Abstract
Fucodiphlorethol G (6’-[2,4-dihydroxy-6-(2,4,6-trihydroxyphenoxy)phenoxy]biphenyl-2,2’,4,4’,6-pentol) is a compound purified from Ecklonia cava, a brown alga that is widely distributed offshore of Jeju Island. This study investigated the protective effects of fucodiphlorethol G against oxidative damage-mediated apoptosis induced by ultraviolet B (UVB) irradiation. Fucodiphlorethol G attenuated the generation of 2, 2-diphenyl-1-picrylhydrazyl radicals and intracellular reactive oxygen species in response to UVB irradiation. Fucodiphlorethol G suppressed the inhibition of human keratinocyte growth by UVB irradiation. Additionally, the wavelength of light absorbed by fucodiphlorethol G was close to the UVB spectrum. Fucodiphlorethol G reduced UVB radiation-induced 8-isoprostane generation and DNA fragmentation in human keratinocytes. Moreover, fucodiphlorethol G reduced UVB radiation-induced loss of mitochondrial membrane potential, generation of apoptotic cells, and active caspase-9 expression. Taken together, fucodiphlorethol G protected human keratinocytes against UVB radiation-induced cell damage and apoptosis by absorbing UVB radiation and scavenging reactive oxygen species.
Keywords: Fucodiphlorethol G, Ultraviolet B, Mitochondria membrane potential, Reactive oxygen species, Human keratinocytes
INTRODUCTION
The ozone layer in the stratosphere has been progressively depleted since the late 1970s, leading to an excess amount of ultraviolet (UV) B irradiation reaching the earth’s surface (Bolaji and Huan, 2013). Exposure to UVB radiation is the key factor in the initiation of several skin disorders, such as wrinkling, scaling, dryness, mottled pigment abnormalities including hypo/hyperpigmentation, and skin cancer (Drouin and Therrien, 1997). UVB radiation may lead to apoptosis via directly and indirectly causing DNA damage (Ravanat et al., 2001), although it may also lead to production of vitamin D, which is essential for the prevention of osteoporosis via calcium metabolism in skin cells (Holick, 2004). UVB radiation can modify molecular structures and damage cellular components, and thereby disrupt cellular functions (Petrova et al., 2011; Wölfle et al., 2011). UVA irradiated from sunlight can contribute to skin cancer via indirect DNA damage induced by reactive oxygen species (ROS) and free radicals. While the damage caused by UVB induced both indirect DNA damage like as ROS generation and direct DNA damage such as formation of pyrimidine dimers, and double-strand DNA breakage (Svobodová et al., 2012). UVB has higher energy per photon than UVA, but most UVB radiation was blocked by ozone layer. However, the rate of UVB reached to earth surface was increased by ozone depletion induced by green-house gases. While the increasing of UVB radiation exposure to human being, the researches of UVB protection have been important. As the risk of UVB radiation causing skin damage, such as inflammation, erythema, and skin carcinomas, has increased, investigations have sought to identify natural extracts and compounds that can block UVB radiation and protect against its damaging effects. Brown alga polyphenols have an anti-photocarcinogenic effect, which may be associated with the prevention of UVB radiation-induced oxidative stress, inflammation, and cell proliferation in the skin (Hwang et al., 2006). For example, alga extract from Phaeodactylum tricornutum stimulates the activity of proteasome peptidase, which is responsible for the degradation of most oxidized proteins, in human keratinocytes exposed to UVB irradiation (Bulteau et al., 2006). A Chlorella extract prevents UVB radiation-induced matrix metalloproteinase 1 expression and UVB radiation-suppressed ela stin protein expression and pro-collagen gene expression in skin fibroblasts (Shih and Cherng, 2008). Our recent studies reported that eckol and triphlorethol-A compounds purified from Ecklonia cava protect against UVB radiation-induced cell damage and apoptosis in human keratinocytes (Piao et al., 2012a, 2012b).
Fucodiphlorethol G (6’-[2,4-dihydroxy-6-(2,4,6-trihydroxyphenoxy)phenoxy]biphenyl-2,2’,4,4’,6-pentol), a phlorotannin component of Ecklonia cava, scavenged 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical (Ham et al., 2007). Although the effects of radical scavenging by fucodiphlorethol G have been studied, the cytoprotective effect of this compound against UVB exposure has not been investigated. The current study investigated the protective properties of fucodiphlorethol G against UVB radiation-induced damage and apoptosis in human keratinocytes.
