Abstract
Purpose
In general, tumor cells display a more glycolytic phenotype compared to the corresponding normal tissue. However, it is becoming increasingly clear that tumors are composed of a heterogeneous population of cells. Breast cancers are organized in a hierarchical manner, with the breast cancer stem cells (BCSCs) at the top of the hierarchy. Here, we investigate the metabolic phenotype of BCSCs and their differentiated progeny. In addition, we determine the effect of radiation on the metabolic state of these two cell populations.
Methods
Luminal, basal, and claudin-low breast cancer cell lines were propagated as mammospheres enriched in BCSCs. Lactate production, glucose consumption and ATP content was compared with differentiated cultures. A metabolic flux analyzer was used to determine the oxygen consumption, extracellular acidification rates, maximal mitochondria capacity and mitochondrial proton leak. The effect of radiation treatment of the metabolic phenotype of each cell population was also determined.
Results
BCSCs consume more glucose, produce less lactate and have higher ATP content compared to their differentiated progeny. BCSCs have higher maximum mitochondrial capacity and mitochondrial proton leak compared to their differentiated progeny. Radiation treatment enhances the higher energetic state of the BCSCs, while decreasing mitochondrial proton leak.
Conclusions
Our study indicated that breast cancer cells are heterogeneous in their metabolic phenotypes and BCSCs reside in a distinct metabolic state compared to their differentiated progeny. BCSCs display a reliance on oxidative phosphorylation, while the more differentiated progeny display a more glycolytic phenotype. Radiation treatment affects the metabolic state of BCSCs. We conclude that interfering with the metabolic requirements of BCSCs may prevent radiation-induced reprogramming of breast cancer cells during radiation therapy, thus improving treatment outcome.
Keywords: Cancer Stem Cells, Metabolism, Radiation
Introduction
Radiation therapy is a key component of multimodal treatment concepts against localized breast cancer. If applied after breast conserving surgery, radiotherapy improves loco-regional control as well as overall survival [1]. Despite recent advances in breast cancer radiotherapy techniques and drug treatments, about 7% of breast cancer patients still relapse loco-regionally indicating a need for further improvements [2].
Recent preclinical and clinical data have confirmed the heterogeneity of cells within breast tumors. Prospective identification of highly tumorigenic subpopulations of breast cancer cells has supported a hierarchical organization of breast cancer and the existence of a small number of breast cancer stem cells (BCSCs) [3]. These BCSCs have the unique ability to self-renew indefinitely, regrow a breast tumor and give rise to the different types of progeny found in the original tumor. Consequently, a successful anti-cancer therapy needs to eliminate all BCSCs in order to cure a patient [4].
In the 1920s, Otto Warburg reported that cancer cells, in contrast with most normal tissues, do not metabolize glucose mainly through oxidative phosphorylation, but rather through aerobic glycolysis (the “Warburg effect”) [5]. Despite the fact that aerobic glycolysis is less efficient in producing ATP, and requires 16 times more glucose than oxidative phosphorylation to produce the same amount of ATP, it provides rapidly growing cancer cells with metabolites needed for the synthesis of macro-molecules during doubling of the cell mass during mitosis. This general difference between most cancer cells and normal cells has led to the development of PET imaging for visualization of metabolically active tumor cells [6]. Naturally, this profound difference in the metabolic state of cancer cells and normal cells has stirred interest in designing drugs that specifically target the aerobic glycolysis pathway.
We recently reported that BCSCs contain a large number of quiescent cells that lack the need for massive macromolecule synthesis. However, when exposed to ionizing radiation, quiescent BCSCs were redistributed into the cell cycle [7]. We have also reported that the bulk of the tumor cells in glioblastoma multiforme (GBM) differ in their metabolic requirements from GBM stem cells.
In the present report, we hypothesize that BCSCs would differ from their differentiated progeny with respect to their metabolic state and that irradiation would interfere with glucose metabolism in BCSCs.
