Abstract
Background
The impact of arm morbidity following breast cancer surgery on patient-observed changes in daily functioning and health-related quality of life (HRQoL) have not been well-studied.
Objective
To examine the association of objective measures such as range of motion (ROM) and lymphedema, with patient-reported outcomes (PROs) in the arm and breast, upper extremity function, activities, and HRQoL.
Methods
The National Surgical Adjuvant Breast and Bowel Project Protocol B-32 was a randomized trial comparing sentinel node resection (SNR) with axillary dissection (AD) in women with node-negative breast cancer. ROM and arm volume were measured objectively. PROs included symptoms; arm function; limitations in social, recreational, occupational, and other regular activities; and a global index of HRQoL. Statistical methods included cross-tabulations and multivariable linear regression models.
Results
In all, 744 women provided at least 1 postsurgery assessment. About one-third of the patients experienced arm mobility restrictions. A similar number of patients avoided the use of the arm 6 months after surgery. Limitations in work and other regular activities were reported by about a quarter of the patients. In this multivariable analysis, arm mobility and sensory neuropathy were predictors of patient-reported arm function and overall HRQoL. Predictors for activity limitations also included side of surgery (dominant vs nondominant). Edema was not significant after adjustment for sensory neuropathy and ROM.
Limitations
Arm mobility and edema were measured simultaneously only once during the follow-up (6 months).
Conclusion
Clinical measures of sensory neuropathy and restrictions in arm mobility following breast cancer surgery are associated with self-reported limitations in activity and reductions in overall HRQoL.
Keywords: Breast Cancer, Axillary Dissection, Sentinel Node Resection, Arm Morbidity, Patient-Reported Outcomes, Quality of Life
BACKGROUND
Restricted arm mobility, lymphedema, peripheral neuropathy, and other symptoms are frequent complications of axillary lymph node dissection (AD) in women undergoing breast cancer surgery.1–6 Over the past decade, sentinel node resection (SNR) has become a common alternative to AD in early–stage breast cancer.7,8 The results from 4 large randomized trials,9–14 2 smaller trials,15–17 and numerous observational studies18–23 have demonstrated that patients undergoing SNR report less arm morbidity and better health-related quality of life (HRQoL) than do patients undergoing AD. In the recently published analyses of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial in women with node-negative breast cancer, significant differences in favor of SNR were reported with respect to arm morbidity; symptoms in the ipsilateral arm and breast; arm function; participation in occupational and social activities; and overall HRQoL.13,14
The results of studies comparing SNR and AD have demonstrated that axillary surgery impacts patient-reported outcomes (PROs). However, the mechanism for this association is not entirely clear. Axillary surgery is associated with several major side effects, such as limited range of motion (ROM), lymphedema, neuropathy, and pain or discomfort in the arm, breast, and chest. The independent effects of these factors on arm function; participation in work, social, and recreational activities; and overall HRQoL are not completely understood.
Arm mobility and symptoms correlated with PROs in several studies.1–6 The effect of lymphedema was observed by some authors,2,3 but was less evident in other studies.4–6 Furthermore, studies that included both objective measures of ROM and edema as well as subjective measures of function and HRQoL on a large sample of patients have been rare. Most published studies of the relationship between arm morbidity and PROs following axillary surgery were relatively small and the methods of measuring arm mobility, edema, neuropathy, and PROs varied across studies. A question that has not been extensively studied is whether the side of breast cancer surgery (dominant vs nondominant) has any effect on PROs.
Understanding which of the side effects of axillary surgery have the greatest impact on HRQoL is important for optimizing treatment and providing accurate prognostic information to the patient. In this study, we have analyzed data from NSABP B-32, a randomized trial comparing SNR and conventional AD in women with early breast cancer. The purpose of the current analysis was to examine the relationships between (1) objective measures of arm morbidity, such as ROM and edema, clinical assessment of neuropathy, and (2) patient-reported symptoms in the arm and breast, upper extremity function, participation in life activities, and overall HRQoL after breast cancer surgery. Because comparisons of self-reported outcomes and arm morbidity between SNR and AD in NSABP B-32 have been reported previously,13,14 in the current analysis the 2 arms of the trial were combined.
METHODS
Participants
Women participating in NSABP B-32 had operable breast cancer and clinically negative lymph nodes. After stratification by age at entry (≤49 years,≥ 50 years), tumor size (≤2.0 cm, 2.1 through 4.0 cm, ≥ 4.1 cm), and surgical treatment plan (lumpectomy vs mastectomy), these women were randomly assigned to AD vs SNR. Those who were sentinel node positive underwent AD. Use of systemic adjuvant therapy was left to the discretion of the treating physician.