MATERIALS AND METHODS
Reagents
Fucodiphlorethol G was provided by Professor Nam Ho Lee (Jeju National University, Jeju, Korea) (Fig. 1). DPPH, 2, 7-dichlorodihydrofluorescein diacetate (DCF-DA), [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium] bromide (MTT), Hoechst 33342, and an anti-actin antibody were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). A caspase-9 antibody was purchased from Cell Signaling Technology (Beverly, MA, USA).
Fig. 1.
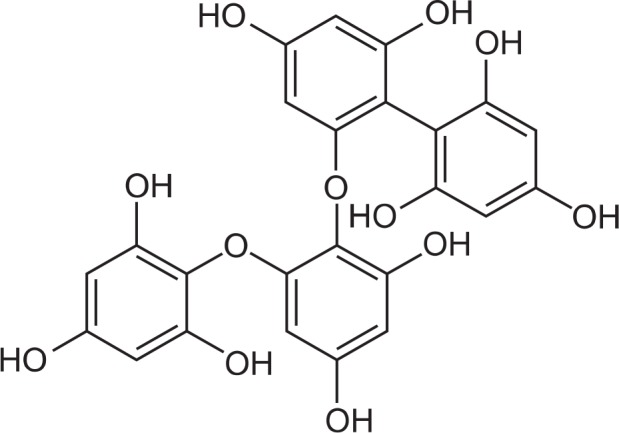
Chemical structure of fucodiphlorethol G (6’-[2,4-dihydroxy-6-(2,4,6-trihydroxyphenoxy)phenoxy]biphenyl-2,2’, 4,4’,6-pentol).
Cell culture and UVB irradiation
Human keratinocytes (HaCaT cells), obtained from Amore Pacific Company (Yongin, Korea), were maintained at 37°C in an incubator in a humidified atmosphere of 5% CO2. The cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated fetal calf serum, streptomycin (100 μg/ml), and penicillin (100 U/ml). The UVB source was a CL-1000M UV Crosslinker (UVP, Upland, CA, USA), which was used to deliver an energy spectrum of UVB radiation (280–320 nm; peak intensity, 302 nm).
DPPH radical detection
Fucodiphlorethol G (1, 10, 20, 40, 60, 80, or 100 μM) and 1 mM N-acetylcysteine (NAC) was added to 0.1 mM DPPH and mixed well. The mixture was incubated for 30 min, after which the amount of residual DPPH was determined by measuring absorbance at 520 nm using a spectrophotometer.
Intracellular reactive oxygen species (ROS) detection
The DCF-DA method was used to detect intracellular ROS levels in HaCaT keratinocytes (Rosenkranz et al., 1992). H2O2-treated or UVB-irradiated cells were seeded at a density of 0.5×105 cells/well in 24-well culture plates. Sixteen hours after plating, cells were treated with fucodiphlorethol G at a concentration of 1, 10, 20, 40, 60, 80, or 100 μM or with 1 mM NAC. To analyze 2, 7-dichlorofluorescein (DCF) generated by intracellular ROS, cells were treated with 1 mM H2O2 or irradiated with 30 mJ/cm2 UVB. After incubating for 30 min or 12 h, 25 μM DCF-DA was added and cells were incubated for a further 10 min. Fluorescence of DCF was detected using a PerkinElmer LS-5B spectrofluorometer (PerkinElmer, Waltham, MA, USA).
Cell viability
Cells were seeded in a 24-well plate at a density of 0.5×105 cells/well. Sixteen hours after plating, cells were treated with fucodiphlorethol G at a concentration of 1, 10, 20, 40, 60, 80, or 100 μM or with 1 mM NAC. Cells were exposed to 30 mJ/cm2 UVB radiation 1 h later and incubated at 37°C for 48 h. Thereafter, 150 μl of MTT stock solution (2 mg/ml) was added to each well to yield a total reaction volume of 500 μl. After incubating cells for 2 h, the plate was centrifuged at 800×g for 5 min, and the supernatants were aspirated. The formazan crystals in each well were dissolved in dimethylsulfoxide (DMSO), and the absorbance at 540 nm was measuring using a scanning multi-well spectrophotometer (Carmichael et al., 1987).