Material and Methods
Cell culture
Human SUM159PT breast cancer cell line was purchased from Asterand (Detroit, MI). Human MCF-7, T47D and MDA-MB-231 breast cancer cell lines were purchased from American Type Culture Collection (Manassas, VA). SUM159PT-ZsGreen-cODC, MCF-7-ZsGreen-cODC, T47D-ZsGreen-cODC, and MDA-MB-231-ZsGreen-cODC were obtained as described in Vlashi et al. [8]. SUM159PT cells were cultured in log-growth phase in F12 Medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum [Sigma Aldrich, St Louis, MO] and penicillin (100 units/ml) and streptomycin (100 µg/ml) cocktail [Invitrogen], 5 µg/mL insulin (Eli Lilly, Indianapolis, IN) and 1 µg/ml hydrocortisone (Pfizer, New York, NY). T47D and MDA-MD-231 cells were cultured in log-growth phase in Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and penicillin (100 units/ml) and streptomycin (100 µg/ml) cocktail. MCF7 cells were cultured in log-growth phase in Minimum Essential Media (MEM) Alpha (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and penicillin (100 units/ml) and streptomycin (100 µg/ml) cocktail, non-essential amino acids (1×) (NEAA, Invitrogen), 1mM sodium pyruvate (Invitrogen) and 5 µg/mL insulin (Eli Lilly, Indianapolis, IN). All cells were grown in a humidified incubator at 37°C with 5% CO2.
Oxygen Consumption and Extracellular Acidification Rate
The oxygen consumption and extracellular acidification rates of monolayers versus mammospheres were determined using the Seahorse XF Extracellular Flux Analyzer (Seahorse Bioscience Inc., North Billerica, MA). The Extracellular Flux Analyzer allows for analyzing oxygen consumption and extracellular acidification rates of a defined number of cells in a defined small volume of culture media in real-time and for monitoring their response to drug treatment. An overview of the technique has been previously described (11). 24-well plates (Seahorse Bioscience Inc.) were coated with laminin (Sigma) as described previously in order to allow the single cells derived from mammosphere cultures to attach for this assay without differentiating [9]. Briefly, each well of the 24-well plate was coated with 50 µL laminin diluted in PBS (10 µg/mL) overnight. The next day, the wells were washed three times with PBS, and cells from monolayer or sphere cultures were plated at a density of 80,000 cells/well and allowed to attach overnight in either monolayer, or mammosphere media. The following day, the adherent cells were washed and fresh media was added. The sensor cartridge was loaded to dispense three metabolic inhibitors sequentially at specific time points: oligomycin (inhibitor of ATP synthase, 1 µM), followed by FCCP (a protonophore and uncoupler of mitochondrial oxidative phosphorylation, 0.5 µM), followed by the addition of a combination of rotenone (mitochondrial complex I inhibitor, 1 µM) and myxothiazol (inhibitor of cytochrome C reductase, 100 nM). Basal oxygen consumption rate (OCR) and extracellular acidification rates (ECAR) were measured as well as the changes in oxygen consumption caused by the addition of the metabolic inhibitors described above. Several parameters were deducted from the changes in oxygen consumption, such as: basal OCR, maximum mitochondrial capacity, and mitochondrial reserve capacity (=[maximum mitochondrial capacity] − [basal OCR]) as described previously [10] (Supplementary Fig.1A).
Lactate production assays
The amount of lactate present in the media was estimated using the Lactate Assay Kit (BioVision Research Products) according to the manufacturer’s instructions. The amount of lactate produced by the cells in each sample was calculated by subtracting the amount of lactate in the media (without cells) from the amount of lactate in the media from each sample.
Quantitative Reverse Transcription-PCR
Quantitative PCR was performed in the My iQ thermal cycler (Bio-Rad, Hercules, CA) using the 2× iQ SYBR Green Supermix (Bio-Rad). Ct for each gene was determined and ΔΔCt was calculated relative to the designated reference sample. Gene expression values were then set equal to 2−ΔΔCt as described by the manufacturer of the kit (Applied Biosystems). PCR primers were synthesized by Invitrogen and designed for the human sequence of PFK_P, PFK_M, PFK_L, PKM1/2 and PKM1 (Forward primer: GAPDH (Forward primer: 5’-CGATCAACTCACCGCCAACA-3’, and Reverse primer: 5’-GTGGCTTCATCTGCTTCCTGTC-3’) or RPLP0 (Forward primer: 5’-GTGATGTGCAGCTGATCAAGACT-3’, and Reverse primer: 5’-GATGACCAGCCCAAAGGAGA-3’) were used as loading control.
Irradiation
Cells grown as monolayers were sorted into ZsGreen-cODC-neg and ZsGreen-cODC-pos cells and plated on laminin-coated Seahorse plates at 40,000 cells/well. The next day the plates were irradiated at room temperature using an experimental X-ray irradiator (Gulmay Medical Inc. Atlanta, GA) at a dose rate of 2.789 Gy/min for the time required to apply a prescribed dose. Corresponding controls were sham irradiated.
Statistics
All data shown are represented as mean ± standard error mean (SEM) of at least 3 biologically independent experiments. A p-value of ≤0.05 in a two-sided paired t-test indicated a statistically significant difference.