Participants in the current study were women who had sentinel node–negative breast cancer and were enrolled in the HRQoL component of NSABP B-32. Inclusion in the B-32 HRQoL study was limited to community-based NSABP institutions participating in the Community Clinical Oncology Program (CCOP). All participants provided written informed consent approved by the National Cancer Institute and the institutional review boards of all participating institutions. More details about the B-32 design can be found elsewhere.24,25
Clinical Measurements
Members of the institutional clinical staff attended a national workshop and annual meetings that included discussion of the measurement of ROM and arm volume. ROM was assessed by measuring the range of lateral abduction of each arm in degrees, using a standard orthopedic goniometer. To this end, the patient was asked to stand straight and to raise the affected arm laterally as high as possible without assistance. The angle between the lateral chest wall and the humerus was measured. The value was recorded and the measurement was repeated on the opposite arm. Reliability of this method is moderate to high.26
Arm volume was assessed bilaterally by the water displacement method, considered the most accurate method of measuring arm volume.27 First, the lateral epicondyle (elbow) was identified and a distance of 10 cm was measured in a straight line toward the mid-deltoid muscle (shoulder). The spot was marked with a marker. The arm was then placed in an empty plastic graduated cylinder. Next, the cylinder was filled with water until the level reached the mark on the arm. The level on the cylinder was recorded. The patient was then asked to remove the arm slowly to allow all water to drop back into the cylinder and avoid any significant spillage. The water level was recorded after the arm was removed. Arm volume was calculated as the difference between the water levels with and without the arm in the cylinder. The measurement was repeated for the ipsilateral arm. An edema score was calculated as the difference from baseline in the volume of the ipsilateral arm relative to the contralateral arm.
Sensory neuropathy (usually resulting from an injury to the intercostal brachial cutaneous nerves) was assessed by a health professional who asked the patient if she currently experienced any numbness or tingling in each arm, especially on the medial aspect of the upper arm. A neuropathy score was computed as the sum of 2 measurements: numbness in the affected arm, and tingling in that arm. A score of zero indicated no neuropathy, whereas a score of 2 indicated both numbness and tingling.
Patient-Reported Outcomes
Measures of PROs relevant to breast cancer surgery included (1) symptoms in the upper extremity and breast/chest, (2) upper extremity function, (3) overall ratings of limitations in work or other regular daily activities and in social and/or recreational activities, and (4) global HRQoL.28–33 The following symptoms were evaluated: (1) tenderness, (2) swelling, (3) discomfort or pain, (4) numbness and “pins and needles,” (5) skin sensitivity, (6) tightness, (7) pulling or stretching, and (8) weakness. Except weakness, the symptoms were reported separately for the upper extremity (arms, underarms, hands, and fingers) and for the breast/chest. Patients reported symptoms separately for the left and right side. The symptoms were measured on a 0- to 4-point scale (from “Not bothered” to “Very bothered”), and were treated in the analysis as yes/no variables (“bothered” vs “not bothered”).
Items assessing upper extremity function were related to pushing/pulling large objects, lifting objects > 10 lb (left and right arm), reaching above shoulder level, and avoiding use of the arm (left arm and right arm).28–33 The items had 4 options, ranging from “Not difficult” to “I could not do this” in the past 7 days, or – for the avoidance item – from “Never” to “Always.” A functional limitations score (FLS) for the upper extremity was calculated as the sum of the 4 individual questions. A score of zero meant no impairment, whereas a score of 4 indicated impairment in all 4 areas. Overall ratings of limitations in participation in social/recreational and work/other regular daily activities were taken from a well-established instrument, the Disabilities of Arm Shoulder and Hand Scale (DASH).29 The options (0 to 4) ranged from “Not limited” to “Very limited” in the past 7 days. An activity limitation score (ALS) was formed by summing the 2 items (work/other activities and social/recreational activities) to form a 0- to 8-point scale. Finally, overall HRQoL was measured using a global scale (0 to 10) with zero being “Worst possible health” and 10 being “Perfect health”.33,34
Data Collection
All measurements were initially performed at baseline. After surgery, ROM was measured at 1 week, 2 to 3 weeks, and 6 months. Edema and sensory neuropathy were assessed every 6 months for 3 years. The questionnaire was self-administered at the time of each follow-up visit (ie, at 1 week, at 2 to 3 weeks, and every 6 months for 3 years) until the patient was diagnosed as sentinel node positive, had a documented breast cancer recurrence or second primary cancer, died, or withdrew consent from the parent B-32 study. In exceptional cases, we allowed the questionnaire to be administered over the phone or mailed to the participant. A Missing Data Form was required for all instances of missing questionnaires.
Statistical Methods
For the cross-tabulations, all measures were treated as categorical variables. For this purpose, the edema score was dichotomized into < 66th percentile and ≥ 66th percentile; ROM was truncated at 180 degrees and grouped into 4 categories (0 to 139 degrees, 140 to 159 degrees, 160 to 179 degrees, and ≥ 180 degrees); the overall HRQoL score (0 to 10) was dichotomized into < 8 and ≥ 8. Descriptive statistics and modeling were based on the 6-month follow-up data because this was the only point in time when all measurements were taken simultaneously. In regression models, the variables were analyzed as continuous variables. Regression models were fit separately for each of the outcome variables (FLS, ALS, and overall HRQoL score). Potential predictors were ROM, edema, sensory neuropathy, side of surgery (dominant vs nondominant), and interactions with each variable and with side of surgery. Treatment group assignment was not included as a predictor because the treatment was the main cause of the impairments studied and, therefore, would have masked the associations between functional limitations and PROs, which would have significantly reduced variation in the data, thus limiting our ability to show the associations of interest. Each model was run, including all the candidate variables and interactions. Next, terms were removed if the P value was greater than .10 (except in the case where lower-order terms needed to be retained when a higher-order interaction was significant), and the model was rerun. If there were any remaining variables that had P > .05, they were removed and this process was repeated until all the covariates had P < .05, or were necessary to support higher-order interactions. This constituted the reduced model. Analyses were conducted using SAS v9.1.3.
