Abstract
Purpose
Neurotoxicity from adjuvant treatment with oxaliplatin has been studied in colorectal patients in short-term studies, but this is the first long-term assessment from the National Surgical Adjuvant Breast and Bowel Project (NSABP) investigating whether excess neurotoxicity persists beyond 4 years.
Patients and Methods
As part of a colorectal cancer long-term survivor study (LTS-01), long-term neurotoxicity was assessed in 353 C-07 patients (cross-sectional sample). Ninety-two of these LTS-01 patients also had longitudinal data and were re-assessed at 5-8 (median 7) years from randomization (longitudinal sample). Contingency tables compared cohorts, a mixed model compared neurotoxicity between treatments over time, and a Wilcoxon rank sum test compared neurotoxicity between treatments (cross-sectional sample).
Results
In the cross-sectional sample, the increase in mean total neurotoxicity scores of 1.8 with oxaliplatin was statistically significant (P= .005), but not clinically significant (minimally important difference was 4 at the long-term assessment. Patients treated with oxaliplatin had increased odds of numbness and tingling in hands (OR= 2.00, P= .015) and feet (OR= 2.78, P< .001) versus patients treated without oxaliplatin. The magnitude of the oxaliplatin effect varied with time (P< .001) in the longitudinal sample such that oxaliplatin-treated patients did not have significantly greater total neurotoxicity scores by 7 years.
Conclusion
At the long-term endpoint, there was no clinically significant increase in total neurotoxicity scores for patients treated with oxaliplatin, but the specific neurotoxicities of numbness and tingling of the hands and feet remained significantly elevated for oxaliplatin-treated patients.
Keywords: colon cancer, oxaliplatin, neurotoxicity, stage II, stage III, randomized clinical trial
INTRODUCTION
Even though colorectal cancer (CRC) rates have decreased in the past two decades due to increased screening, CRC remains the third leading cause of cancer deaths among men and women.1 Surgery is the mainstay treatment for stage II and III colon cancer while the addition of adjuvant chemotherapy has been shown to improve disease-free and overall survival.2-12 Due to the high incidence of CRC and improvements in its treatment, 10% of the estimated 11.7 million people living with cancer in the United States in 2007, were survivors of CRC.13 Almost half (4.7 million) of all cancer survivors were diagnosed at least 10 years earlier, requiring assessments of the long-term effects of cancer treatment. To address this need, the National Surgical Adjuvant Breast and Bowel Project (NSABP) designed a long-term survivorship study (LTS-01) in 2006 of CRC survivors from five completed NSABP trials (Protocols C-05, C-06, C-07, R-02, and R-03) to assess quality of life and late toxicity from adjuvant treatment more than 4 years after random assignment to the parent trial.
One of the specific aims of this long-term follow-up study was to compare neurotoxicity between treatment groups from the C-07 trial. C-07 was a randomized, multicenter, phase III trial with the primary aim to compare the efficacy of fluorouracil plus leucovorin (FULV) with the same regimen plus oxaliplatin (FLOX) in prolonging disease-free survival in patients with stage II or III colon cancer. Initial results with 4 years median follow-up reported an increase in disease-free survival in favor of FLOX (hazard ratio [HR] 0.80; P< .004).11 Updated results with 8 years median follow-up further support the benefit of oxaliplatin reporting an increase in disease-free survival in favor of FLOX (HR = 0.82; P= .002); overall survival showed a non-significant trend towards improved outcome with FLOX (HR= 0.88; P = .08).14
Although oxaliplatin has been linked with improved disease-free survival, it has also been associated with important acute and chronic toxicity and thus, the need for long-term neurotoxicity assessment. Oxaliplatin has been found safe with the exception of dose-limiting neurotoxicity characterized by peripheral sensory neuropathy (PSN), with dysesthesia and/or distal paresthesia often triggered or exacerbated by cold. Due to anticipated PSN, the NSABP monitored self-reported neurotoxicity outcomes in a sample of C-07 patients at a long-term endpoint in the LTS-01 study. A subset of these LTS-01 patients also had longitudinal neurotoxicity data. This report provides the first long-term comparison of the severity and duration of neurotoxicity in patients treated with and without oxaliplatin.
METHODS
Selection of Participants
This paper compares long-term neurotoxicity in two relevant groups of patients. The first group of patients is the cross-sectional sample which consists of 353 patients from the C-07 trial who participated in the LTS-01 study. Within this group, 92 patients had longitudinal neurotoxicity information, composing the second group of interest, the longitudinal sample. See Figure 1 for the patient flow diagram.
Figure 1.