UV/visible light absorption analysis
An absorption analysis of fucodiphlorethol G (20 μM) was performed by scanning with UV/visible light of 200–400 nm using an HP-8453E UV-visible spectroscopy system (Hewlett Packard, Palo Alto, CA, USA) and a standard quartz cuvette with a 1 cm path length. Fucodiphlorethol G was diluted in DMSO at a ratio of 1:500 before scanning.
Analysis of the level of 8-isoprostane
Lipid peroxidation was assayed by determining the level of 8-isoprostane in culture medium (Belli et al., 2005). Cells were treated with 20 μM fucodiphlorethol G, exposed to UVB radiation 1 h later, and incubated for an additional 48 h at 37°C. An enzyme immunoassay (Cayman Chemical, Ann Arbor, MI, USA) was used to measure the level of 8-isoprostane according to the manufacturer’s protocol.
DNA fragmentation analysis
Cells were seeded in a 24-well plate at a density of 0.5×105 cells/well. At sixteen hours after plating, cells were treated with 20 μM fucodiphlorethol G, exposed to UVB radiation 1 h later, and incubated for an additional 48 h at 37°C. Cellular DNA fragmentation was assessed by analyzing cytoplasmic histone-associated DNA fragmentation. A kit from Roche Diagnostics (Portland, OR, USA) was used according to the manufacturer’s protocol.
Mitochondrial membrane potential (Δψm) analysis
Cells were treated with fucodiphlorethol G (20 μM), exposed to UVB radiation (30 mJ/cm2) 1 h later, and then incubated for another 12 h at 37°C. Cells were stained with JC-1 (15 μM). For image analysis, JC-1-stained cells were mounted in mounting medium (DAKO, Carpinteria, CA, USA). Microscopic images were collected using the Laser Scanning Microscope 5 PASCAL program (Carl Zeiss, Jena, Germany) on a confocal microscope. In addition, JC-1-stained cells were analyzed by flow cytometry (Troiano et al., 2007).
Western blot analysis
Cell lysates were collected and protein concentrations were determined using the Bradford reagent. Aliquots of the lysates (20 μg of protein) were boiled for 5 min and electrophoresed on 10% SDS-polyacrylamide gels. Gels were transferred to nitrocellulose membranes (Bio-Rad, CA, USA). Membranes were incubated with a caspase-9 antibody and further incubated with secondary immunoglobulin G-horseradish peroxidase conjugates. Protein bands were visualized by developing the blots using an Enhanced Chemiluminescence Western Blotting Detection Kit (Amersham, Buckinghamshire, UK) and exposing the membranes to X-ray film.
Nuclear staining with Hoechst 33342
Cells were treated with 20 μM fucodiphlorethol G and exposed to 30 mJ/cm2 UVB radiation 1 h later. After incubation for a further 48 h at 37°C, 1 μl of the DNA-specific fluorescent dye Hoechst 33342 (stock, 15 mM) was added to each well of the 6-well plate. The plate was then incubated for 10 min at 37°C. The degree of nuclear condensation in the stained cells was determined by visualization with a fluorescence microscope equipped with a CoolSNAP-Pro color digital camera.
Statistical analysis
All measurements were made in three independent experiments, and all values represent the mean ± standard error. The results were subjected to an analysis of variance using Tukey’s test for analysis of differences. Statistical significance was set at p<0.05.
RESULTS
Effects of fucodiphlorethol G on free radical scavenging and on the viability in UVB-irradiated cells
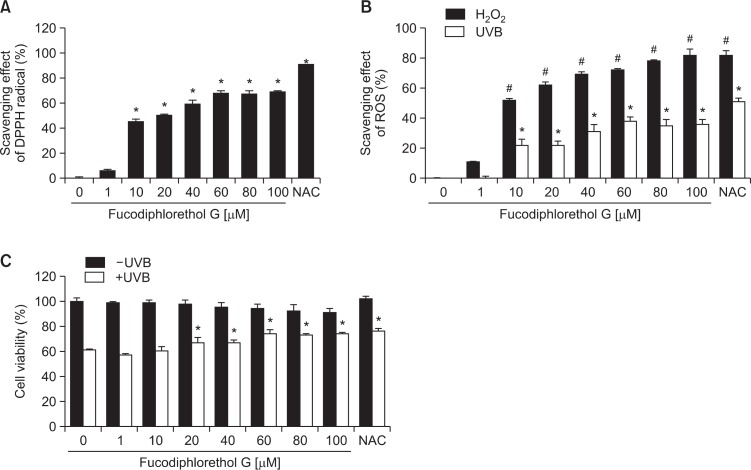
Fucodiphlorethol G significantly scavenged DPPH radical in a concentration-dependent manner (Fig. 2A). The intracellular ROS scavenging effect of fucodiphlorethol G in H2O2 (1 mM)-treated cells or UVB (30 mJ/cm2)-irradiated cells increased in a concentration-dependent manner (Fig. 2B). Moreover, th ese antioxidant effects of fucodiphlorethol G increased cell viability reduced by UVB in a concentration-dependent manner; the survival rates of UVB-irradiated cells pre-treated with 20, 40, 60, 80, and 100 μM fucodiphlorethol G were 67%, 67%, 74%, 73%, and 74%, respectively in comparison to that of cells only exposed to UVB radiation (61%) (Fig. 2C), suggesting that ROS scavenging effect of fucodiphlorethol G are related to protection of cells against oxidative stress induced by UVB radiation. Moreover, treatment with 20 μM fucodiphlorethol G was non-toxic to cells. Thus, we chose 20 μM as the optimal concentration of fucodiphlorethol G for subsequent experiments. These data suggest that fucodiphlorethol G not only scavenges free radicals and intracellular ROS generated by UVB irradiation, but also protects cells from UVB radiation-induced cell death.