RESULTS
Descriptive Results
Of the 749 patients enrolled in the B-32 HRQoL substudy, 744 provided at least 1 postsurgery assessment, 678 (91.1%) of whom also provided data at 6 months. About 87% of the participants were white and 78% were 50 years of age or older. (See Table 1.) The vast majority (84%) received a lumpectomy. Tumor size was ≤ 2 cm in 87% of the patients. Of these, 52% were randomly assigned to SNR and 48% to AD.
Table 1.
Characteristics of the NSABP B-32 HRQoL Study Population at Baseline
| Characteristic | No. of Patients (N = 744) | % of Patients |
|---|---|---|
|
| ||
| Race | ||
| White | 650 | 87.4a |
| Black | 69 | 9.3a |
| Other | 25 | 3.4a |
|
| ||
| Age | ||
| < 50 years | 161 | 21.6 |
| ≥50 years | 583 | 78.4 |
|
| ||
| Treatment arm | ||
| AD | 354 | 47.6 |
| SNR | 390 | 52.4 |
|
| ||
| Tumor size | ||
| ≤2 cm | 648 | 87.1 |
| 2.1–4 cm | 91 | 12.2 |
| ≥4.1 cm | 5 | 0.7 |
|
| ||
| Surgery | ||
| Lumpectomy | 625 | 84.0 |
| Modified radical mastectomy | 119 | 16.0 |
Abbreviations: AD, axillary dissection; SNR, sentinel node resection.
Percentages do not add up to 100% because of rounding.
The evolution of arm morbidity measures, symptoms, and other PROs in the AD and SNR groups up to 3 years post surgery was described by Land et al13 and Ashikaga et al.14 Reductions in HRQoL in both groups were most severe in the postoperative period and the scores essentially stabilized at 12 months. In the current analysis in which the 2 treatment groups were combined, 30% of the patients had ROM < 160 degrees at 6 months post surgery, 34% had arm edema, and 32% experienced some sensory neuropathy. (See Table 2.) With respect to PRO, 31% of the patients had difficulties pushing or pulling large objects, 32% had problems lifting objects > 10 lb, 19% had difficulties reaching above shoulder level, and 31% avoided using the affected arm. Furthermore, 16% of the patients reported limitations with social/recreational activities and 24% reported limitations in work/other regular activities. Finally, 25% of the patients had HRQoL scores < 80% of the maximum score.
Table 2.
Frequency of Arm Problems, Limitations in Social and Work Activities, and Decreased Global HRQoL in the NSABP B-32 Study Population at 6 Months After Surgery
| Characteristic | No. of Patients (N = 744) | % of Patients |
|---|---|---|
|
| ||
| ROM (degrees) | ||
| < 140 | 54 | 8.3 |
| 140 – 159 | 138 | 21.2 |
| 160 – 179 | 287 | 44.0 |
| ≥180 | 173 | 26.5 |
| Total | 652 | 100.0 |
|
| ||
| Edema | ||
| No | 424 | 66.2 |
| Yes | 217 | 33.9 |
| Total | 641 | 100.1b |
|
| ||
| Neuropathy | ||
| 0 (no sensory neuropathy) | 446 | 67.9 |
| 1 (either numbness or tingling) | 121 | 18.4 |
| 2 (numbness and tingling) | 90 | 13.7 |
| Total | 657 | 100.0 |
|
| ||
| Difficulty pushing/pulling large objects | ||
| Yes | 210 | 31.2 |
| No | 464 | 68.8 |
| Total | 674 | 100.0 |
|
| ||
| Difficulty lifting > 10 lb | ||
| Yes | 213 | 31.7 |
| No | 459 | 68.3 |
| Total | 672 | 100.0 |
|
| ||
| Difficulty reaching above shoulder level | ||
| Yes | 127 | 18.8 |
| No | 548 | 81.2 |
| Total | 675 | 100.0 |
|
| ||
| Avoidance of arm use | ||
| Yes | 205 | 30.6 |
| No | 465 | 69.4 |
| Total | 670 | 100.0 |
|
| ||
| Restricted social and recreational activity | ||
| Yes | 108 | 16.0 |
| No | 567 | 84.0 |
| Total | 675 | 100.0 |
|
| ||
| Restricted work and other activities | ||
| Yes | 163 | 24.1 |
| No | 513 | 75.9 |
| Total | 676 | 100.0 |
|
| ||
| Global HRQoL scorea | ||
| < 8 | 170 | 25.1 |
| ≥8 | 508 | 74.9 |
| Total | 678 | 100.0 |
Abbreviations: ROM, range of motion; HRQoL, health-related quality of life.
0 = worst possible health; 10 = perfect health.
Percentage does not equal 100% because of rounding.
