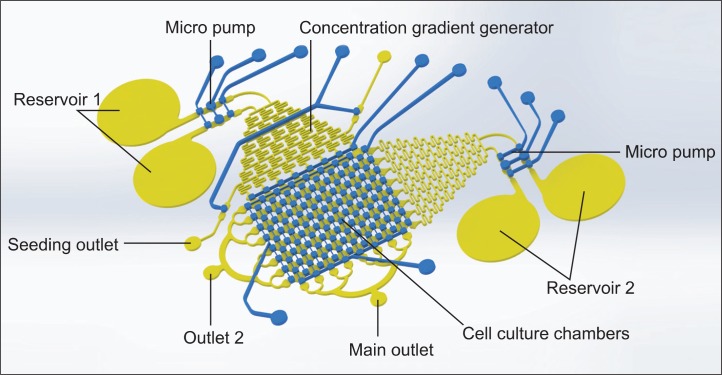
Soft lithography (the previously reported protocol [Whitesides et al., 2001; Kim et al., 2010a; Kim et al., 2010b; Kim et al., 2012a; Kim et al., 2012b]) was used for t fabrication of the microfluidic device in poly dimethyl siloxane (PDMS (Sylgard 184, Dow Corning)). This device consists of two layers – one for the fluidic layer that contains the diffusive mixer to generate a concentration gradient and microchambers to culture the cells and the other for the pneumatic layer that has 10 channels to control the microvalves on the fluidic layer independently (Fig. 1). The fluidic channel has 8×8 chamber array and two concentration gradient generators with two micropumps for the sequential drug treatment. Each micropump has a reservoir holding 100 μl volume to introduce the reagent into the microchannels. Pneumatic layer is composed of three parts; two valve groups to operate the micropump for generating the flow and maintaining the cell culture, gradient valves to isolate the diffusive mixer from injected cells through two outlets during seeding process, and directional valves to determine the flow direction of the generated gradient between two orthogonal direction in culture chamber arrays. Using the soft-lithography technology, a channel structures on silicon wafer were pattered using SU-8 2050 (Microchem, Newton, MA, USA) photo resistant as a master mold. PDMS was mixed with a 10:1 ratio between the base and curing agent, and after that the precursor PDMS mixture was poured on the master mold in a petri dish to obtain pneumatic layer with 4 mm thickness. The master mold for fluidic layer was spin-coated at 800 rpm for 30 seconds after pouring the PDMS precursor to fabricate the thin membrane with uniform thickness of 150 μm. In order to make a flat surface, these PDMS coated master molds were placed on a hotplate without heating for 10 minutes prior to baking at 70°C for 2 hrs. After baking, patterned PDMS bas-relief layer for pneumatic channel and membrane layer for fluidic channel were peeled from the master molds. PDMS bas-relief layer was punched with 19 gauge sharpened flat top needle to connect the pneumatic controlling tygon tubing. Two PDMS layers were then assembled after exposing to oxygen plasma aligned between the pneuma tic channel and the fluidic channel. Methanol was used as a lubricant to freely move around layers during aligning, and then was baked at 70°C for 8 hrs. Assembled PDMS device was punched to create the reservoirs prior to bonding it with a glass slide with oxygen plasma treatment. Before attaching the PDMS device to the slide glass, all pneumatic valves were keep opened by applying the negative pressure to prevent the surface of mi crovalves from permanent bonding to the glass slide. This process provides two layer PDMS structure with integrated microvalves fabricated without additional complex and time-consuming processes. The peristaltic micropump was composed of two microvalves and one actuator powered by pneumatic pressure was controlled by LabVIEW program. The detailed operating scheme of the peristaltic pump system is described in previously published research paper (Unger et al., 2000; Li et al., 2005; Lai and Folch, 2011; Graf and Bowser, 2013). The flow rate was controlled with a diameter of actuator and also the frequency of operating cycles of micropump pneumatic components. Since to assemble the micropump on microfluidic chip, any complex tubing and bulky syringe pump were not required to support the flow, it is so easy to handle the system for moving between microscope and cell culture incubator compared to previous systems (Kim et al., 2010a; Kim et al., 2010b). In addition, using the peristaltic pump provides much more stable fluidic flow in generating gradient with the diffusive mixer compared to syringe pumps where uneven back flow possibly causes the flow irregularity such as droplet generation. (Garstecki et al., 2006) The commercial pneumatic controller (NV-PNEUMARO 4, NavibioTech [Cheonan, Korea]) having 10 independent controlled pneumatic ports to operate the system automatically were used and the system was synchronized with LabVIEW software to automatically operate the pneumatic components in the microfluidic device during the entire experiment.