Fig. 2.
Fucodiphlorethol G scavenges DPPH radical and intracellular ROS and protects against cell death induced by UVB. (A) DPPH radical was incubated with various concentrations of fucodiphlorethol G. The amount of DPPH radical was determined by measuring absorbance at 520 nm using a spectrophotometer. *Significantly different from control (p<0.05). (B) Cells were treated with various concentrations of fucodiphlorethol G for 1 h and then treated with 1 mM H2O2 or 30 mJ/cm2 UVB radiation. Cells were incubated for a further 24 h and intracellular ROS were detected by fluorescence spectrophotometry. #,*Significantly different from each control in H2O2 or UVB-treated cells, respectively (p<0.05). (C) Cells were treated with various concentrations of fucodiphlorethol G for 1 h and then treated with 30 mJ/cm2 UVB radiation. Cell viability was determined using the MTT assay. *Significantly different from UVB-irradiated cells (p<0.05).
Effects of fucodiphlorethol G on absorption of light within the UVB spectrum
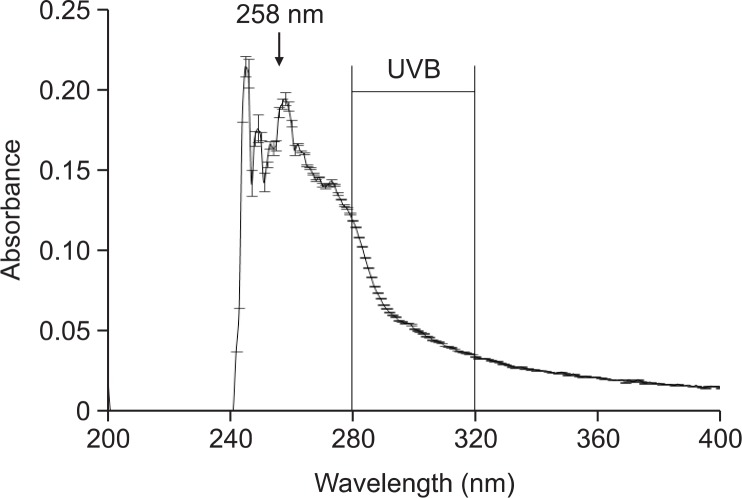
UVB radiation has a wavelength of 280–320 nm. The effect of fucodiphlorethol G on absorption of UVB was determined using a UV/visible spectrophotometer. Fucodiphlorethol G showed a high absorptive capacity at a peak position of 258 nm, which is close to the range of UVB radiation (Fig. 3). Thus, the light-absorbing property of fucodiphlorethol G might be closely associated with its cytoprotective effect against UVB radiation. This data show that fucodiphlorethol G absorbed UVB radiation and may reduce the level of UVB radiation that reaches cells.
Fig. 3.
Fucodiphlorethol G absorbs UVB light. UV/visible spectroscopic measurements were conducted in the spectral range of 200–400 nm to measure absorbance by fucodiphlorethol G.