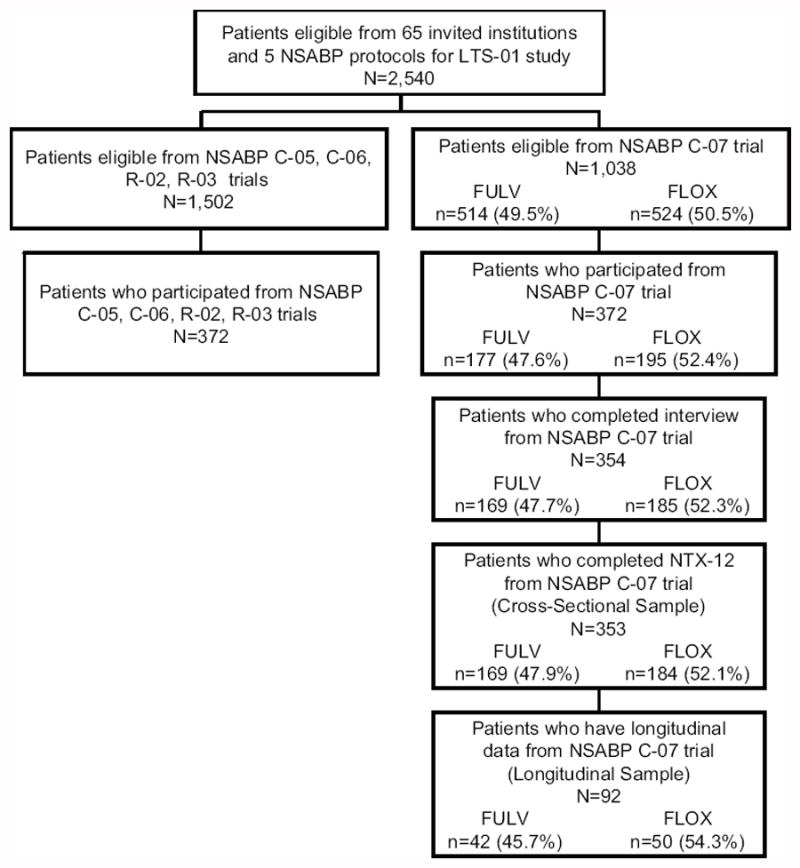
Patient Flow from the National Surgical Adjuvant Breast and Bowel Project C-07 trial to the Patient-Reported Outcomes (PRO) substudy and Long-Term Survivors study (LTS-01) to the overlapping subset available for long-term neurotoxicity analysis. FULV, fluorouracil and leucovorin; FLOX, FULV plus oxaliplatin.
Cross-Sectional Sample
The cross-sectional sample is comprised of 353 patients who had participated in NSABP C-07 trial and were re-assessed in the LTS-01 study at a long term end-point (ranging from 4.2 to 8.6, median 6.0, years from random assignment). The LTS-01 study invited long-term survivors that had previously participated in NSABP C-05, C-06, C-07, R-02, and R-03 CRC trials from sixty-five institutions to enroll. Eligibility required that patients had survived at least 3 years after study entry and had been in contact with institutional staff within the prior two years. (See Ganz et al.15 for the institutional and patient recruitment process).
This manuscript is focused on LTS-01 patients who had participated in the C-07 trial. The C-07 trial accrued stage II or stage III colon cancer patients from 2000 to 2002 who had undergone resection with curative intent. Patients were randomly assigned to receive FULV (1,245) or FLOX (1,247). Patients with clinically significant PSN (National Cancer Institute Common Toxicity Criteria version 2.016 grade 2 or higher) were excluded from the C-07 study. Informed consent was obtained from all participants for both the C-07 and LTS-01 studies. Protocols and consent forms were approved by the National Institutes of Health and the institutional review boards of all participating institutions.
Of 65 invited institutions in the LTS-01 study, 60 institutions agreed to offer LTS-01 to their patients. Of 2,408 eligible patients at participating institutions, 744 agreed to participate, of which, 708 completed the interview. The remaining patients either were not successfully contacted by institutional staff or did not agree to participate. C-07 supplied 1,038 (40.9%) of the 2,540 LTS-01 eligible patients and 372 (50.0%) of the 744 LTS-01 patients who agreed to participate. Similar proportions of patients who were eligible to participate in LTS-01 by C-07 treatment arm actually participated. Interviews were completed by 354 of these patients and 353 patients provided neurotoxicity information at the long-term endpoint (48% FULV and 52% FLOX).
Longitudinal Sample
The longitudinal sample consists of 92 C-07 patients who had participated in LTS-01 and had longitudinal neurotoxicity information from the patient-reported outcomes (PRO) study. The PRO study was a substudy of NSABP C-07 enrolling the first 400 patients from participating Clinical Community Oncology Programs. Of the 400 patients, 395 were eligible for neurotoxicity analysis over 18 months (Land et al.17 described this subset) and 92 participated in the LTS-01 study. These 92 patients (46% FULV, 54% FLOX) reported neurotoxicity results at least at baseline and at the long-term endpoint, which for this group ranged from 5.5 to 8.1 (median 7.0) years from randomization.