Fig. 1.
Schematic of the microfluidic array. Different concentrations of sensitizer and drug are generated in the diffusive gradient mixers sequentially to perfuse cells cultured in downstream microchambers. The fluidic and the pneumatic channels are represented with yellow and blue color respectively.
Cell experiment in 96-well plates
Controlled combination chemotherapy experiments were performed in a 96 well plate. PC3 cells were seeded with density of 8000 cells/ml (1600 cells per well). After culturing the cells for 24 hrs at 5% CO2 incubator, previous growth medium was changed to fetal bovine serum free medium and incubated for 5 hrs. PC3 cells were incubated with 0 to 40 μM curcumin contained medium for 24 hrs followed by 20 ng/ml TRAIL treatment for 24 hrs.
Generation of two drug concentrations in the microdevice
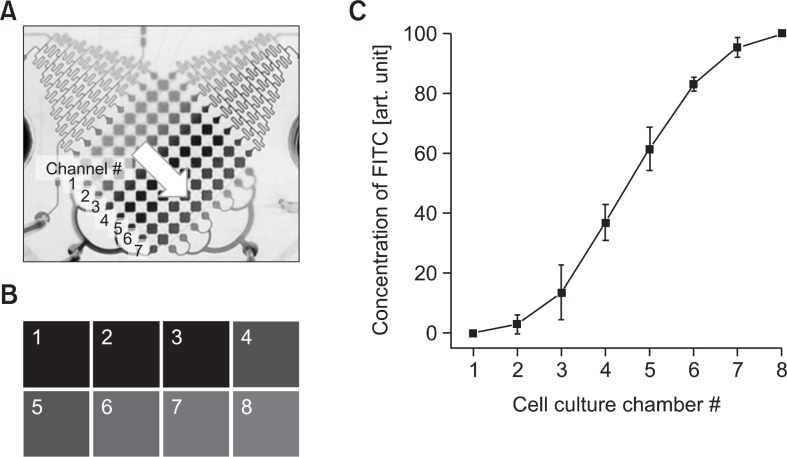
The microfluidic device has orthogonal cell culture chambers of 8×8 arrays. Each directional chamber arrays were connected with a concentration gradient generator containing reservoir. 8 different concentrations of drugs were generated by the flow by the micropump. 8 different concentrations of drug were introduced into cell culture chambers by opening the gradient valve and the directional valves with horizontal direction. The cell culture chamber arrays that are orthogonally positioned were exposed to 8 different concentrations with the same method after pretreated with sensitizer for 24 hrs. Therefore, 64 cell culture chambers were exposed 64 different combinations of sensitizer and drug. The valve and the pump actuation were automatically controlled by LabVIEW interface, enabling fully automated multi-day sequential cancer drug assay. The gradient generators were profiled through FITC by changing the parameter of micropump on LabVIEW system. (Fig. 3) The intensity of fluorescent shows the linear gradient profile increasing the FITC concentration in Fig. 3C.
Fig. 3.
The concentration gradient profile of diffusive mixer in microfluidic system using FITC with adjustment of the parameters of micro-pump on LabVIEW system for (A) the position of cell culture chambers, (B) fluorescent image of FITC in cell culture chamber array with concentration gradient, and (C) intensity of fluorescent depends on FITC concentration.