Protective effects of fucodiphlorethol G against damage to cellular components caused by UVB radiation
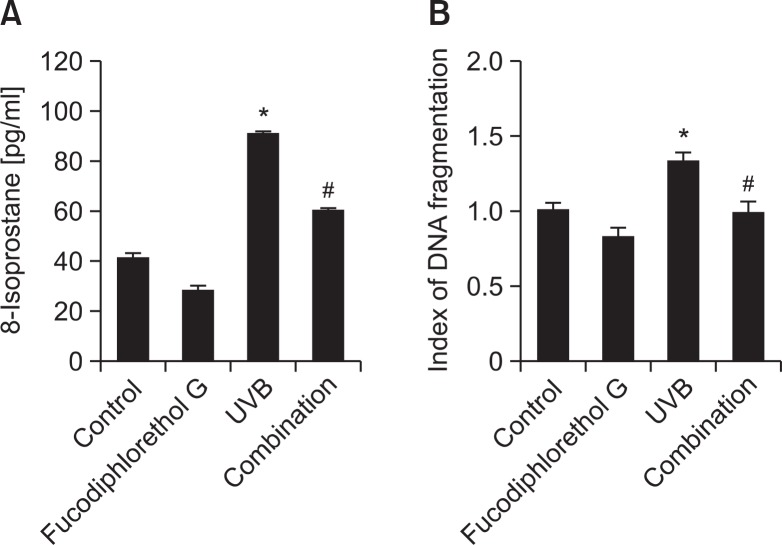
To investigate the protective effects of fucodiphlorethol G against membrane lipid peroxidation and cellular DNA damage induced by UVB irradiation, cells were treated with fucodiphlorethol G, exposed to UVB 1 h later, and then incubated for a further 48 h. 8-Isoprostane is a by-product of ROS-induced lipid peroxidation and is used as a specific index of cellular ipoperoxidation (Belli et al., 2005). The concentration of 8-isoprostane in UVB-treated cells was significantly increased to 91 pg/ml, whereas this was reduced to 60 pg/ml in UVB-irradiated cells pretreated with fucodiphlorethol G (Fig. 4A). Mo reover, while the level of DNA fragmentation was higher in UVB-irradiated cells than in control cells, it was significantly reduced in UVB-irradiated cells pretreated with fucodiphlorethol G (Fig. 4B). These data indicate that fucodiphlorethol G attenuated lipid peroxidation and DNA fragmentation induced by UVB irradiation in HaCaT cells.
Fig. 4.
Fucodiphlorethol G protects HaCaT keratinocytes against UVB radiation-induced lipid peroxidation and DNA fragmentation. (A) The level of 8-isoprostane, an indication of lipid peroxidation, was measured in culture medium. *Significantly different from control cells (p<0.05); #significantly different from UVB-irradiated cells (p<0.05) (B) DNA fragmentation was quantified with an enzyme-linked immunosorbent assay kit. *Significantly different from control cells (p<0.05); #significantly different from UVB-irradiated cells (p<0.05).
Effects of fucodiphlorethol G on UVB radiation-induced disruption of Δψm and apoptosis
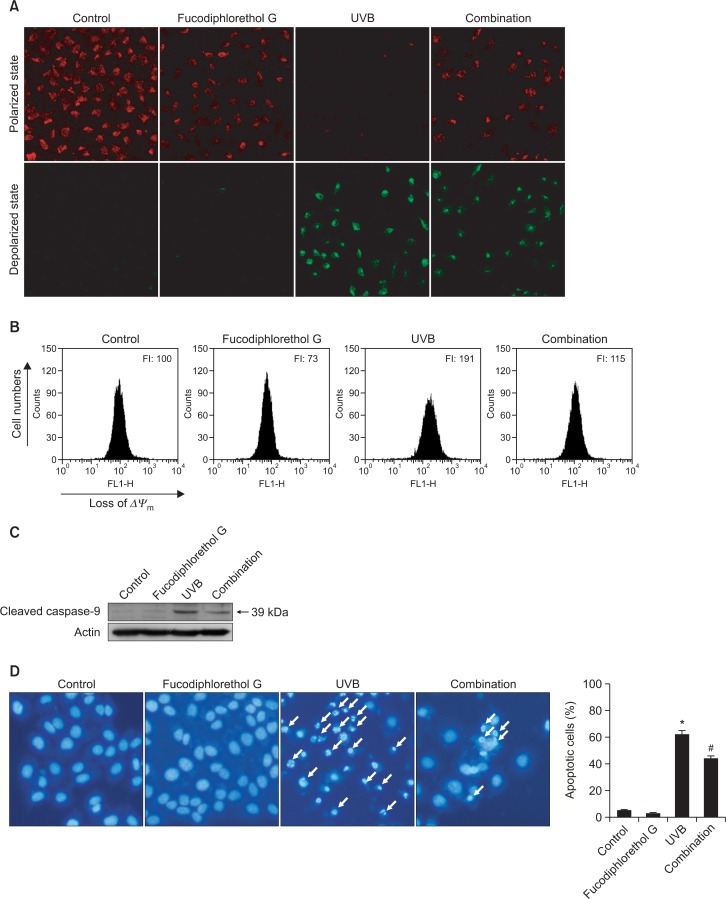
Mitochondria are the site at which oxidative phosphorylation occurs and are intimately involved in regulation of apoptosis, ROS production during respiration, and regulation of cellular metabolism. To understand the mechanism by which fucodiphlorethol G protects against UVB radiation-induced apoptosis, Δψm was examined. Strong red fluorescence, indicative of JC-1 aggregates and mitochondrial polarization, was exhibited in control cells and fucodiphlorethol G-treated cells (Fig. 5A). However, in cells exposed to UVB radiation, the level of