Treatment
The FULV regimen included weekly 500mg/m2 intravenous (IV) bolus of FU for 6 weeks plus 500 mg/m2 IV of LV weekly for 6 weeks of each 8-week cycle for 3 cycles. The FLOX regimen added to FULV 85mg/m2 IV of oxaliplatin administered on weeks 1, 3, and 5 of each 8-week cycle for 3 cycles.11 Oxaliplatin dose modifications were required by the protocol based on the NCI-Sanofi grade. Dose modification was required for grade 2 toxicities that persisted between cycles or any grade 3 toxicities. Grade 4 toxicities or persistent grade 3 toxicities required termination of oxaliplatin.
Assessment of Neurotoxicity
To evaluate patient-reported neurotoxicity, patients answered a 12-item questionnaire (NTX-12) from the validated Functional Assessment of Cancer Therapy (FACT)/Gynecologic Oncology Group Oxaliplatin-Specific Neurotoxicity questionnaire.18-20 Patients completed the questionnaire before random assignment (baseline), at week 4 of cycle 2, at 6, 12, and 18 month follow-up visits, and at the LTS-01 long-term assessment. Thus, the assessment of neurotoxicity was the same for the cross-sectional sample and for those who had longitudinal assessments. The NTX-12 was comprised of statements intended to measure the severity and impact of PSN on patients’ lives including numbness/tingling in hands or feet, discomfort in hands or feet, joint pain, general weakness, trouble hearing, ringing or buzzing in ears, trouble with buttons, trouble feeling shape of objects in hands, trouble walking, and pain in hands or feet when exposed to cold temperatures. Patients were instructed to choose the number corresponding to how true each statement was for them in the past week using a Likert-type scale (0=“not at all,” 1=“a little bit,” 2=“somewhat,” 3=“quite a bit,” 4=“very much”). The NTX-12-score at each evaluation refers to the sum of the ratings from the 12 items. This sum can range from 0 to 48 where lower scores indicate less neuropathy. We have previously proposed that a difference in 4 points in the NTX-12 scale be considered a minimal clinically important difference (proposed in the C-07 protocol based on historical data).17
Statistical Methods
Using χ2 goodness of fit tests, characteristics of the LTS-01 cross sectional and longitudinal samples were compared to the C-07 population to test the representativeness of the subsets to the C-07 population from which they came. Differences in patient characteristics between treatment groups for patients in LTS-01 longitudinal sample were computed using the χ2 test of independence. A Wilcoxon rank sum test compared neurotoxicity scores between treatments for participants from the LTS-01 cross-sectional sample. The NTX-12 responses were dichotomized by severity (higher severity included the categories “somewhat,” “quite a bit,” or “very much,” while lesser severity included “not at all” or “a little bit”) and were analyzed by logistic regression at the long-term endpoint. Comparisons of the distributions of severity level between treatment groups were computed using the χ2 test of independence. A mixed effects longitudinal analysis compared long-term total neurotoxicity scores for patients in the longitudinal study between treatment groups from cycle 2 of treatment out to the long-term endpoint controlling for baseline score and including continuously measured time from baseline to each evaluation for each patient, quadratic and cubic terms of time, and a term for the interaction of time and treatment. Computations were performed in SAS v. 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics and Assessments
A full description of all patients who participated in the LTS-01 and C-07 trials have been reported elsewhere.11, 16, 21 The long-term endpoint for the 353 patients in the cross-sectional sample ranged from 4.2 to 8.6 years from randomization (median 6.0 years), while the long-term endpoint for the 92 patients in the longitudinal sample ranged from 5.5 to 8.1 years from randomization (median 7.0 years). In both the cross-sectional and longitudinal samples, the median time to long-term endpoint did not vary between treatment groups (for the cross-sectional sample, the median time to endpoint was 6.04 years for those who received FLOX and 6.07 years for those who received FULV, p=0.60; for the longitudinal sample the median time to endpoint was 6.92 years for those who received FLOX and 6.84 years for those who received FULV, p=0.42). The subsets of C-07 patients in the cross-sectional and longitudinal LTS-01 samples are representative of the C-07 population in terms of age, sex, and site of colon cancer (Table 1). Both patient samples, however, significantly differ from the C-07 population with respect to number of positive nodes. In the full C-07 population, 26% had four or more positive nodes, whereas this decreased to 20% and 13% for the cross-sectional and longitudinal samples, respectively. Since a larger number of positive nodes is associated with poorer prognosis, a decreased representation of these patients is expected in studies of long-term survivors. There were no significant imbalances between treatment groups of patient characteristics for the 92 patients in the longitudinal sample (Table 2).
Table 1.