Cell culture and drug treatment
The microfluidic device was sterilized under UV for 20 min. Slide glass surface of fluidic channel was coated with 50 μg/ml collagen I and kept at 4°C for 12 hrs followed by removal of excessive collagen I with RPMI 1640 medium. PC3 cells were seeded into the cell culture chambers by controlling the micro valves using syringe pump at 12 μl/min flow rate, the valves around the cell culture chamber array were closed to prevent the invasion of cells into unexpected area such as diffusive mixer. After seeding, all valves were closed to help attach the cells on collagen I coated surface in cell culture chambers. The seeded cells were then incubated in 37ºC, 5% CO2 incubator for 18 hrs with refreshing medium every 4 hrs. After cell loading, each reservoir of a concentration gradient generator was filled with fresh medium and curcumin as the sensitizer respectively with closing all valves. For the drug treatment, all micro pumps for cell culture array were opened, introducing 8 different concentrations (from 0 μM to 40 μM) of curcumin. This treatment process was repeated every 4 hrs for refreshment. Also, TRAIL as anticancer drug was exposed into cell culture chambers through the other concentration gradient generator by same way after finishing the treatment of sensitizer.
Cell apoptosis assay
Post drug exposed cells were washed with 1×PBS (phosphate buffered saline) to remove dead cells and then Live/ Dead kit was introduced into cell culture chambers through left or right micro pump to stain the cells. Calcein AM indicates live cells with green fluorescent and ethidium homodimer indicates dead cells with red fluorescent. After introducing Live/ Dead kit, cells were kept in the dark for 30 min and washed with 1×PBS to rinse the cells. Stained cells were visualized and observed under a fluorescence microscope (AxioObserver D1, Carl Zeiss). In 96 well plate experiments, MTT assay was performed after combination drug treatment to verify cell viability. This result was compared with experiment in micro-fluidic device.
RESULTS
Characterization of concentration gradients in the device
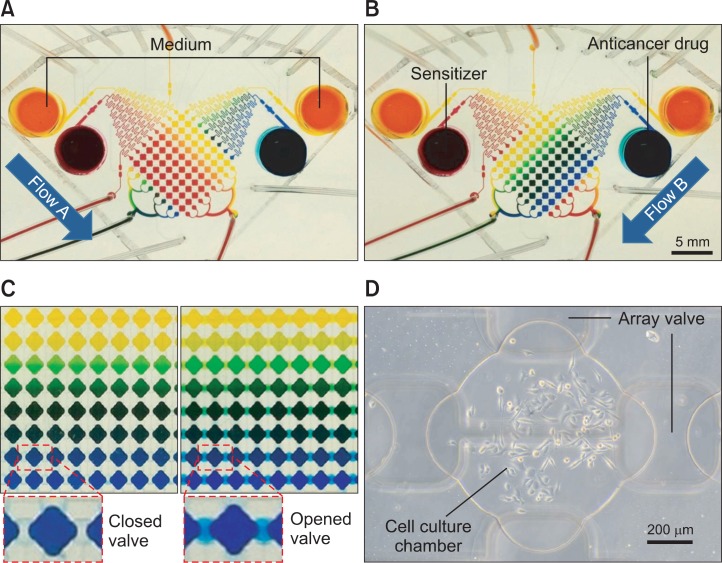
The microfluidic channel has 8×8 chamber array and two con centration gradient generators with two micropumps for the sequential drug treatment (Fig. 1). Different concentrations of curcumin and TRAIL were generated in the array using two diffusive mixers in conjunction with micropumps in the single microfluidic system. Fig. 2. demonstrates the combination array of two color dyes of 8 different concentration gradients generated in the microfluidic device sequentially. The single concentration gradient can be generated in a standard diffusive mixer where the linear concentration gradient from the minimum to the maximum concentration is represented as different colors (from yellow to blue dye solutions shown in Fig. 2A). In this system, the other concentration gradient by different solution in perpendicular to flow of sensitizer is re presented as the different colors of dye solution (yellow to red representing yellow as minimum concentration). During gradient generation, the microvalve array separates the each neighbor concentration solution with unique concentration at one row and column respectively. Fig. 2C demonstrates operation of microvalve with closing and opening. Quantitation of 64 combinations generated using 0-10 mM each of FITC and Rhodamine B was performed (not shown). A