red fluorescence was reduced and green fluorescence was observed, indicative of the JC-1 monomer and mitochondrial depolarization. Pretreatment with fucodiphlorethol G prevented both the reduction in red fluorescence and the induction of green fluorescence in UVB-irradiated cells. To confirm this data, it was investigated the levels of green fluorescence of JC-1, indicative of a loss of Δψm, using flow cytometry (Fig. 5B). UVB-irradiated cells showed a loss of Δψm, as evidenced by an increase in green fluorescence, whereas pre-treatment with fucodiphlorethol G suppressed the loss of Δψm in UVB-irradiated cells. Disruption of Δψm causes early apoptosis via production of the apoptosome. The level of active caspase-9, one of the components of the apoptosome, was measured by western blotting. Treatment with fucodiphlorethol G markedly attenuated the level of active caspase-9 in comparison to that in cells only exposed to UVB radiation (Fig. 5C). To further study the protective effect of fucodiphlorethol G against UVB radiation-induced apoptosis in HaCaT cells, nuclei were stained with Hoechst 33342 and visualized by fluorescence microscopy. Control cells showed intact nuclei, whereas large amounts of nuclear shrinkage and fragmentation were observed in UVB-irradiated cells. Although UVB irradiation caused apoptotic nuclei in HaCaT cells, pre-treatment with fucodiphlorethol G significantly reduced the amounts of nuclear shrinkage and fragmentation (Fig. 5D). Taken together, these data indicate that treatment with fucodiphlorethol G protected HaCaT cells against UVB radiation-induced apoptotic cell death.
Fig. 5.
Fucodiphlorethol G suppresses UVB radiation-induced disruption of Δψm, activation of caspase-9, and apoptosis in HaCaT keratinocytes. Cells were pre-treated with 20 μM fucodiphlorethol G and then treated with 30 mJ/cm2 UVB radiation 1 h later. Cells were stained with JC-1, and Δψm was analyzed by (A) confocal microscopy and (B) flow cytometry. (C) Cleaved caspase-9 was detected using a specific antibody. (D) Apoptotic body formation was observed under a fluorescence microscope following Hoechst 33342 staining. Apoptotic bodies are indicated by arrows. *Significantly different from control cells (p<0.05); #Significantly different from UVB-irradiated cells (p<0.05).