Results
Breast cancer stem cells differ metabolically from their differentiated progeny
Recently, we reported that radiation treatment can reprogram breast cancer cells into breast cancer stem ceIls (BCSCs), and that these reprogramming events correlate with an increase in expression of reprogramming factors Oct4, Sox2, Nanog and Klf4 [11]. In an attempt to determine whether BCSCs are affected by hypoxic conditions, we tested the reprogramming of breast cancer cells under hypoxic conditions versus normoxia. Propagating breast cancer cells as mammospheres is a common method for enriching the cell cultures for breast cancer stem cells (BCSCs). However, mammosphere cultures contain not only BCSCs but also differentiated cells. Therefore, we used an imaging system recently developed by our laboratory to sort BCSCs from non-tumorigenic cells using Fluorescence Activated Cell Sorting (FACS). This system takes advantage of the fact that BCSCs lack 26S proteasome activity and therefore, accumulates a fusion protein of ZsGreen and the C-terminal degron of ornithine decarboxylase (cODC). In cells with intact proteasome function, cODC directs the fusion protein to ubiquitin-independent proteasomal degradation. This allows for detection of BCSCs without the need for additional staining, which could potentially perturb metabolic function. We have demonstrated that this fluorescent reporter identifies breast cancer cells with a stem cell phenotype [7, 8, 11] and that breast cancer cells with low proteasome activity overlap with cells expressing high levels of ALDH1 and cells with a CD24−/low/CD44high phenotype, markers commonly used for identifying BCSCs [11]. Furthermore, more recent data indicate that breast cancer cells with low proteasome activity (ZsGreen-cODC-pos) are significantly more tumorigenic compared to the ZsGreen-cODC-neg cells [12], and are necessary for tumor initiation and maintenance [13]. Initially, we characterized the intrinsic numbers of cells with low proteasome activity (ZsGreen-cODC-pos) in four different breast cancer cell lines: two luminal cell lines, MCF-7 and T47D, a basal cell line MDA-MB-231 and the claudin-low cell line, SUM159PT. The percentage of ZsGreen-cODC-pos cells increased in the more aggressive breast cancer lines, MDA-MB-231 and SUM159PT (Fig. 1A). Next, we determined if the percentage of ZsGreen-cODC-pos cells in these lines is increased after a single dose of radiation treatment. As shown in Fig. 1B, radiation treatment indeed increases the number of BCSCs in a dose-dependent manner in all four cell lines tested. Motivated by recent reports demonstrating that BCSCs are stimulated in a hypoxic tumor microenvironment [14], we investigated if hypoxic conditions further enhance the radiation-induced enrichment of BCSCs. To our surprise, hypoxic conditions partially prevented the radiation-induced enrichment of BCSCs (Fig. 1C). These surprising results led us to hypothesize that the metabolism of breast cancer stem cells differs from that of the differentiated cells, given that hypoxic conditions affect the metabolism of a cell.
Figure 1. Radiation-induced enrichment of breast cancer stem cells favors normoxia.
Four different breast cancer cell lines, MCF7, MDA-MB-231, T47D and SUM159PT stably expressing the fusion protein ZsGreen-cODC were grown in normoxic or hypoxic conditions for 5 days. On day 6, the percentage of BCSCs (ZsGreen-cODC-pos) cells was analyzed using FACS. (A) The percentage of ZsGreen-cODC-pos cells increases in more aggressive breast cancer lines (triple-negative, MDA-MB-231 and claudin-low, SUM159PT) compared to the luminal lines (MCF7 and MDA-MB-231). In all four lines analyzed under normoxia (B) the percentage of ZsGreen-cODC-pos cells was increased in a dosedependent manner after radiation treatment. (C) Hypoxia (dotted lines) significantly reduced the efficiency of the radiation-induced enrichment of BCSCs (ZsGreen-cODC-pos) in all the lines.