concentration gradient of solution was established in the diffusive mixer which whole solution in the chip was flowed by micropump from the reservoir to the cell culture chamber and the solution was trapped in the cell culture chamber array for 4 hrs by closing the valves before refreshing the concentration gradient. This operation mimics the replenishment of media in the cell culture chamber with fresh media. Most microfluidic systems (Neils et al., 2004; Lee et al., 2006) using a diffusive mixer require continuous perfusion of solutions to maintain concentration gradients. However, continuous perfusion has several li mitations. First, a large amount of drug is consumed for each experiment. Second, cells cultured in the microfluidic channel are continuously exposed to significant shear stress. On the other hand, our system does not require continuous perfusion during the process that cells are cultured and also is always exposed to drug except for the period when generating the concentration gradient (30 seconds). Therefore, this system would be advantageous for culturing cells that are sensitive to shear stress and has benefits in using expensive or less amount of drug for high throughput screening.
Fig. 2.
Demonstration of concentration gradient in microfluidic system using color dye solution (A) Trapping of 8 different concentration gradient of a sensitizer through a diffusive mixer by driven micropump from two reservoirs (sensitizer and medium are represented by red and yellow respectively) (B) The second trapping of 8 different concentration gradient of drug (drug was represented by blue color dye) (C) Concentration gradient of color dye was maintained 4 hours after closing valves without perfusion of reagent (D) Representative cell culture chamber with PC3 cells trapped and grown for 24 hrs.
Culture of PC3 cells in the microfluidic device
In this study, the prostate cancer cell line PC3 was used for combinatorial drug treatment with the developed system. PC3 cells were introduced and captured into the cell culture chamber array and maintained for 24 hrs with media being refreshed every 3.5 hrs in humidity incubator with 5% CO2 at 37ºC to adapt at the microfluidic environment prior to drug treatment. PC3 cells were cultured in the device without any adverse effect from the microfluidic environment for 7 days (not shown). Fig. 2D shows that a representative PC3 cells grown in culture chamber array for 24 hrs.
Drug treatment with TRAIL and curcumin as sensitizer
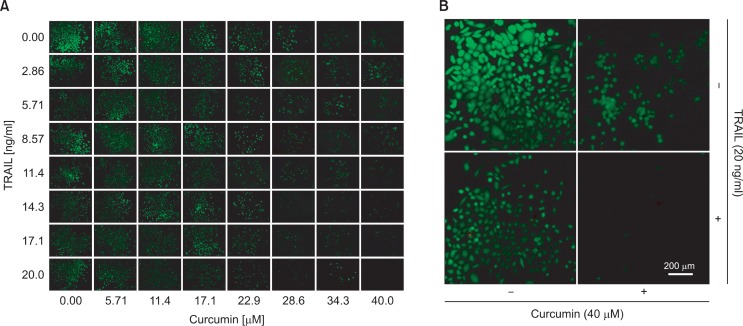
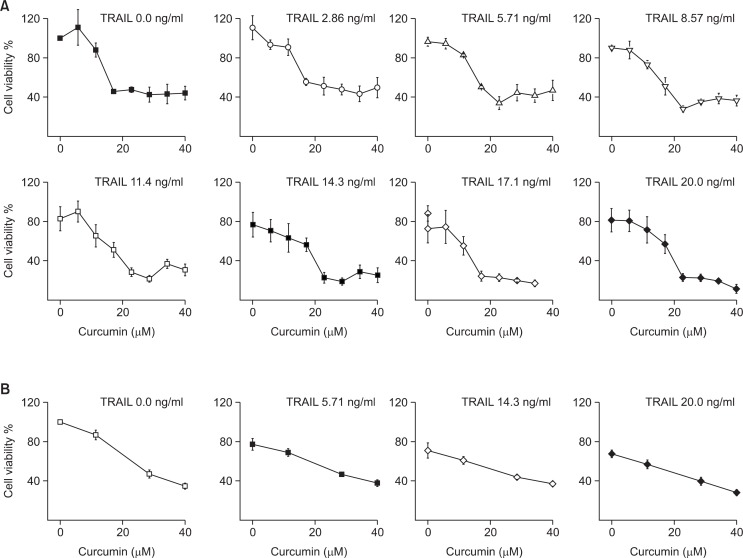
Microfluidic cell array system was investigated to understand e the effect of the combinatorial treatment of TRAIL and curcumin as a sensitizer in the viability of PC3 human prostate cancer cell line. PC3 cells were cultured in microfluidic cell cul ture array and treated sequentially with different concentrations combination of TRAIL and curcumin. First, PC3 cells in cell culture chamber were treated with different concentrations of curcumin. Based on the lethal concentration 50 (LC50) values for PC3 cells in prior reports, the concentration range of curcumin was decided (Adam et al., 2012; Heger et al., 2014). The concentration range was less than that of the LC50 value to investigate the potential sensitizing activity of curcumin as sensitizer to TRAIL. The concentration gradient of curcumin was generated by the diffusive mixer with two streams of 0 and 40 μM curcumin. After 