DISCUSSION
Abnormal weather conditions have become a serious problem, along with global warming caused by the accumulation of greenhouse gases (McKenzie et al., 2011). These conditions exacerbate degradation of the ozone layer, leading to exposure of the earth’s surface to sunlight containing a large amount of UVB radiation (Bais et al., 1993; McKenzie et al., 2011). Although UVB radiation has beneficial effects, such as vitamin D synthesis, on organisms, exposure to excessive amounts of UVB radiation can cause cellular damage and cell death (Drouin and Therrien, 1997; Ali et al., 2011; Wacker and Holick, 2013). In particular, upon exposure to excessive amounts of UVB radiation, skin cells can undergo death and skin cancer often develops (Drouin and Therrien, 1997; Ali et al., 2011). These effects of UVB radiation are due to the induction of DNA damage and oxidative stress via ROS generation in skin cells (Ravanat et al., 2001; Fagot et al., 2002; Ichihashi et al., 2003). In the current study, we focused on UVB radiation-induced ROS and the protective effects of fucodiphlorethol G against oxidative stress. DPPH radical and intracellular ROS induced by treatment with hydrogen peroxide or UVB irradiation were scavenged by fucodiphlorethol G in a concentration-dependent manner (Fig. 2). Additionally, fucodiphlorethol G restored the UVB radiation-induced reduction in cell viability. In addition to its ROS-scavenging effects, fucodiphlorethol G also directly absorbed UVB radiation (Fig. 3). Oxidative stress induced by UVB radiation modifies various cellular components such as lipid membranes, proteins, DNA, and mitochondria. These modifications lead to perturbation of the functions of these structures, after which repair or apoptotic mechanisms are initiated (Kulms and Schwarz, 2002; Sander et al., 2002; Caricchio et al., 2003). UVB-irradiated cells exhibited increased lipid peroxidation and DNA fragmentation (Fig. 4). Meanwhile, fucodiphlorethol G pretreatment suppressed UVB radiation-induced lipid peroxidation and DNA fragmentation. Furthermore, Δψm was disrupted upon exposure to UVB irradiation and restored by pre-treatment with fucodiphlorethol G. A decreased Δψm should induce release of cytochrome c from mitochondria, which is a critical component of the intrinsic apoptotic pathway (Arends et al., 1990; Tsujimoto, 1998). The release of cytochrome c from mitochondria into the cytosol triggers formation of the apoptosome, which is composed of apoptotic protease activating factor 1, cytochrome c, and active caspase-9. Fucodiphlorethol G suppressed UVB radiation-induced active caspase-9 and apoptotic cell death (Fig. 5).
In summary, UVB radiation induces oxidative stress in human keratinocytes and eventually damages cells and causes apoptosis. However, fucodiphlorethol G blocks UVB radiation-induced oxidative stress and cell damage via reducing the level of ROS, directly absorbing UVB radiation, and blocking mitochondrial dysfunction, resulting in the cytoprotection against UVB radiation-induced apoptosis.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-C1ABA001-2011-0021037).
REFERENCES
- Ali D, Verma A, Mujtaba F, Dwivedi A, Hans RK, Ray RS. UVB-induced apoptosis and DNA damaging potential of chrysene via reactive oxygen species in human keratinocytes. Toxicol Lett. 2011;204:199–207. doi: 10.1016/j.toxlet.2011.04.033. [DOI] [PubMed] [Google Scholar]
- Arends MJ, Morris RG, Wyllie AH. Apoptosis. The role of the endonuclease. Am J Pathol. 1990;136:593–608. [PMC free article] [PubMed] [Google Scholar]
- Bais AF, Zerefos CS, Meleti C, Ziomas IC, Tourpali K. Spectral measurements of solar UVB radiation and its relations to total ozone, SO2, and clouds. J Geophys Res. 1993;98:5199–5204. [Google Scholar]
- Belli R, Amerio P, Brunetti L, Orlando G, Toto P, Proietto G, Vacca M, Tulli A. Elevated 8-isoprostane levels in basal cell carcinoma and in UVA irradiated skin. Int J Immunopathol Pharmacol. 2005;18:497–502. doi: 10.1177/039463200501800309. [DOI] [PubMed] [Google Scholar]
- Bolaji B, Huan Z. Ozone depletion and global warming: Case for the use of natural refrigerant-a review. Renew Sustain Energy Rev. 2013;18:49–54. [Google Scholar]
- Bulteau AL, Moreau M, Saunois A, Nizard C, Friguet B. Algae extract-mediated stimulation and protection of proteasome activity within human keratinocytes exposed to UVA and UVB irradiation. Antioxid Redox Signal. 2006;8:136–143. doi: 10.1089/ars.2006.8.136. [DOI] [PubMed] [Google Scholar]