In an attempt to acquire a better understanding of the metabolic state of BCSCs we first compared lactate production levels in all four breast cancer lines. Cells were seeded at clonal densities into ultra-low adhesion plates under serum-free conditions to allow formation of mammospheres and to enrich for BCSCs [15]. Lactate production from mammosphere cultures was compared to the corresponding differentiated cells cultured as monolayers. Lactate production was significantly decreased under mammosphere conditions that favor selection for BCSCs (Fig. 2A). Next, we tested if these results correlated with glucose uptake. Interestingly, mammosphere cultures utilized significantly more glucose than the differentiated monolayer cultures (Fig. 2B–C). In order to determine whether there was a qualitative difference in functional mitochondria in the BCSC population in these cell lines compared to the differentiated cells, we labeled the mitochondria of all the cell lines expressing the ZsGreen-cODC reporter for BCSCs, with a mitochondria-specific red-fluorescent dye (MitoTracker CMXRos) and analyzed the mitochondria-associated fluorescence via Flow Cytometry. When the ZsGreen-cODC-pos population was compared to ZsGreen-cODC-neg population of cells in mammosphere cultures of all four cell lines, the mitochondria specific dye consistently labeled the BCSCs (ZsGreen-cODC-pos) more efficiently than the non-stem cell (ZsGreen-cODC-neg) population (Fig. 2D). These results suggested that BCSCs may have a greater number of mitochondria. However, it should be noted that the accumulation of the red-fluorescent MitoTracker Red CMXRos depends on the mitochondrial membrane potential. Therefore, the latter results could alternatively suggest that BCSCs have more efficient mitochondria thus, making them more efficient at performing oxidative phosphorylation. Finally, we measured the ATP content of cells propagated in mammosphere cultures, and found that they had more than a two-fold increase in ATP content compared to the monolayer cultures (Fig. 2E). Taken together, these results suggested that the BCSCs reside in a different metabolic state compared to their more differentiated progeny.
Figure 2. Breast cancer stem cells produce less lactate, consume more glucose, and have higher ATP levels than their differentiated progeny.
Four breast cancer lines were propagated as monolayers or mammospheres and their lactate production (A) and glucose consumption (B–C) from the same number of cells was measured (n≥3). (B) The glucose uptake in mammospheres was compared to that of monolayers by measuring labeling with the fluorescent glucose analogue, 2-NBDG, for 15 minutes via Flow Cytometry (n≥3). (C) Representative FACS analysis for the uptake of 2-NBDG. (D) Breast cancer cells stably expressing the ZsGreen-cODC reporter for BCSCs were labeled with a mitochondria specific red fluorescent dye (MitoTracker-RED CMXRos), and the red fluorescence associated with the ZsGreen-cODC-neg cells was compared with the ZsGreen-cODC-pos cells. (E) The ATP content of BCSCs enriched in mammosphere cultures is higher compared to the ATP content of differentiated cells in monolayer cultures.
Breast cancer stem cells up-regulate phosphofructokinase
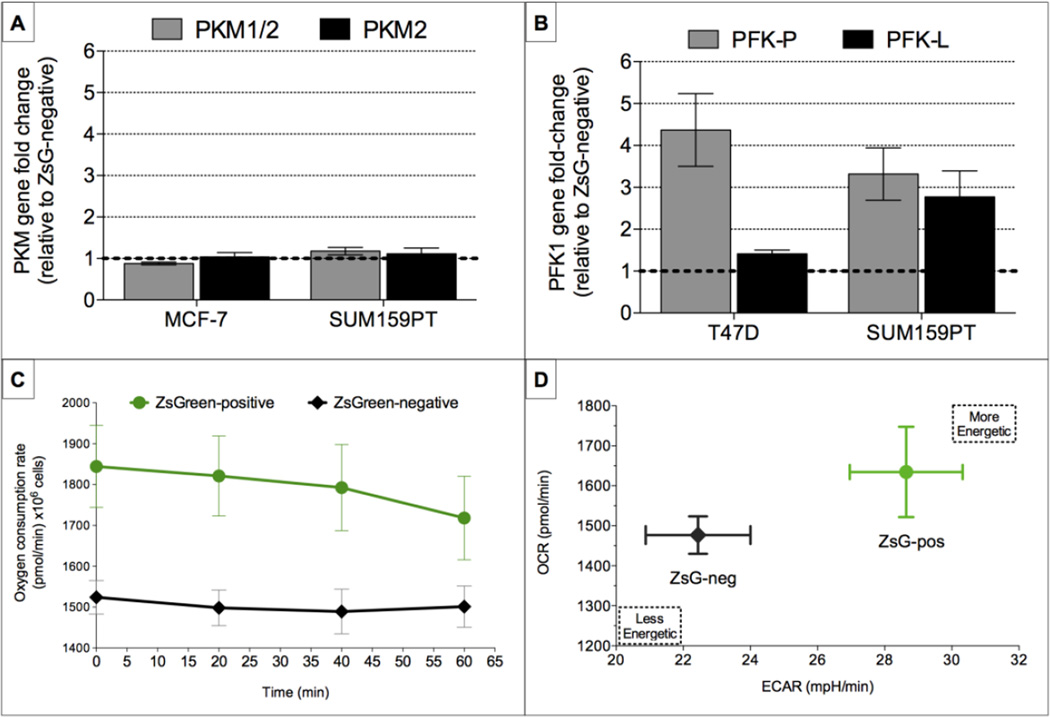
In an attempt to explore the underlying mechanism of the observed differences between the bulk of the breast cancer cells and BCSCs, we measured the gene expression levels of two pyruvate kinase muscle isozymes - PK-M1 and PK-M2 - key enzymes that have been implicated in the Warburg effect [16, 17]. Surprisingly, we were not able to detect differences in the expression levels of both enzymes between BCSCs and non-tumorigenic cells, in a luminal breast cancer line (MCF7) and the claudin-low line (SUM159PT) (Fig. 3A).