Symptoms
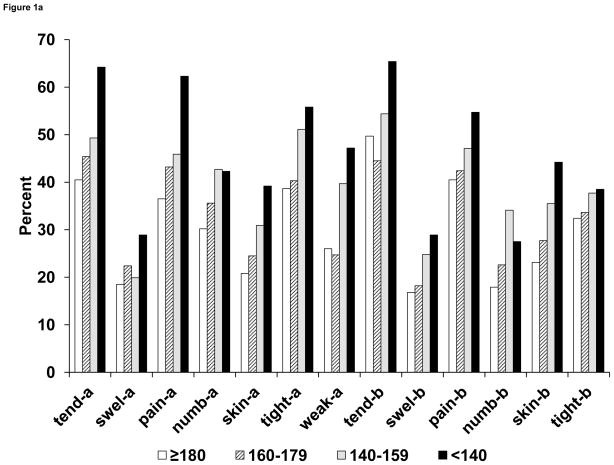
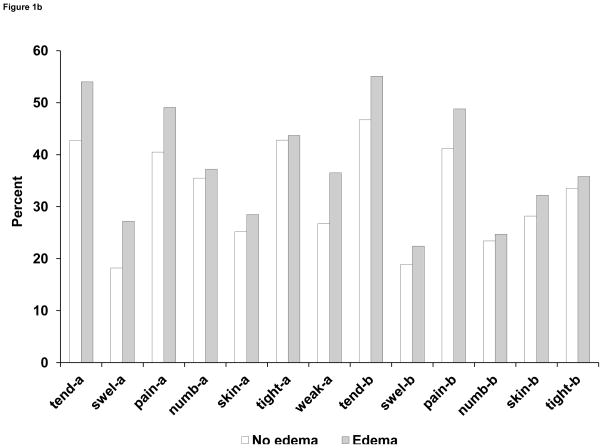
Tenderness, pain, and tightness were the arm and breast symptoms reported most frequently at 6 months. The frequency of most symptoms increased monotonically across the 4 levels of ROM. (See Figure 1A.) For example, the proportion of patients reporting tenderness in the arm increased from 40.5% in the ≥ 180-degrees group to 64.2% in the < 140-degrees group, whereas swelling of the arm was reported by 18.5% of those in the ≥ 180-degrees group and 28.9% in the < 140-degrees group. Interestingly, similar gradients in symptom frequency were observed for symptoms in the breast. Proportions of those reporting a given symptom were higher in patients with edema than in those without, but the differences were small for numbness and tightness in the arm and for most of the breast symptoms. (See Figure 1B.)
Figure 1.
Figure 1A. Proportion (%) of patients in NSABP B-32 reporting symptoms (all levels of severity) in the arm and breast at 6 months after breast cancer surgery, according to 4 categories of ROM. ROM categories refer to maximal arm abduction in degrees. Symptoms were measured on a 5-point scale and dichotomized as bothered (any level) vs not bothered.
Abbreviations: ROM, range of motion; tend-a, arm tenderness; swel-a, arm swelling; pain-a, arm pain; numb-a, arm numbness; skin-a, arm skin sensitivity; tight-a, arm tightness; weak-a, arm weakness; tend-b, breast tenderness; swel-b, breast swelling; pain-b, breast pain; numb-b, breast numbness; skin-b, breast skin sensitivity; tight-b, breast tightness.
Figure 1B. Proportion (%) of patients in NSABP B-32 reporting symptoms (all levels of severity) in the arm and breast at 6 months after breast cancer surgery, according to arm edema (yes/no). Symptoms were measured on a 5-point scale and dichotomized as bothered (any level) vs not bothered.
Abbreviations: ROM, range of motion; tend-a, arm tenderness; swel-a, arm swelling; pain-a, arm pain; numb-a, arm numbness; skin-a, arm skin sensitivity; tight-a, arm tightness; weak-a, arm weakness; tend-b, breast tenderness; swel-b, breast swelling; pain-b, breast pain; numb-b, breast numbness; skin-b, breast skin sensitivity; tight-b, breast tightness.
Arm Function
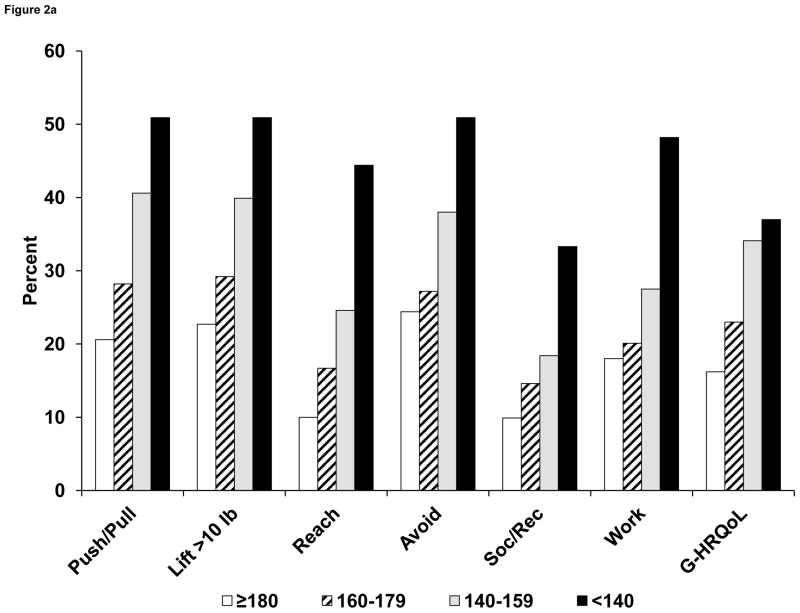
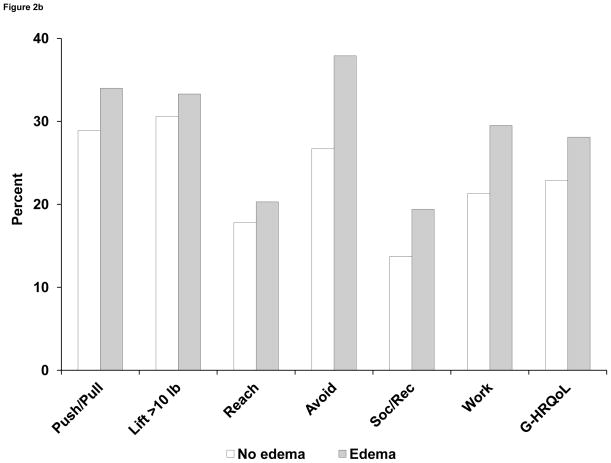
In bivariable analyses, self-reported arm function was related to ROM. (See Figure 2A.) The proportion of patients with difficulties in lifting objects weighing > 10 lb ranged from 23% among those with ROM ≥ 180 degrees to 51% in persons with ROM < 140 degrees. Similar monotonic relationships were seen for other measures of arm function (pushing/pulling objects, reaching above shoulder level, and use avoidance). Mean scores on a 0- to 4-point scale combining these 4 items (FLS) ranged from 0.77 to 1.94 for the 4 levels of ROM. Arm function was lower among patients with arm edema, compared with those who did not have edema (Figure 2B), although the differences were relatively minor for lifting and reaching and were greater for pushing/pulling objects and especially avoidance. Finally, arm function was strongly related to neuropathic symptoms, with a clear gradient across the 3 levels of symptoms for all measures of function. (See Figure 3.)