24 hrs exposure to these concentrations of curcumin, a gradient of 0-10 ng/ml TRAIL was generated and treated for 24 hrs. The cell viability was determined with counting the live cells after Live/Dead staining. Fig. 4A shows representative fluorescent micrographs of cell chamber array in a single experiment. The data in the insets (Fig. 4B) show that cells in cell culture chamber without curcumin and TRAIL have proliferated and are confluent (top left as control). Treatment with 40 μM of curcumin (top right) or 10 ng/ ml of TRAIL (bottom left) alone shows less viability, whereas both exposure to 40 μM curcumin and 20 ng/ml TRAIL with sequential treatment shows very few live cells. The fraction of live cells in each cell culture chamber was determined. Fig. 5A shows that single treatment of TRAIL without curcumin as sensitizer leads to a dose-dependent decrease with ∼30% in cell viability compared to highest concentration (20 ng/ml). Curcumin also induces loss cell viability in PC3 as seen from a dose-dependent decrease compared with the absence of TRAIL. The effect of curcumin as sensitizer shows from 23 μM concentration of curcumin exposed on PC3 cell with TRAIL. The cell viability decreases from 50% in the curcumin alone to 20% with an increase in the concentration of TRAIL. This ex tent of decrease at 23 μM concentration of curcumin is significant compared with smaller amount at lower and higher concentrations of curcumin, as could be expected. In the lower concentration, the concentration of curcumin is not enough to fully sensitize PC3 cells to TRAIL-mediated cell death. On the other hand, curcumin by itself decreases cell viability in the higher concentration. The objective of combinational chemotherapy is to find out the optimized combination conditions among the concentrations of sensitizer with the lowest concentration of drug to minimize the side effect by the drug. In this curcumin as sensitizer on PC3 cells, the combination of 23 μM curcumin with 14 ng/ml of TRAIL is sufficient to reduce cell viability by 90%. Our results imply that the optimal concentration of curcumin for sensitizing PC3 cells at lower dose TRAIL treatment is about ∼23 μM and these results obtained with the microfluidic device were compared to those in 96-well plate (Fig. 5B). The combination treatment in the device results in rather smaller increase in PC3 cell viability than those in well plate. The above difference was estimated with comparing the LC50 values obtained for combination treatment in microfluidic device and 96-well plate (Table 1) which means that using microfluidic system for cell based combination therapy is more effective than well plate. There are several possible differences in drug efficacy between two formats. First, both the periodic media replenishment with fresh reagent (sensitizer and anticancer drug) and the removal of waste products every 4 hrs were used for the microfluidic device. It is not possible to do in well plate with replenishment every 4 hrs during a couple of days with manual operation. The consumption of reagent using well plate (especially expensive anticancer drug) is also much bigger than that in microfluidic system. The volume of a cell culture chamber in the microfluidic device is only 48 nl, so total volume inside is under 4 μl. In this single replenishment for 8×8 combination treatment in microfluidic system, the total reagent had been spent only 10 μl including media with highest concentration.