Comparison of Patient Characteristics for those in NSABP trial C-07 and the Cross-sectional and Longitudinal LTS-01 Samples
| Characteristic | C-07 | LTS-01 | ||||
|---|---|---|---|---|---|---|
| Cross-Sectional Sample | Longitudinal Sample | |||||
|
| ||||||
| No. of Patients (%) | No. of Patients (%) | P* | No. of Patients (%) | P* | ||
| N | 2467 | 353 | 92 | |||
| Age, years | ||||||
| < 50 | 561 (22.7) | 79 (22.4) | 0.46 | 20 (21.7) | 0.53 | |
| 50-59 | 712 (28.9) | 110 (31.2) | 32 (34.8) | |||
| 60-69 | 794 (32.2) | 117 (33.1) | 29 (31.5) | |||
| ≥ 70 | 400 (16.2) | 47 (13.3) | 11 (12.0) | |||
| Sex | ||||||
| Male | 1392 (56.4) | 214 (60.6) | 0.11 | 60 (65.2) | 0.09 | |
| Female | 1075 (43.6) | 139 (39.4) | 32 (34.8) | |||
| Positive Nodes† | ||||||
| 0 | 714 (28.9) | 112 (31.7) | 0.03 | 30 (32.6) | 0.02 | |
| 1-3 | 1121 (45.4) | 172 (48.7) | 50 (54.4) | |||
| 4+ | 631 (25.6) | 69 (19.6) | 12 (13.0) | |||
| Unknown | 1 (<0.1) | 0 (0) | 0 (0) | |||
| Site | ||||||
| Left Colon | 499 (20.2) | 78 (22.1) | 0.79 | 20 (21.7) | 0.39 | |
| Right Colon | 1085 (44.0) | 150 (42.5) | 46 (50.0) | |||
| Sigmoid | 855 (34.7) | 122 (34.6) | 26 (28.3) | |||
| Multiple | 28 (1.1) | 3 (0.9) | 0 (0) | |||
Abbreviations: NSABP, National Surgical Adjuvant Breast and Bowel Project; LTS-01, Long-Term Survivors study; FULV, fluorouracil and leucovorin; FLOX, FULV plus oxaliplatin.
P-value from the χ2 goodness of fit test.
χ2 goodness of fit test does not include unknown category.
Table 2.
Patient Characteristics for those in NSABP LTS-01 Longitudinal Sample by Treatment
| Characteristic | FULV | FLOX | P* (FULV vs. FLOX) |
|---|---|---|---|
|
| |||
| No. of Patients (%) | No. of Patients (%) | ||
| N | 42 | 50 | |
| Age, years | |||
| < 50 | 11 (26.2) | 9 (18.0) | 0.72 |
| 50-59 | 15 (35.7) | 17 (34.0) | |
| 60-69 | 12 (28.6) | 17 (34.0) | |
| ≥ 70 | 4 (9.5) | 7 (14.0) | |
| Sex | |||
| Male | 30 (71.4) | 30 (60.0) | 0.25 |
| Female | 12 (28.6) | 20 (40.0) | |
| Positive Nodes | |||
| 0 | 15 (35.7) | 15 (30.0) | 0.17 |
| 1-3 | 19 (45.2) | 31 (62.0) | |
| 4+ | 8 (19.1) | 4 (8.0) | |
| Site | |||
| Left Colon | 10 (23.8) | 10 (20.0) | 0.22 |
| Right Colon | 17 (40.5) | 29 (58.0) | |
| Sigmoid | 15 (35.7) | 11 (22.0) | |
Abbreviations: NSABP, National Surgical Adjuvant Breast and Bowel Project; LTS-01, Long-Term Survivors study; FULV, fluorouracil and leucovorin; FLOX, FULV plus oxaliplatin.
P-value from χ2 test of independence.
Neurotoxicity submission rates were acceptable in the longitudinal sample. The lowest number of completed questionnaires occurred during week 4 of cycle 2, where 76 of 92 patients, (83%), completed the questionnaire. There was no significant difference by treatment in the number of questionnaires completed over the six evaluation time points (P= .996) and the reasons given for missing forms were similar across treatments. In the FULV and FLOX groups, respectively, 72% and 67% of missed assessments were due to staff oversight or reasons unrelated to the patients’ condition or choice. The median dose of oxaliplatin received for patients on the FLOX arm was 677 mg/m2, with interquartile range from 320 to 763 mg/m2.11
Patient-Reported Neurotoxicity: Cross-Sectional Sample
The NTX-12-score for the 169 FULV patients ranged from 0-33 with mean 5.7 and median 4, while for the 184 FLOX patients the score ranged from 0-28 with mean 7.5 and median 6. While there was a statistically significant difference in NTX-12-scores between treatment groups at the long-term endpoint (P= .005), the difference in means of 1.8 and in medians of 2 were not clinically significant because the differences were less than the 4 points prospectively considered clinically important.
Dichotomized responses (at least “somewhat” versus lesser severity) for the individual neurotoxicity questions were examined to provide greater insight into specific long-term effects of adjuvant therapy. Odds ratios [OR] for each of the 12 questions are presented in the forest plot in Figure 2 and depict the odds of higher severity for those who received FLOX as compared to the odds for those who received FULV. There was no significant difference in odds for all questions except for the two which asked about the severity of numbness and tingling in hands and feet.
Figure 2.