Figure 2.
Figure 2A. Proportion (%) of patients in NSABP B-32 reporting any limitations in arm function, social/recreational activities, work activities, and overall HRQoL at 6 months after breast cancer surgery, according to 4 categories of ROM. ROM categories refer to maximal arm abduction in degrees. Arm function items had 4 options and were dichotomized as yes (any level) vs no. Social/recreational and work/other activity questions had 5 options and were dichotomized as yes (any level) vs no. Global HRQoL (0–10) was dichotomized as < 8 vs ≥ 8.
Abbreviations: ROM, range of motion; Push/Pull, pushing or pulling large objects; Lift > 10 lb, lifting objects > 10 lb; Reach, reaching above shoulder level; Avoid, avoiding use of the arm; Soc/Rec, limitation in social or recreational activities; Work, limitation in work or other activities; G-HRQoL, global HRQoL score (0–10).
Figure 2B. Proportion (%) of patients in NSABP B-32 reporting any limitations in arm function, social/recreational activities, work/other activities, and overall HRQoL at 6 months after breast cancer surgery, according to arm edema (yes/no). Arm function items had 4 options and were dichotomized as yes (any level) vs no. Social/recreational and work/other activity questions had 5 options and were dichotomized as yes (any level) vs no. Global HRQoL (0–10) was dichotomized as < 8 vs ≥ 8.
Abbreviations: Push/Pull, pushing or pulling large objects; Lift > 10 lb, lifting objects >10 lb; Reach, reaching above shoulder level; Avoid, avoiding use of the arm; Soc/Rec, limitation in social or recreational activities; Work, limitation in work or other activities; G-HRQoL, global HRQoL score (0–10).
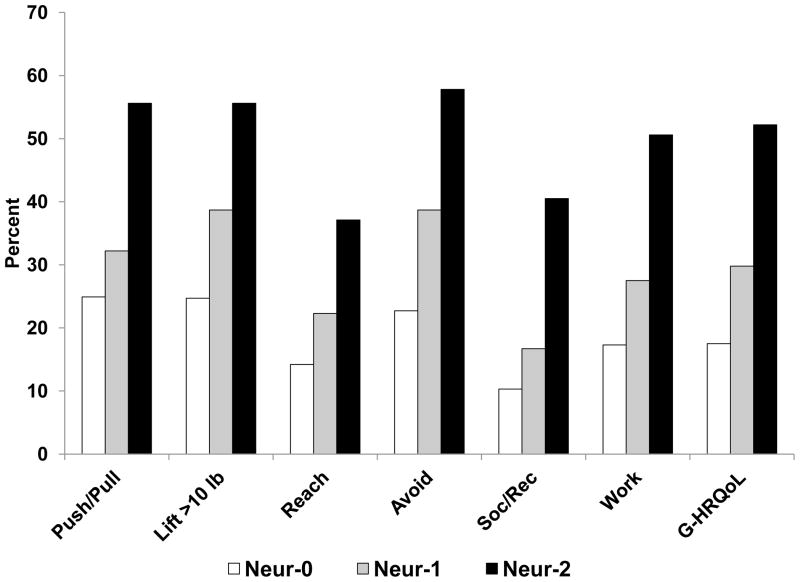
Figure 3.
Proportion (%) of patients in NSABP B-32 reporting any limitations in arm function, social/recreational activities, work/other activities, and overall HRQoL at 6 months after breast cancer surgery, according to sensory neuropathy. Arm function items had 4 options and were dichotomized as yes (any level) vs no. Social/recreational and work/other activity questions had 5 options and were dichotomized as yes (any level) vs no. Global HRQoL (0–10) was dichotomized as < 8 vs ≥ 8.
Abbreviations: Neur-0, no numbness or tingling on clinical assessment; Neur-1, either numbness or tingling; Neur-2, both numbness and tingling; Push/Pull, pushing or pulling large objects; Lift > 10 lb, lifting objects > 10 lb; Reach, reaching above shoulder level; Avoid, avoiding use of the arm; Soc/Rec, limitation in social or recreational activities; Work/Other, limitation in work or other activities; G-HRQoL, global HRQoL score (0 – 10).