Figure 3. Breast cancer stem cells overexpress two different isoforms of the glycolytic enzyme phosphofructokinase (PFK), but consume more oxygen and exist in a more energetic state.
Relative expression levels of two glycolytic enzymes, (A) the two muscle isoforms of the pyruvate kinase (PKM1/2 and PKM2, A), and (B) isoforms P and L of phosphofructokinase (PFK-P and PFK-L, B) in ZsGreen-cODC-pos breast cancer cells are compared to the ZsGreen-cODC-negative population. (C) Breast cancer stem cells with low proteasome activity (SUM159-ZsGreen-cODC-positive) consume more oxygen (C) and exist in a more energetic state (D) when compared to the ZsGreencODC- negative population. The same number of both populations of cells were plated on Seahorse plates and oxygen consumption and extracellular acidification rates were measured as a function of time.
However, a recent study demonstrated a link between the platelet isoform of the glycolytic enzyme, phosphofructokinase (PFK-P) and KLF4, a transcription factor important in maintaining stemness, as well as in reprogramming of somatic cells into iPSCs [18]. In these studies it was demonstrated that in breast cancer cells, KLF4 activates the transcription of the PFK-P gene directly by binding to its promoter [19]. To investigate the expression of the PFK gene in our system we performed qRT-PCR analysis in two different breast cancer lines in sorted differentiated cells (ZsGreen-cODC-neg) and sorted BCSCs (ZsGreen-cODC-pos). In both cases, two isoforms of the PFK1 enzyme were upregulated in the ZsGreen-cODC-pos population compared to the ZsGreen-cODC-neg population in the luminal breast cancer line, T47D and the claudin-low line, SUM159PT (Fig. 3B).
Next, in order to determine whether the glucose molecules committed to glycolysis are oxidized all the way to lactate or are shuttled into the mitochondria for oxidative phosphorylation, we measured oxygen consumption rates in the SUM159PT-ZsGreen-cODC-pos cells versus ZsGreen-cODC-neg cells using a metabolic flux analyzer. Indeed, if left undisturbed the ZsGreen-cODC-pos BCSC population consumed more oxygen compared to the ZsGreen-cODC-neg population (Fig. 3C). Finally, when the extracellular acidification rate was plotted against oxygen consumption rate for BCSCs and non-tumorigenic cells, BCSCs were more energetic than their non-tumorigenic progeny (Fig. 3D).
Effect of radiation treatment on the metabolic state of BCSCs
Next, we sought to examine the metabolic state of BCSCs more quantitatively using the metabolic flux analyzer and addition of a series of metabolic inhibitors, as described previously (Supplementary Fig. 1A) [20]. These experiments revealed that BCSCs and non-tumorigenic cells did not differ in ATP turnover, mitochondrial reserve capacity, or non-mitochondrial respiration (Supplementary Fig. 1B). However, BCSCs showed a significant increase in maximum mitochondrial capacity and mitochondrial proton leak (Fig. 4A). In general maximal mitochondrial capacity is associated with mitochondria content, therefore these results are in line with our observations that BCSCs have higher number of mitochondria (Fig. 2D). These results indicated again that BCSCs depend more on oxidative phosphorylation.
Figure 4. Radiation treatment changes the metabolic phenotype of breast cancer stem cells.
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured in sorted SUM159-ZsGreen-cODC-neg and SUM159-ZsGreen-cODC-pos cells in the absence and presence of a series of metabolic inhibitors as described in Vlashi et al. [20]. Maximum mitochondrial capacity and mitochondrial proton leak were calculated, and both these parameters were significantly higher in the BCSCs (ZsGreen-pos) compared to the non-tumorigenic cells (A). (B) A single dose of 8 Gy enhanced the energetic state of BCSCs (ZsGreen-pos), but it had no effect on the ZsGreen-neg population. (C) A single dose of 8 Gy did not have an effect on maximum mitochondrial capacity (C, left panel), however it significantly decreased mitochondrial proton leak (C, right panel).