Fig. 4.
Cell culture in the combinatorial array (A) Representative fluorescent micrographs of PC3 cells after sequential treatment with curcumin and TRAIL for 24 hours each. (B) Top left: no curcumin, no TRAIL; top right: curcumin, no TRAIL; bottom left: no curcumin, TRAIL; bottom right: curcumin, TRAIL.
Fig. 5.
PC3 cell viability after sequential exposure to curcumin and TRAIL. PC3 cells were initially exposed to (A) in microfluidic system with eight concentrations (0.0, 5,71, 11.4, 17.1, 22.9, 28.6, 34.3, and 40.0 mM) of curcumin for 24 hrs, followed by exposure to eight concentrations (0.0, 2.86, 5.71, 8.57, 11.4, 14.3, 17.1, and 20.0 ng/ml) of TRAIL for 24 hrs. (B) in 96-well plate. Data is taken after averaging three independent experiments.
Table 1.
Comparison of LC50 values of curcumin for chemosensitizer treated with TRAIL in the microfluidic device and 96-well plate
| TRAIL [ng/ml] | LC50 values of curcumin (μM) | |
|---|---|---|
|
| ||
| Microfluidic device | 96-well plate | |
| 0.00 | 26.3 ± 3.06 | 27.1 ± 3.14 |
| 5.71 | 20.1 ± 1.77* | 25.3 ± 4.39 |
| 14.3 | 14.5 ± 3.72* | 22.2 ± 2.88 |
| 20.0 | 12.1 ± 2.95* | 18.3 ± 2.48 |
p<0.05 by Mann–Whitney U-test. The Shapiro-Wilk test was performed to check normal distribution.
DISCUSSION
In this paper, we have developed a fully automated and programmable high throughput drug screening system based on microfluidic chip to perform combinational chemotherapy with 64 pair-wise concentration combinations between sensitizer and drug (Fig. 2). The consumption of reagent was minimized under 100 μl for entire experiment by including micropump on microfluidic system. Our results have benefits for some usual biological experiments having limitation of reagent which always involving a small amount or expensive purified natural component or anticancer drug. In addition, the cost for this cell array microfluidic device with massive production is not expensive comparing to that of conventional multi-well plate. The microfluidic system has been characterized using a natural curcumin component as a sensitizer for TRAIL-induced cell death against prostate cancer PC3 cells. Previous researches have shown that sensitization of prostate cancer cells by curcumin inhibits the activation of NF-κB in cells to sensitize prostate cancer PC3 cells to TRAIL (Deeb et al., 2004; Deeb et al., 2006; Deeb et al., 2007). We have also demonstrated that the optimal concentration of curcumin for sensitizing prostate cancer PC3 cells at lower dose TRAIL treatment is about ∼23 μM (Fig. 5). Other researches have shown that characterizing the in vitro LC50 concentration of curcumin alone is against diverse cancer cell types, because of pharmacologically safety of curcumin comparing to the toxicity of synthetic reagents (Heger et al., 2014). This system can be useful to verify the optimization of curcumin concentration against the other cancer cells with low dose TRAIL treatment. Our results in microfluidics system are more sensitive than in well plate because of frequent replenishment of fresh media and drug solution (Table 1). These facts have an advantage in deciding the range of drug dosage for animal experiments. The another aspect of benefits is that this method does not require large consumption of labor and time for combinational drug screening among multi-drug because of fully automated system. It could help minimize the individual deviation by experimenter with standardization. This HTDS should be useful for screening experiment from cloud to specific ranges. This system can be extended to identifying combinatorial drug treatments for a variety of in-vitro disease models. Finally, the ability to carry out sequential treatment also facilitates exploration of diverse dosing studies for new drug development.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-116436).