In a multivariable model, scores on the 4-item FLS at 6 months were significantly affected by ROM (Regression Coefficient [RC] = −0.021 per 1-degree change; P < .001) and neuropathy (RC = 0.516; P < .001). (See Table 3.) However, arm edema was not related to arm function. Arm function was not affected by the side of surgery (dominant vs nondominant).
Table 3.
Regression Coefficients for the Effects of Sensory Neuropathy and ROM on Limitations in Arm Function (Model 1), Limitations in Work and Social Activities (Model 2), and Global HRQoL (Model 3) in Multivariable Regression Models: NSABP B-32 at 6 Months After Breast Cancer Surgery
| Model 1 Arm function limitations | Model 2 Activity limitations | Model 3 Global HRQoL | |
|---|---|---|---|
| Predictor | |||
| Sensory neuropathy | 0.516 | 0.514 | −0.715 |
| ROM | −0.021 | −0.023 | 0.017 |
| Dominant side | NA | −2.532 | NA |
| ROM × Dominant side | NA | 0.016 | NA |
Potential predictor variables in all models included sensory neuropathy, range of motion, edema, side of surgery (dominant vs nondominant), and interactions between side and other variables. Results from the reduced model are presented. All coefficients were statistically significant with P < 0.01.
Abbreviations: ROM, range of motion; HRQoL, health-related quality of life.
Activity Limitations
Overall ratings of limitations in work or other daily activities were related to ROM in a bivariable analysis. (See Figure 2B.) Among those with ROM ≥ 180 degrees, 18% reported any limitations in work/other activities. This proportion increased to 48% among women with a severe mobility restriction (ROM < 140 degrees). Similarly, the overall rating of participation in social and recreational activities was related to ROM, with proportions reporting limitations in these activities ranging from 9.9% to 33% across the 4 levels of ROM. Women with arm edema reported more limitations in work/other activities (29% vs 21%) and in social/recreational activities (19% vs 14%) than did those without edema. (See Figure 2B.) There was a strong effect of neuropathy on both work and other activities, as well as social/recreational activities. The proportions of women reporting any limitations in work/other activities increased from 17% to 51%, and the proportions reporting limitations in social/recreational activities increased from 10% to 41% across the 3 levels of neuropathy. (See Figure 3.)
In a multiple regression model, scores on a 2-item ALS combining work/other and social/recreational participation were significantly related to ROM (RC = −0.023 per degree; P < .001), neuropathy (RC = 0.514; P < .001), dominant vs nondominant side of surgery (RC = −2.532; P = .002), and side of surgery × ROM interaction (RC = 0.016; P = .002). (See Table 3.)
Overall Health-Related Quality of Life
The global HRQoL score was related to measures of ROM, edema, and neuropathy in bivariable analyses. There was an increase in the proportion of women reporting QoL < 8, from 16% in normal ROM to 37% in ROM < 140 degrees. (See Figure 2A.) For edema, the frequency increased from 23% to 28% among those without vs those with edema, respectively. (See Figure 2B.) For neuropathy, 17% had HRQoL scores < 8 among those without numbness or tingling, compared with 52% among those with both numbness and tingling. (See Figure 3.)
In multivariable analyses with a global HRQoL score as a continuous variable, significant predictors of HRQoL were ROM (RC = 0.017 per degree; P < .001) and neuropathy (RC = −0.715; P < .001). (See Table 3.) Neither edema nor side of surgery was associated with HRQoL.
DISCUSSION
About one-third of the women in the current study experienced significant arm mobility restrictions (ROM < 160 degrees) and a similar number avoided use of the arm 6 months after breast cancer surgery. About 25% of the participants reported limitations in work or other regular activities, and 16% were limited in social or recreational activities.
Objectively measured arm mobility and clinically assessed sensory neuropathy were predictors of patient-reported arm function; limitations in work/other and social/recreational activities; and overall HRQoL. Moreover, surgery on the dominant side was associated with significantly more restrictions in social/recreational and work/other activities than was surgery on the nondominant side. Patients with arm edema reported poorer outcomes than did those without edema, but the effect was not significant in the multivarible analysis. This does not necessarily mean that edema is not important; rather, the impact of edema could be mediated by symptoms and/or restricted mobility.
The results of regression modeling indicate that a decrease of 40 degrees in ROM (from no restriction to severe restriction) was associated with an almost 0.7-point decrease in overall HRQoL on a scale of 0 to10. Sensory neuropathy (numbness and tingling) was associated with a 1.4-point decrease in overall HRQoL. These are substantial effects, as a change of 0.3 points on this scale could be considered clinically important.35
From a methodological standpoint, the global HRQoL scale (anchored by worst possible health = 0 and perfect health = 10) can be considered to measure health values, and could be converted to health utilities for the purpose of a decision analysis.36 It is worth noting that the scale correlated significantly with objective measures of arm morbidity. In our previous report, this scale was highly sensitive to the impact of axillary surgery.13
Our results are generally consistent with previous studies of PROs in women undergoing breast cancer surgery. Hack and colleagues1 reported a survey of 222 women with AD in which self-reported pain/discomfort and the sensation point of pain/discomfort on arm movement (as assessed by a physiotherapist) were significantly related to a global HRQoL index. Neuropathy and edema were not evaluated. The largest study to examine the impact of arm edema on HRQoL in a population-based cohort of breast cancer survivors was the Iowa Women’s Health Study.2 Scores on the physical component of the SF-36 (36-item Short Form) health survey were worse among women with lymphedema than among those without lymphedema or symptoms. However, lymphedema was measured by self-report, and the authors did not report a comparison of women with edema vs without edema while they controlled for symptoms. Arm mobility was not assessed.