We have recently reported that ionizing radiation reprogrammed non-tumorigenic breast cancer cells into induced BCSCs (induced-BCSCs) [11]. This reprogramming step was mediated by radiation-induced re-expression of the embryonic stem cell transcription factors Oct4, Sox2, Nanog, and Klf4 [21] and our data indicate that induced-BCSCs could be a major cause for treatment failure [11]. Interestingly, irradiation with a single dose of 8 Gy significantly enhanced the more energetic phenotype of BCSCs in an acute manner (30 min post irradiation exposure), while the metabolic state of non-tumorigenic cells was not affected by radiation treatment (Fig. 4B). Further analysis demonstrated that radiation treatment with a single dose of 8 Gy did not have an effect on the maximum mitochondrial capacity of non-tumorigenic cells or BCSCs (Fig. 4C, left panel) however, it significantly reduced the mitochondrial proton leak for both cell populations (Fig. 4C, right panel). Although surprising, these results indicate that radiation treatment restores mitochondrial membrane potential, thus improving oxidative phosphorylation efficiency in the ZsGreen-cODC-population, and making this population of cells more energetic, as shown in Fig. 4B.
Discussion
The Warburg effect is one of the most striking differences observed between normal tissues and tumors. The increased glucose uptake in tumors is now effectively used in PET-imaging in oncology for tumor detection and response to therapy. More recently, targeting metabolic pathways that confer cancer cells a survival advantage has become an area of increasing interest. The hope that metabolic differences between cancer cells and normal tissue will offer opportunities for therapeutic intervention is experiencing a rebirth since Warburg first described the unique metabolic state of cancer cells [22].
Pre-clinical and clinical data support a hierarchical organization of solid cancers with a small population of radiotherapy- and chemotherapy-resistant cancer stem cells that exhibit increased self-renewal capacity and the ability to re-grow a tumor after sublethal treatment. In breast cancer and glioma, a significant number CSCs are quiescent and only recruited into the pool of cycling cells during the repopulation of the tumor [7, 23]. The quiescent state of a cell is considered a catabolic state and associated with autophagy, suggesting that the metabolic pathways utilized in CSCs may differ from those in differentiated cells. We have previously reported that in glioma this is indeed the case [24]. Lagadinou et al. have reported similar results in leukemia, and were able to use the dependency of quiescent leukemia stem cells on mitochondrial metabolism for their elimination [25].
Here, we report that, similar to glioma, breast cancer stem cells also depend more on oxidative phosphorylation for their energy needs. The experimental evidence suggesting that BCSCs depend more on oxidative phosphorylation are several-fold. BCSCs, (1) produce less lactate than their differentiated progeny (Fig. 2A), (2) have a higher number of mitochondria or more functional mitochondria (as determined by the mitochondria-specific fluorescent stain MitoTracker-RED CMXRos (Fig. 2D)), and (3) have higher ATP content (Fig. 2E), which correlates with higher oxygen consumption rates (Fig. 3C), and a more energetic state (Fig. 3D) (Schematic 1). Interestingly, to our surprise, BCSCs in our system also consumed more glucose than their differentiated counterparts (Fig. 2B–C) behaving more like a cell with a glycolytic phenotype. However, while there was no difference in relative expression of the M1 and M2 isoforms of pyruvate kinase, a glycolytic enzyme that has been implicated in the Warburg effect [16], there was a significant difference in the relative expression of the phosphofructokinase (PFK)-P and L isoforms. PFK is a glycolytic enzyme that mediates a commitment step in glycolysis. Therefore, taken together these results indicate that BCSCs are not only taking up more glucose and are committing more of it down the glycolytic pathway, but are also shuttling more of the resulting pyruvate into the mitochondria, as indicated by the lower lactate production and increased oxygen consumption rates, mitochondrial numbers/mass and ATP content in BCSCs compared to their differentiated progeny (Schematic 1). Previously, we have reported that glioblastoma multiforme (GBM) stem cells exhibited higher lactate production, less glucose consumption and lower extracellular acidification rate [20], results that seem contradictory to the findings reported here for BCSCs. It should be noted that lactate production levels in the current study were measured in breast cancer cells propagated in differentiation media (as monolayers) and compared to cultures propagated as mammospheres enriched in BCSCs. In contrast, the GBM stem cell study compared ZsGreen-cODC-neg and ZsGreen-cODC-pos cells sorted from neurosphere cultures enriched in GBM-SCs, thus these two data sets cannot be directly compared and do not necessarily contradict each other. In the case of glucose consumption, both studies (in breast and GBM) compared differentiated cultures (monolayers) with CSC-enriched cultures (mammospheres in breast, and neurospheres for GBM). However, we indeed observed opposite results, as mammosphere cultures had higher glucose uptake compared to their differentiated cultures, while in glioma neurosphere cultures consumed less glucose than the corresponding differentiated glioma cultures. Such a discrepancy cannot readily be explained, and further studies on levels of glucose transporters would be needed to shed light on these observed differences.