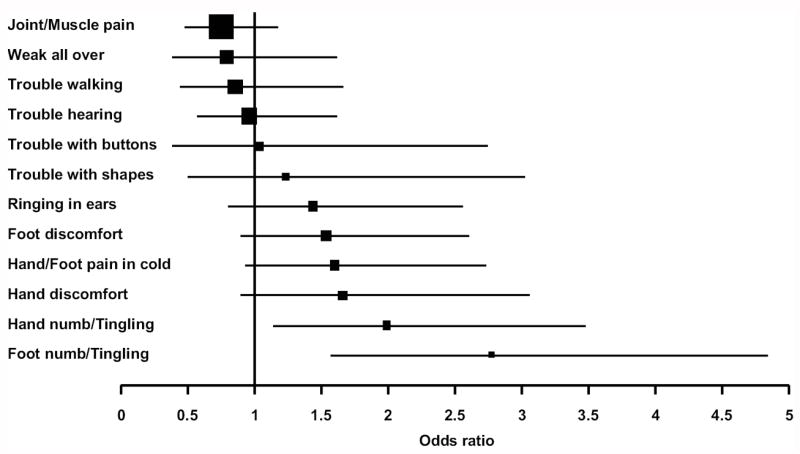
Forest plot depicting odds ratio for each neurotoxicity question for the 353 C-07 and Long-Term Survivors, LTS-01, patients; Odds of reporting the symptom (with severity of “somewhat,” “quite a bit,” or “very much” vs. “not at all” or a “little bit”) for FLOX divided by the odds for FULV; FULV, fluorouracil and leucovorin; FLOX, FULV plus oxaliplatin.
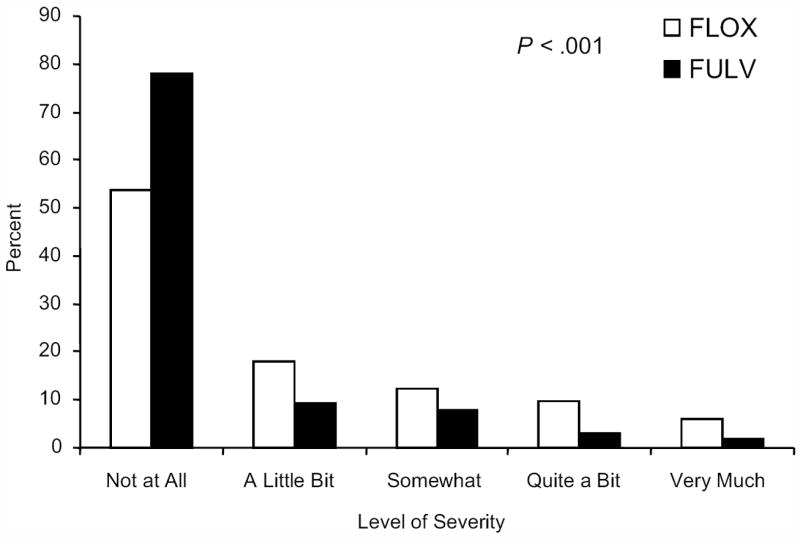
Patients who received FLOX reported higher severity of numbness or tingling in hands and feet than those in the FULV group at the long-term endpoint. The OR of at least higher severity of numbness or tingling in hands for the FLOX group compared to the FULV group was 2.00 (95% CI, 1.15 to 3.48, P= .015). While 24% of FLOX patients had at least “somewhat” severity of numbness or tingling in their hands, only 14% of FULV patients did so (Figure 3A). The difference in distributions of the five levels of severity of numbness or tingling in hands between treatment groups in Figure 3A was not significant at the .05 level (P= .07). The OR of higher severity of numbness or tingling in feet for the FLOX group compared to the FULV group was 2.78 (95% CI, 1.59 to 4.85, P< .001). Twenty-nine percent of FLOX patients had at least “somewhat” severity of numbness or tingling in feet versus 13% for FULV patients. The difference in distributions across all five severity levels of numbness or tingling in feet in Figure 3B was significant between treatments (P< .001, FLOX vs. FULV: 13% vs. 8% “somewhat,” 10% vs. 3% “quite a bit,” and 6% vs. 2% “very much”).
Figure 3.
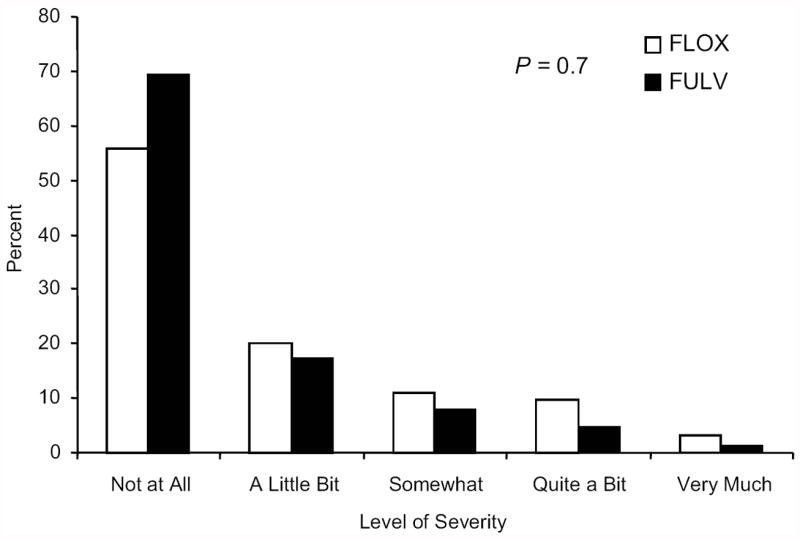
Panel A: Distribution of severity of numbness or tingling in hands, for 353 NSABP C-07 patients participating in the Long-Term Survivors, LTS-01, long-term assessment of neurotoxicity; P-value compares distributions by treatment across the 5 levels of severity; FULV, fluorouracil and leucovorin; FLOX, FULV plus oxaliplatin.