A few recent studies in breast cancer survivors included measures of both arm mobility and edema. Rietman et al3 studied 55 women at a mean of 2.7 years after breast cancer surgery with AD, in which pain was a significant predictor of 6 domains of the SF-36 and arm volume was a predictor of 2 domains, but active ROM was not significant. Nesvold and colleagues4 studied the associations of restricted shoulder abduction and lymphedema with multiple domains of the SF-36 in 256 breast cancer patients with lymph node metastases and AD at a mean of 4.1 years post surgery. In this multivariable analysis, all domains of the SF-36 were significantly associated with having impaired shoulder abduction, whereas none of the associations with lymphedema were statistically significant. In a study by Kaya and colleagues6 among 67 breast cancer patients, arm pain on motion, anterior chest wall pain, loss of grip strength, and shoulder flexion were related to various domains of the FACT (Functional Assessment of Cancer Therapy)–B+4, a cancer-specific measure of HRQoL.
Descriptive results from our study as shown in Table 2 can serve as a benchmark for both clinicians and patients. Because our analysis was done in the full B-32 sample (SNR and AD), the effect of surgery was less severe than would have been expected in a sample of women undergoing conventional AD. However, SNR is becoming a common procedure.7,8 Furthermore, because the study sample was selected from the CCOP sites, the results are representative of patients treated by community physicians. Important additional advantages of our study are a large sample, high follow-up rate, objective measures of arm mobility and edema, and comprehensive assessment of PROs including symptoms in the upper extremity, breast, and chest; arm function; limitations in work/other daily activities and social/recreational participation; and overall HRQoL.
Some limitations of the study should be mentioned. Although the assessment of PROs was comprehensive, some measures employed in the current study – such as limitations in social/recreational participation and work/other regular daily activities – were difficult to quantify and were assessed by a single item (overall rating) for each. Although these items were previously validated, the ability of such instruments to capture the complex constructs being measured is limited. Another important limitation of the study is that arm mobility and volume were both measured at the same time only once during the follow-up (at 6 months), which limited the multivariable analysis of the impact of these factors to a single time point. (The time point of greatest clinical interest had been specified a priori as 6 months.)
In conclusion, a substantial proportion of women with early breast cancer complain of arm/breast symptoms and functional impairments 6 months after surgical treatment. Clinical measures of arm morbidity correlate with patient-reported measures of function and HRQoL, but the correlations are not strong, suggesting that both types of variables are important to assess. Sensory neuropathy and restrictions in arm mobility are key predictors of social, recreational, work, and other regular daily activity limitations as well as overall HRQoL after breast cancer surgery.
Acknowledgments
Supported by: Grants U10-CA-12027, U10-CA-69651, U10-CA-37377, and U10-CA-69974 from the National Cancer Institute, Department of Health and Human Services, Public Health Service. The funding source had no further role.
Footnotes
The authors retain the right to provide a copy of the final manuscript to the NIH upon acceptance for journal publication, for public archiving in PubMed Central as soon as possible but no later than 12 months after publication by the journal.
CONFIDENTIAL
Not to be distributed or submitted without explicit permission of the NSABP Operations Office.
The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hack TF, Cohen L, Katz J, Robson LS, Goss P. Physical and psychological morbidity after axillary lymph node dissection for breast cancer. J Clin Oncol. 1999;17(1):143–149. doi: 10.1200/JCO.1999.17.1.143. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed RL, Prizment A, Lazovich DA, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol. 2008;26(35):5689–5696. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rietman JS, Dijkstra PU, Debreczeni R, Geertzen JH, Robinson DP, DeVries J. Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. Disabil Rehabil. 2004;26(2):78–84. doi: 10.1080/09638280310001629642. [DOI] [PubMed] [Google Scholar]
- 4.Nesvold IL, Fosså SD, Holm I, Naume B, Dahl AA. Arm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of life. Acta Oncol. 2010;49(3):347–353. doi: 10.3109/02841860903302905. [DOI] [PubMed] [Google Scholar]
- 5.Hormes JM, Bryan C, Lytle LA, et al. Impact of lymphedema and arm symptoms on quality of life in breast cancer survivors. Lymphology. 2010;43(1):1–13. [PubMed] [Google Scholar]
- 6.Kaya T, Karatepe AG, Günaydn R, Yeti H, Uslu A. Disability and health-related quality of life after breast cancer surgery: relation to impairments. South Med J. 2010;103(1):37–41. doi: 10.1097/SMJ.0b013e3181c38c41. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Niland JC, Bookman MA, et al. Emergence of sentinel node biopsy in breast cancer as standard of care in academic comprehensive cancer centers. J Natl Cancer Inst. 2003;95(20):1514–1521. doi: 10.1093/jnci/djg076. [DOI] [PubMed] [Google Scholar]
- 8.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23(30):7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial [published correction appears in J Natl Cancer Inst. 2006;98(12):876] J Natl Cancer Inst. 2006;98(9):599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 10.Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95(3):279–293. doi: 10.1007/s10549-005-9025-7. [DOI] [PubMed] [Google Scholar]