Schematic 1.
We recently reported that BCSCs, identified by our fluorescent reporter system, express higher levels of the Yamanaka transcription factors [18] responsible for inducing pluripotent stem cells (iPS) from differentiated cells [11], including Krüppel-like Factor (KLF4). Interestingly, a recent report demonstrates that KLF4 activates the transcription gene for the platelet isoform of phosphofructokinase (PFKP) in breast cancer [19]. The specific up-regulation of the PFKP enzyme in the BCSC population diverts most of the glucose away from the pentose phosphate shunt, which feeds into nucleotide synthesis. We have also previously demonstrated that the BCSCs with low proteasome activity exhibit a quiescent phenotype [7], and thus do not have a need for efficient nucleotide synthesis required for rapid proliferation. Furthermore, in general, cells that are not proliferating depend more on oxidative phosphorylation, and only switch to glycolysis upon requirements for rapid proliferation [26], which is consistent with our findings. One of the most interesting findings resulting from these studies was the observation that radiation treatment pushes BCSCs into a more energetic state while the non-stem cell population remains undisturbed. Further studies are necessary to shed light into the possible mechanisms for such a differential effect of radiation treatment on the metabolic state of BCSCs and the non-stem cell compartment of breast tumors.
In summary, our data have important implications in the context of studies aiming at targeting tumor cell metabolism while considering tumors as a bulk population of cells. We have now demonstrated via two different tumor models, previously in glioblastoma multiforme [20] and presently in breast cancer that cancer stem cells populations, within solid tumors that have a hierarchical organization, differ in their metabolic state from the differentiated progeny. Our studies demonstrate that breast cancer stem cells rely more on oxidative phosphorylation than glycolysis when compared to their more differentiated counterparts. The findings presented here add to the body of literature on the metabolic state of cancer stem cells. Other laboratories have reported metabolic differences in the tumor initiating cell population for different solid tumors [27–29], and there is contradicting evidence on the metabolic state of BCSCs [30]. It is evident that more studies are needed to further characterize the metabolic state of cancer stem cells to clearly define whether interference with the metabolic requirements of this important cancer cell population will translate into therapeutic benefit for cancer patients.
Supplementary Material
(A) Representative oxygen consumption rates measured as a function of time on a Seahorse platform, as different metabolic inhibitors are added to the cell media. (B) Several parameters were deducted from the changes in oxygen consumption (A), such as: basal OCR, maximum mitochondrial capacity, and mitochondrial reserve capacity (=[maximum mitochondrial capacity] − [basal OCR]) as described previously in [10]. BCSCs and non-tumorigenic cells did not differ in ATP turnover, mitochondrial reserve capacity, or non-mitochondrial respiration
Acknowledgements
FP was supported by a generous gift from Steve and Cathy Fink and by grants from the National Cancer Institute (1RO1CA137110, 1R01CA161294) and the Army Medical Research & Materiel Command’s Breast Cancer Research Program (W81XWH-11-1-0531).
LV and KR were supported by S10RR026744 (National Center for Research Resources) and P01 HL028481 (National Institutes of Health).