REFERENCES
- Adam V, Ekblad M, Sweeney K, Muller H, Busch KH, Johnsen CT, Kang NR, Lemoine NR, Hallden G. Synergistic and selective cancer cell killing mediated by the oncolytic adenoviral mutant addeltadelta and dietary phytochemicals in prostate cancer models. Hum Gene Ther. 2012;23:1003–1015. doi: 10.1089/hum.2012.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–2725. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua S, Linton RS, Gamboa J, Banerjee I, Yarmush ML, Rege K. Lytic peptide-mediated sensitization of TRAIL-resistant prostate cancer cells to death receptor agonists. Cancer Lett. 2010;293:240–253. doi: 10.1016/j.canlet.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Beltran H, Beer TM, Carducci MA, De Bono J, Gleave M, Hussain M, Kelly WK, Saad F, Sternberg C, Tagawa ST, Tannock IF. New therapies for castration-resistant prostate cancer: efficacy and safety. Eur Urol. 2011;60:279–290. doi: 10.1016/j.eururo.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Bouralexis S, Findlay DM, Evdokiou A. Death to the bad guys: targeting cancer via Apo2L/TRAIL. Apoptosis. 2005;10:35–51. doi: 10.1007/s10495-005-6060-0. [DOI] [PubMed] [Google Scholar]
- Deeb D, Gao X, Jiang H, Divine G, Dulchavsky SA, Gautam SC. Vaccination with leukemia-loaded dendritic cells eradicates residual disease and prevent relapse. J Exp Ther Oncol. 2006;5:183–193. [PubMed] [Google Scholar]
- Deeb D, Jiang H, Gao X, Al-Holou S, Danyluk AL, Dulchavsky SA, Gautam SC. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nu clear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J Pharmacol Exp Ther. 2007;321:616–625. doi: 10.1124/jpet.106.117721. [DOI] [PubMed] [Google Scholar]
- Deeb D, Jiang H, Gao X, Hafner MS, Wong H, Divine G, Chapman RA, Dulchavsky SA, Gautam SC. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther. 2004;3:803–812. [PubMed] [Google Scholar]
- Garstecki P, Fuerstman MJ, Stone HA, Whitesides GM. Formation of droplets and bubbles in a microfluidic T-junction-scaling and mechanism of break-up. Lab. Chip. 2006;6:437–446. doi: 10.1039/b510841a. [DOI] [PubMed] [Google Scholar]
- Graf NJ, Bowser MT. Effect of cross sectional geometry on PDMS micro peristaltic pump performance: comparison of SU-8 replica molding vs. micro injection molding. Analyst. 2013;138:5791–5800. doi: 10.1039/c3an00671a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heger M, Van Golen RF, Broekgaarden M, Michel MC. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol Rev. 2014;66:222–307. doi: 10.1124/pr.110.004044. [DOI] [PubMed] [Google Scholar]
- Hung PJ, Lee PJ, Sabounchi P, Aghdam N, Lin R, Lee LP. A novel high aspect ratio microfluidic design to provide a stable and uniform microenvironment for cell growth in a high throughput mammalian cell culture array. Lab. Chip. 2005a;5:44–48. doi: 10.1039/b410743h. [DOI] [PubMed] [Google Scholar]
- Hung PJ, Lee PJ, Sabounchi P, Lin R, Lee LP. Continuous perfusion microfluidic cell culture array for high-through put cell-based assays. Biotechnol Bioeng. 2005b;89:1–8. doi: 10.1002/bit.20289. [DOI] [PubMed] [Google Scholar]
- Kelley SK, Ashkenazi A. Targeting death receptors in can cer with Apo2L/TRAIL. Curr Opin Pharmacol. 2004;4:333–339. doi: 10.1016/j.coph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Kim J, Hegde M, Jayaraman A. Co-culture of epithelial cells and bacteria for investigating host-pathogen interactions. Lab. Chip. 2010a;10:43–50. doi: 10.1039/b911367c. [DOI] [PubMed] [Google Scholar]