- 11.Zavagno G, De Salvo GL, Scalco G, et al. A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg. 2008;247(2):207–213. doi: 10.1097/SLA.0b013e31812e6a73. [DOI] [PubMed] [Google Scholar]
- 12.Gill G SNAC Trial Group of the Royal Australasian College of Surgeons (RACS) and NHMRC Clinical Trials Centre. Sentinel lymph node–based management or routine axillary clearance? One-year outcomes of sentinel node biopsy versus axillary clearance (SNAC): a randomized controlled surgical trial. Ann Surg Oncol. 2009;16(2):266–275. doi: 10.1245/s10434-008-0229-z. [DOI] [PubMed] [Google Scholar]
- 13.Land SR, Kopec JA, Julian TB, et al. Patient-reported outcomes in sentinel node–negative adjuvant breast cancer patients receiving sentinel-node biopsy or axillary dissection: National Surgical Adjuvant Breast and Bowel Project phase III protocol B-32 [published correction appears in J Clin Oncol. 2010;28(36):5350] J Clin Oncol. 2010;28(25):3929–3936. doi: 10.1200/JCO.2010.28.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102(2):111–118. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy as a staging procedure in breast cancer: Update of a randomised controlled study. Lancet Oncol. 2006;7(12):983–990. doi: 10.1016/S1470-2045(06)70947-0. [DOI] [PubMed] [Google Scholar]
- 16.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 17.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005;23(19):4312–4321. doi: 10.1200/JCO.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 18.Schrenk P, Rieger R, Shamiyeh A, Wayand W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer. 2000;88(3):608–614. doi: 10.1002/(sici)1097-0142(20000201)88:3<608::aid-cncr17>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Haid A, Kuehn T, Konstantiniuk P, et al. Shoulder-arm morbidity following axillary dissection and sentinel node only biopsy for breast cancer. Eur J Surg Oncol. 2002;28(7):705–710. doi: 10.1053/ejso.2002.1327. [DOI] [PubMed] [Google Scholar]
- 20.Burak WE, Hollenbeck ST, Zervos EE, Hock KL, Kemp LC, Young DC. Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Am J Surg. 2002;183(1):23–27. doi: 10.1016/s0002-9610(01)00848-0. [DOI] [PubMed] [Google Scholar]
- 21.Rietman JS, Dijkstra PU, Geertzen JH, et al. Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancer. Ann Surg Oncol. 2004;11(11):1018–1024. doi: 10.1245/ASO.2004.03.512. [DOI] [PubMed] [Google Scholar]
- 22.Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245(3):452–461. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Husted Madsen A, Haugaard K, Soerensen J, et al. Arm morbidity following sentinel lymph node biopsy or axillary lymph node dissection: a study from the Danish Breast Cancer Cooperative Group. Breast. 2008;17(2):138–147. doi: 10.1016/j.breast.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel lymph node resection and conventional axillary lymph node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 25.Krag DN, Anderson SJ, Julian TB, et al. Sentinel lymph node resection compared with conventional axillary lymph node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muir SW, Corea CL, Beaupre L. Evaluating change in clinical status: reliability and measures of agreement for the assessment of glenohumeral range of motion. N Am J Sports Phys Ther. 2010;5(3):98–110. [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor R, Jayasinghe UW, Koelmeyer L, Ung O, Boyages J. Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther. 2006;86(2):205–214. [PubMed] [Google Scholar]
- 28.Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L. Breast cancer survivors: psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38(2):183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 29.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [published correction appears in Am J Ind Med. 1996;30(3):372]: The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29(6):602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Shimozuma K, Ganz PA, Petersen L, Hirji K. Quality of life in the first year after breast cancer surgery: rehabilitation needs and patterns of recovery. Breast Cancer Res Treat. 1999;56(1):45–57. doi: 10.1023/a:1006214830854. [DOI] [PubMed] [Google Scholar]
- 31.Westrup JL, Lash TL, Thwin SS, Silliman RA. Risk of decline in upper-body function and symptoms among older breast cancer patients. J Gen Intern Med. 2006;21(4):327–333. doi: 10.1111/j.1525-1497.2006.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopec JA, Yothers G, Ganz PA, et al. Quality of life in operable colon cancer patients receiving oral compared with intravenous chemotherapy: results from National Surgical Adjuvant Breast and Bowel Project Trial C-06 [published correction appears in J Clin Oncol. 2007;25(34):5540–5541] J Clin Oncol. 2007;25(4):424–430. doi: 10.1200/JCO.2005.05.2597. [DOI] [PubMed] [Google Scholar]
- 33.Land SR, Kopec JA, Yothers G, et al. Health-related quality of life in axillary node–negative, estrogen receptor–negative breast cancer patients undergoing AC versus CMF chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel Project B-23. Breast Cancer Res Treat. 2004;86(2):153–164. doi: 10.1023/B:BREA.0000032983.87966.4e. [DOI] [PubMed] [Google Scholar]
- 34.de Boer AG, van Lanschot JJ, Stalmeier PF, et al. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004;13(2):311–320. doi: 10.1023/B:QURE.0000018499.64574.1f. [DOI] [PubMed] [Google Scholar]
- 35.Farivar SS, Liu H, Hays RD. Half standard deviation estimate of the minimally important difference in HRQOL scores? Expert Rev Pharmacoecon Outcomes Res. 2004;4(5):515–523. doi: 10.1586/14737167.4.5.515. [DOI] [PubMed] [Google Scholar]
- 36.Torrance GW, Feeny D, Furlong W. Visual analog scales: do they have a role in the measurement of preferences for health states? Med Decis Making. 2001;21(4):329–334. doi: 10.1177/0272989X0102100408. [DOI] [PubMed] [Google Scholar]