- Caricchio R, McPhie L, Cohen PL. Ultraviolet B radiation-induced cell death: critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. J Immunol. 2003;171:5778–5786. doi: 10.4049/jimmunol.171.11.5778. [DOI] [PubMed] [Google Scholar]
- Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- Drouin R, Therrien JP. UVB-induced cyclobutane pyrimidine dimer frequency correlates with skin cancer mutational hot-spots in p53. Photochem Photobiol. 1997;66:719–726. doi: 10.1111/j.1751-1097.1997.tb03213.x. [DOI] [PubMed] [Google Scholar]
- Fagot D, Asselineau D, Bernerd F. Direct role of human dermal fibroblasts and indirect participation of epidermal keratinocytes in MMP-1 production after UV-B irradiation. Arch Dermatol Res. 2002;293:576–583. doi: 10.1007/s00403-001-0271-1. [DOI] [PubMed] [Google Scholar]
- Ham YM, Baik JS, Hyun JW, Lee NH. Isolation of a new phlorotannin, fucodiphlorethol G, from a brown alga Ecklonia cava. Bull Korean Chem Soc. 2007;28:1595–1597. [Google Scholar]
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- Hwang H, Chen T, Nines RG, Shin H, Stoner GD. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int. J. Cancer. 2006;119:2742–2749. doi: 10.1002/ijc.22147. [DOI] [PubMed] [Google Scholar]
- Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. UV-induced skin damage. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- Kulms D, Schwarz T. Molecular mechanisms involved in UV-induced apoptotic cell death. Skin Pharmacol Appl Skin Physiol. 2002;15:342–347. doi: 10.1159/000064539. [DOI] [PubMed] [Google Scholar]
- McKenzie RL, Aucamp PJ, Bais AF, Bjorn LO, Ilyas M, Madronich S. Ozone depletion and climate change: impacts on UV radiation. Photochem Photobiol Sci. 2011;10:182–198. doi: 10.1039/c0pp90034f. [DOI] [PubMed] [Google Scholar]
- Petrova A, Davids LM, Rautenbach F, Marnewick JL. Photoprotection by honeybush extracts, hesperidin and mangiferin against UVB-induced skin damage in SKH-1 mice. J. Photochem. Photobiol. B. 2011;103:126–139. doi: 10.1016/j.jphotobiol.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Piao MJ, Lee NH, Chae S, Hyun JW. Eckol inhibits ultraviolet B-induced cell damage in human keratinocytes via a decrease in oxidative stress. Biol Pharm Bull. 2012a;35:873–880. doi: 10.1248/bpb.35.873. [DOI] [PubMed] [Google Scholar]
- Piao MJ, Zhang R, Lee NH, Hyun JW. Protective effect of triphlorethol-A against ultraviolet B-mediated damage of human keratinocytes. J Photochem Photobiol B. 2012b;106:74–80. doi: 10.1016/j.jphotobiol.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Ravanat JL, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components. J Photochem Photobiol B. 2001;63:88–102. doi: 10.1016/s1011-1344(01)00206-8. [DOI] [PubMed] [Google Scholar]
- Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ. A microplate assay for the de tection of oxidative products using 2’,7’-dichlorofluorescin-diacetate. J. Immunol. Methods. 1992;156:39–45. doi: 10.1016/0022-1759(92)90008-h. [DOI] [PubMed] [Google Scholar]
- Sander CS, Chang H, Salzmann S, Muller CS, Ekanayake-Mudiyanselage S, Elsner P, Thiele JJ. Photoaging is associated with protein oxidation in human skin in vivo. J Invest Dermatol. 2002;118:618–625. doi: 10.1046/j.1523-1747.2002.01708.x. [DOI] [PubMed] [Google Scholar]
- Shih MF, Cherng JY. Potential protective effect of fresh grown unicellular green algae component (resilient factor) against PMA- and UVB-induced MMP1 expression in skin fibroblasts. Eur J Dermatol. 2008;18:303–307. doi: 10.1684/ejd.2008.0393. [DOI] [PubMed] [Google Scholar]
- Svobodová AR, Galandáková A, Sianská J, Doležal D, Lichnovská R, Ulrichová J, Vostálová J. DNA damage after acute exposure of mice skin to physiological doses of UVB and UVA light. Arch Dermatol Res. 2012;304:407–412. doi: 10.1007/s00403-012-1212-x. [DOI] [PubMed] [Google Scholar]
- Troiano L, Ferraresi R, Lugli E, Nemes E, Roat E, Nasi M, Pinti M, Cossarizza A. Multiparametric analysis of cells with different mitochondrial membrane potential during apoptosis by polychromatic flow cytometry. Nat Protoc. 2007;2:2719–2727. doi: 10.1038/nprot.2007.405. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Wacker M, Holick MF. Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111–148. doi: 10.3390/nu5010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfle U, Esser PR, Simon-Haarhaus B, Martin SF, Lademann J, Schempp CM. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic Biol Med. 2011;50:1081–1093. doi: 10.1016/j.freeradbiomed.2011.01.027. [DOI] [PubMed] [Google Scholar]