- Kim J, Hegde M, Jayaraman A. Microfluidic co-culture of epithelial cells and bacteria for investigating soluble signal-mediated interactions. J. Vis. Exp. 2010b doi: 10.3791/1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Hegde M, Kim SH, Wood TK, Jayaraman A. A microfluidic device for high throughput bacterial biofilm studies. Lab. Chip. 2012a;12:1157–1163. doi: 10.1039/c2lc20800h. [DOI] [PubMed] [Google Scholar]
- Kim J, Taylor D, Agrawal N, Wang H, Kim H, Han A, Rege K, Jayaraman A. A programmable microfluidic cell array for combinatorial drug screening. Lab. Chip. 2012b;12:1813–1822. doi: 10.1039/c2lc21202a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H, Folch A. Design and dynamic characterization of “single-stroke” peristaltic PDMS micropumps. Lab. Chip. 2011;11:336–342. doi: 10.1039/c0lc00023j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PJ, Hung PJ, Rao VM, Lee LP. Nanoliter scale microbioreactor array for quantitative cell biology. Biotechnol Bioeng. 2006;94:5–14. doi: 10.1002/bit.20745. [DOI] [PubMed] [Google Scholar]
- Li N, Hsu CH, Folch A. Parallel mixing of photolithographically defined nanoliter volumes using elastomeric microvalve arrays. Electrophoresis. 2005;26:3758–3764. doi: 10.1002/elps.200500171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neils C, Tyree Z, Finlayson B, Folch A. Combinatorial mi xing of microfluidic streams. Lab. Chip. 2004;4:342–350. doi: 10.1039/b314962e. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Ryu H, Minamishima YA, Ryo A, Lee SW. Modulation of p53 and p73 levels by cyclin G: implication of a negative feedback regulation. Oncogene. 2003;22:1678–1687. doi: 10.1038/sj.onc.1206306. [DOI] [PubMed] [Google Scholar]
- Shankar S, Chen X, Srivastava RK. Effects of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo. Prostate. 2005;62:165–186. doi: 10.1002/pros.20126. [DOI] [PubMed] [Google Scholar]
- Shankar S, Siddiqui I, Srivastava RK. Molecular mechani sms of resveratrol (3,4,5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cell Biochem. 2007;304:273–285. doi: 10.1007/s11010-007-9510-x. [DOI] [PubMed] [Google Scholar]
- Shankar S, Singh TR, Srivastava RK. Ionizing radiation enhances the therapeutic potential of TRAIL in prostate cancer in vitro and in vivo: Intracellular mechanisms. Prostate. 2004;61:35–49. doi: 10.1002/pros.20069. [DOI] [PubMed] [Google Scholar]
- Sheikh MS, Fornace AJ., JR Role of p53 family members in apoptosis. J Cell Physiol. 2000;182:171–181. doi: 10.1002/(SICI)1097-4652(200002)182:2<171::AID-JCP5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Malik A, Adhami VM, Asim M, Hafeez BB, Sarfaraz S, Mukhtar H. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene. 2008;27:2055–2063. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]
- Simons JW, Mikhak B, Chang JF, Demarzo AM, Carducci MA, Lim M, Weber CE, Baccala AA, Goemann MA, Clift SM, Ando DG, Levitsky HI, Cohen LK, Sanda MG, Mulligan RC, Partin AW, Carter HB, Piantadosi S, Marshall FF, Nelson WG. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 2000;288:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kim M-C, Marquez M, Thorsen T. High-density microfluidic arrays for cell cytotoxicity analysis. Lab. Chip. 2007;7:740–745. doi: 10.1039/b618734j. [DOI] [PubMed] [Google Scholar]
- Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]