Panel B: Distribution of severity of numbness or tingling in feet, for 353 NSABP C-07 patients participating in the Long-Term Survivors, LTS-01, long-term assessment of neurotoxicity; P-value compares distributions by treatment across the 5 levels of severity; FULV, fluorouracil and leucovorin; FLOX, FULV plus oxaliplatin.
Patient-Reported Neurotoxicity: Longitudinal Sample
For the 92 patients with baseline and long-term data, there were a total of 498 neurotoxicity evaluations over six time points. For all patients at all evaluations, the total neurotoxicity score ranged from 0 to 33 with mean 4.66 and median 3. Ninety percent of the NTX-12-scores were below 12 and only six observations reported very high neurotoxicity (>23).
The mean change from baseline of the NTX-12-score for patients treated with FLOX was initially much larger than the mean change for patients treated with FULV, but this difference dissipated by the long-term assessment. The median changes from baseline of the NTX-12-score followed a similar pattern, but were lower than the mean changes from baseline for both treatment groups at each evaluation. The only difference in median scores deemed clinically important occurred at week 4 of cycle 2 with a difference of 5 (Figure 4).
Figure 4.
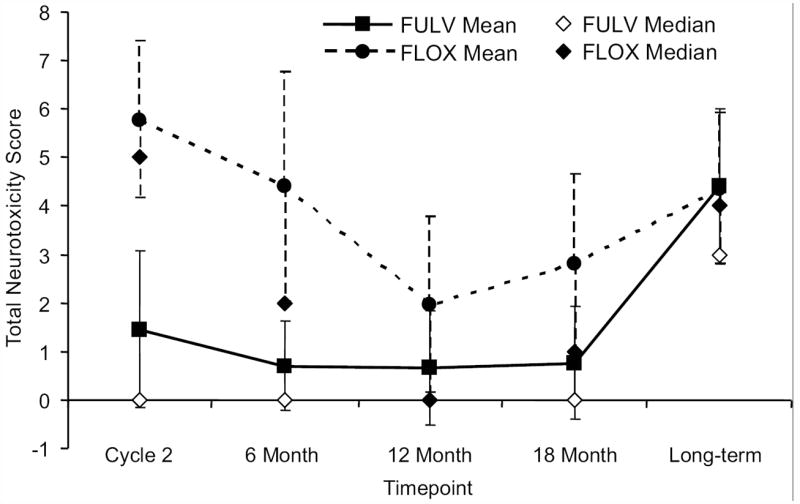
In the longitudinal sample, mean and median changes from baseline of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Oxaliplatin-Specific Neurotoxicity Scale total score (NTX-12-score). FULV, fluorouracil and leucovorin; FLOX, FULV plus oxaliplatin.
For the first four assessments after baseline, the 95% confidence intervals of the mean changes from baseline for patients treated with FULV straddle zero indicating no little or no change from baseline, whereas for patients treated with FLOX, the confidence intervals are greater than zero. The mean changes from baseline and their confidence intervals, however, are almost identical at the long-term assessment for both treatment groups. Also, the 95% confidence intervals around the mean overlap from the 12-month assessment to the long-term endpoint illustrating no significant difference in the mean (median) change from baseline of the neurotoxicity scores between treatment groups (Figure 4).
A mixed effects model verified that the effect of oxaliplatin on the NTX-12-score changed significantly with time (P< .001). For patients with a 0 baseline NTX-12-score treated with FULV, the mean NTX-12-score was predicted to be 0.77 at 6 months from baseline, 2.45 at 5 years from baseline, and 3.65 at 7 years. For similar patients with a 0 baseline NTX-12-score, but treated with FLOX, the mean NTX-12-score was predicted to be 3.35 at 6 months from baseline, 3.63 at 5 years, and 3.92 at 7 years. Thus, the model suggests that for patients with identical baseline neurotoxicity scores, the initial substantial difference in neurotoxicity scores during treatment dissipated by 7 years from baseline.