We would like to thank Ekaterini Angelis, PhD, for careful editing of this manuscript.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, Kamby C, Kjaer M, Gadeberg CC, Rasmussen BB, et al. Postoperative radiotherapy in high-risk postmenopausal breastcancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353(9165):1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Warburg O, Posener K, Negelein E. Ueber den Stoffwechsel der Tumoren. Biochemische Zeitschrift. 1924;152:319–344. [Google Scholar]
- 6.Ter-Pogossian MM, Phelps ME, Hoffman EJ, Mullani NA. A positronemission transaxial tomograph for nuclear imaging (PETT) Radiology. 1975;114(1):89–98. doi: 10.1148/114.1.89. [DOI] [PubMed] [Google Scholar]
- 7.Lagadec C, Vlashi E, Della Donna L, Meng Y, Dekmezian C, Kim K, Pajonk F. Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast cancer research : BCR. 2010;12(1):R13. doi: 10.1186/bcr2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlashi E, Kim K, Lagadec C, Donna LD, McDonald JT, Eghbali M, Sayre JW, Stefani E, McBride W, Pajonk F. In vivo imaging, tracking, and targeting of cancer stem cells. Journal of the National Cancer Institute. 2009;101(5):350–359. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, Bayani J, Head R, Lee M, Bernstein M, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4(6):568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Amo T, Yadava N, Oh R, Nicholls DG, Brand MD. Experimental assessment of bioenergetic differences caused by the common European mitochondrial DNA haplogroups H and T. Gene. 2008;411(1–2):69–76. doi: 10.1016/j.gene.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagadec C, Vlashi E, Della Donna L, Dekmezian C, Pajonk F. Radiation-induced reprogramming of breast cancer cells. Stem cells. 2012;30(5):833–844. doi: 10.1002/stem.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagadec C, Vlashi E, Alhiyari Y, Phillips TM, Dratver MB, Pajonk F. Radiation-Induced Notch Signaling in Breast Cancer Stem Cells. International journal of radiation oncology, biology, physics. 2013 doi: 10.1016/j.ijrobp.2013.06.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlashi E, Lagadec C, Chan M, Frohnen P, McDonald AJ, Pajonk F. Targeted elimination of breast cancer cells with low proteasome activity is sufficient for tumor regression. Breast cancer research and treatment. 2013;141(2):197–203. doi: 10.1007/s10549-013-2688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conley SJ, Gheordunescu E, Kakarala P, Newman B, Korkaya H, Heath AN, Clouthier SG, Wicha MS. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(8):2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65(13):5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 16.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 17.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452(7184):181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Moon JS, Kim HE, Koh E, Park SH, Jin WJ, Park BW, Park SW, Kim KS. Kruppel-like factor 4 (KLF4) activates the transcription of the gene for the platelet isoform of phosphofructokinase (PFKP) in breast cancer. The Journal of biological chemistry. 2011;286(27):23808–23816. doi: 10.1074/jbc.M111.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vlashi E, Lagadec C, Vergnes L, Matsutani T, Masui K, Poulou M, Popescu R, Della Donna L, Evers P, Dekmezian C, et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(38):16062–16067. doi: 10.1073/pnas.1106704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagadec C, Vlashi E, Della Donna L, Dekmezian C, Pajonk F. Radiation-induced Reprograming of Breast Cancer Cells. Stem Cells. 2012 doi: 10.1002/stem.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warburg O. On the metabolism of carcinoma cells. Biochem Z. 1924;152(309–344):309. [Google Scholar]
- 23.Vlashi E, Kim K, Lagadec C, Donna LD, McDonald JT, Eghbali M, Sayre JW, Stefani E, McBride W, Pajonk F. In vivo imaging, tracking, and targeting of cancer stem cells. J Natl Cancer Inst. 2009;101(5):350–359. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlashi E, Lagadec C, Vergnes L, Matsutani T, Masui K, Poulou M, Popescu R, Della Donna L, Evers P, Dekmezian C, et al. Metabolic state of glioma stem cells and nontumorigenic cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(38):16062–16067. doi: 10.1073/pnas.1106704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer KM, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell stem cell. 2013;12(3):329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148(1–2):259–272. doi: 10.1016/j.cell.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 28.Morfouace M, Lalier L, Bahut M, Bonnamain V, Naveilhan P, Guette C, Oliver L, Gueguen N, Reynier P, Vallette FM. Comparison of spheroids formed by rat glioma stem cells and neural stem cells reveals differences in glucose metabolism and promising therapeutic applications. The Journal of biological chemistry. 2012;287(40):33664–33674. doi: 10.1074/jbc.M111.320028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menendez JA, Joven J, Cufi S, Corominas-Faja B, Oliveras-Ferraros C, Cuyas E, Martin-Castillo B, Lopez-Bonet E, Alarcon T, Vazquez-Martin A. The Warburg effect version 2.0: metabolic reprogramming of cancer stem cells. Cell cycle. 2013;12(8):1166–1179. doi: 10.4161/cc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng W, Gentles A, Nair RV, Huang M, Lin Y, Lee CY, Cai S, Scheeren FA, Kuo AH, Diehn M. Targeting Unique Metabolic Properties of Breast Tumor Initiating Cells. Stem cells. 2014 doi: 10.1002/stem.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Representative oxygen consumption rates measured as a function of time on a Seahorse platform, as different metabolic inhibitors are added to the cell media. (B) Several parameters were deducted from the changes in oxygen consumption (A), such as: basal OCR, maximum mitochondrial capacity, and mitochondrial reserve capacity (=[maximum mitochondrial capacity] − [basal OCR]) as described previously in [10]. BCSCs and non-tumorigenic cells did not differ in ATP turnover, mitochondrial reserve capacity, or non-mitochondrial respiration