DISCUSSION
Neurotoxicity due to oxaliplatin had previously been reported by the C-07 and MOSAIC trials. Land, et al. reported significant differences in neurotoxicity scores between treatments up to 18 months after treatment onset in the C-07 trial.17 The MOSAIC trial extended the time-period analyzed by Land, et al., reporting neurotoxic effects up to 48 months, but only for the treatment arm including oxaliplatin, making a long-term treatment comparison impossible.12 Park, et al. also reported oxaliplatin-induced neuropathy results for 24 oxaliplatin-treated patients with follow-up at a median of 25 months post-treatment.22 At follow-up, 79.2% of patients reported residual neuropathic symptoms where the severity of neurotoxicity scores correlated with cumulative dose. Park et al.’s manuscript, however, only reports neurotoxicity information for a small sample of patients who received oxaliplatin, again making a treatment comparison impossible and follow-up is only a median of just over two years.
As the first long-term comparison of the severity and duration of neurotoxicity in patients treated with and without oxaliplatin, this report found that differences by treatment in mean and median neurotoxicity scores in the cross-sectional sample (n=353) was half that deemed clinically important at the long-term assessment, a median of 6.0 years from random assignment. While there were differences in neurotoxicity scores between treatment groups in the first 18 months of the C-07 colon cancer trial, the longitudinal sample (n=92) established that these differences dissipated by the long-term evaluation. The increase in the mean total neurotoxicity score for patients treated with FULV, at the long-term endpoint, is most likely due to the general increase of PSN in an aging population.23
Findings from shorter-term studies, such as Haller et al.24 and André et al.,12 corroborate that some neurotoxic effects from oxaliplatin are reversible. Haller et al.24 reported grade 3 or 4 neurosensory toxicity in 11% of patients treated with oxaliplatin (versus 8% treated without oxaliplatin) where persistent symptoms in 5% of patients resolved after a median period of 1 month after completion of treatment. André et al.12 reported that 92% of patients from the MOSAIC trial treated with oxaliplatin experienced PSN of any grade during treatment, but 4 years after completion of therapy, only 15.5% reported PSN, with <1% having symptoms graded as severe.
In Land et al.’s 2007 paper, foot discomfort, feet numbness/tingling, and hand/foot pain in the cold were significantly more severe in those treated with oxaliplatin at 18 months.17 From our long-term analysis, sensitivity to the cold and foot discomfort were no longer significantly different between the treatment groups, demonstrating that treatment-related differences in these effects disappear or at least decrease after 6 years from randomization. Hand/foot numbness/tingling appeared to be the longest lasting side-effects for those treated with oxaliplatin. The odds of hand numbness/tingling were significantly greater for the FLOX group during therapy and, while no longer significant at 18 months, were significantly greater at the long-term endpoint. The odds of feet numbness/tingling remained significantly greater for those in the FLOX group during therapy, at 18 months, and at the long-term assessment.
Specific countermeasures to address these neurotoxic effects, which occur during and continue after treatment, are important to develop. Additional studies, which utilize a pharmacogenetic approach to assessing toxicity and survival in CRC patients treated with oxaliplatin, could shed light on those who are more susceptible to PSN, provide early detection and supplemental treatment strategies for those who develop PSN, and create specific medications to address the lingering effects of hand/foot numbness/tingling.25-28
We note that since neurotoxicity scores correlate with cumulative oxaliplatin dose, the expected cumulative oxaliplatin exposure in the C-07 trial (765 mg/m2) was less than that from the trials reported in Haller et al. (1,040 mg/m2) and Andre et al. (1,020 mg/m2), as expected, based on the regimen. Thus, our findings may underestimate the long-term results in other long-term survivors treated with regimens using different schedules of oxaliplatin. Along with the lower cumulative dose of oxaliplatin from the C-07 study, other limitations include the relatively small sample size of the longitudinal sample, the use of a relatively new patient-reported outcome measure to assess neurotoxicity, and a lack of comparison between the treatment groups of the longitudinal, cross-sectional, and original cohorts with respect to comorbidities that increase neuropathy.
The longitudinal sample provided adequate evidence, especially when combined with the comparison of mean scores from cross-sectional samples and other studies, that there was not a clinically significant difference in long-term NTX-12-scores for patients treated with or without oxaliplatin. Numbness and tingling of hands and feet persisted at an elevated incidence for oxaliplatin-treated patients 6 years from randomization. This study enhances physicians’ and patients’ understanding of the severity and duration of neurotoxic effects of oxaliplatin. Efforts should be made to minimize peripheral sensory neuropathy during and after oxaliplatin treatment so that patients can benefit from extended disease-free survival while maintaining their quality of life.
Acknowledgments
Supported by: NIH Public Health Service grants U10-CA-12027, U10-CA-69651, U10-CA-37377, and U10-CA-69974 from the National Cancer Institute, Department of Health and Human Services; and Grant No. RSGPB-05-236-01-CPPB from the American Cancer Society.
Footnotes
The work described in this manuscript is original research and has not been previously published. Related work was published as follows:
Kuebler JP, Wieand HS, O’Connell MJ, et al: Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol 25:2198-2204, 2007.
Kopec JA, Land SR, Cecchini RS, et al: Validation of a self-reported neurotoxicity scale in patients with operable colon cancer receiving oxaliplatin. J Supp Oncol 2006; 4(8):W1-W8.
Yothers G, O’Connell MJ, Colangelo L, et al: 5-FU and leucovorin (Lv) with or without xaliplatin (Ox) for adjuvant treatment of stage II and III colon cancer: Long term follow-up of NSABP C-07 with survival analysis. ASCO GI Cancers Symposium 2010. Abstr ASCO GI 401.
Land SR, Kopec JA, Cecchini RS, et al: Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol 25: 2205-2211, 2007
The authors declare no conflicts of interest,
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics. CA: A cancer journal for clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resented colon carcinoma. N Engl J Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15:246–250. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 4.Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: Results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–1887. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 5.Benson AB, III, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 6.Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of Intergroup 0089. J Clin Oncol. 2005;23:8671–8678. doi: 10.1200/JCO.2004.00.5686. [DOI] [PubMed] [Google Scholar]
- 7.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 8.Lembersky BC, Wieand HS, Petrelli NJ, et al. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol. 2006;24:2059–2064. doi: 10.1200/JCO.2005.04.7498. [DOI] [PubMed] [Google Scholar]
- 9.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 10.de Gramont A, Boni C, Navarro M, et al. Oxaliplatin/5FU/LV in the adjuvant treatment of stage II and stage III colon cancer: Efficacy results with a median follow-up of 4 years. J Clin Oncol. 2005;23:16S. suppl; abstr 3501. [Google Scholar]
- 11.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 12.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC Trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) Cancer survivors—United States, 2007. [11-29-11];MMWR. 2011 60:269–272. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6009a1.htm. [PubMed] [Google Scholar]
- 14.Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 including survival and subset analyses. J Clin Oncol. 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganz P, Land SR, Antonio C, et al. Cancer survivorship research: The challenge of recruiting adult long term cancer survivors from a cooperative clinical trials group. J Cancer Surviv. 2009;3:137–147. doi: 10.1007/s11764-009-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute. [11-29-11];NCI Cancer Therapy Evaluation Program: Common Toxicity Criteria, Version 2.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf.
- 17.Land SR, Kopec JA, Cecchini RS, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol. 2007;25:2205–2211. doi: 10.1200/JCO.2006.08.6652. [DOI] [PubMed] [Google Scholar]
- 18.Kuroi K, Shimozuma K. Neurotoxicity of taxanes: Symptoms and quality of life assessment. Breast Cancer. 2004;11:92–99. doi: 10.1007/BF02968010. [DOI] [PubMed] [Google Scholar]
- 19.Cella D, Peterman A, Hudgens S, et al. Measuring the side effects of taxane therapy in oncology: The functional assessment of cancer therapy taxane (FACT-taxane) Cancer. 2003;98:822–831. doi: 10.1002/cncr.11578. [DOI] [PubMed] [Google Scholar]
- 20.Kopec JA, Land SR, Cecchini RS, et al. Validation of a self-reported neurotoxicity scale in patients with operable colon cancer receiving oxaliplatin. [11-29-11];J Support Oncol. 2006 4:W1–W8. http://www.supportiveoncology.net/jso/journal/abstracts/0408396.html. [Google Scholar]
- 21.Kunitake H, Zheng P, Yothers G, et al. Routine preventive care and cancer surveillance in long-term survivors of colorectal cancer: Results from National Surgical Adjuvant Breast and Bowel Project protocol LTS-01. J Clin Oncol. 2010;28:5274–5279. doi: 10.1200/JCO.2010.30.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SB, Lin CS, Krishnan AV, et al. Long-term neuropathy after oxaliplatin treatment: challenging the dictum of reversibility. The Oncologist. 2011 Apr 8; doi: 10.1634/theoncologist.2010-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mold JW, Vesely SK, Keyl BA, et al. The Prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract. 2004;17:309–318. doi: 10.3122/jabfm.17.5.309. [DOI] [PubMed] [Google Scholar]
- 24.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 25.Park SB, Lin CS-Y, Krishnan AV, et al. Oxaliplatin-induced neurotoxicity: Changes in axonal excitability precede development of neuropathy. Brain. 2009;132:2712–2723. doi: 10.1093/brain/awp219. [DOI] [PubMed] [Google Scholar]
- 26.Boige V, Mendiboure J, Pignon JP, et al. Pharmacogenetic assessment of toxicity and outcome in patients with metastatic colorectal cancer treated with LV5FU2, FOLFOX, and FOLFIRI: FFCD 2000-05. J Clin Oncol. 2010;28:2556–2564. doi: 10.1200/JCO.2009.25.2106. [DOI] [PubMed] [Google Scholar]
- 27.Park SB, Lin CS-Y, Krishnan AV, et al. Utilizing natural activity to dissect the pathophysiology of acute oxaliplatin-induced neuropathy. Exp Neurol. 2011;227:120–127. doi: 10.1016/j.expneurol.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Braun MS, Seymour MT. Molecular markers of chemotherapy toxicity in colorectal cancer. Curr Colorectal Cancer Rep. 2011;7:105–111. [Google Scholar]


