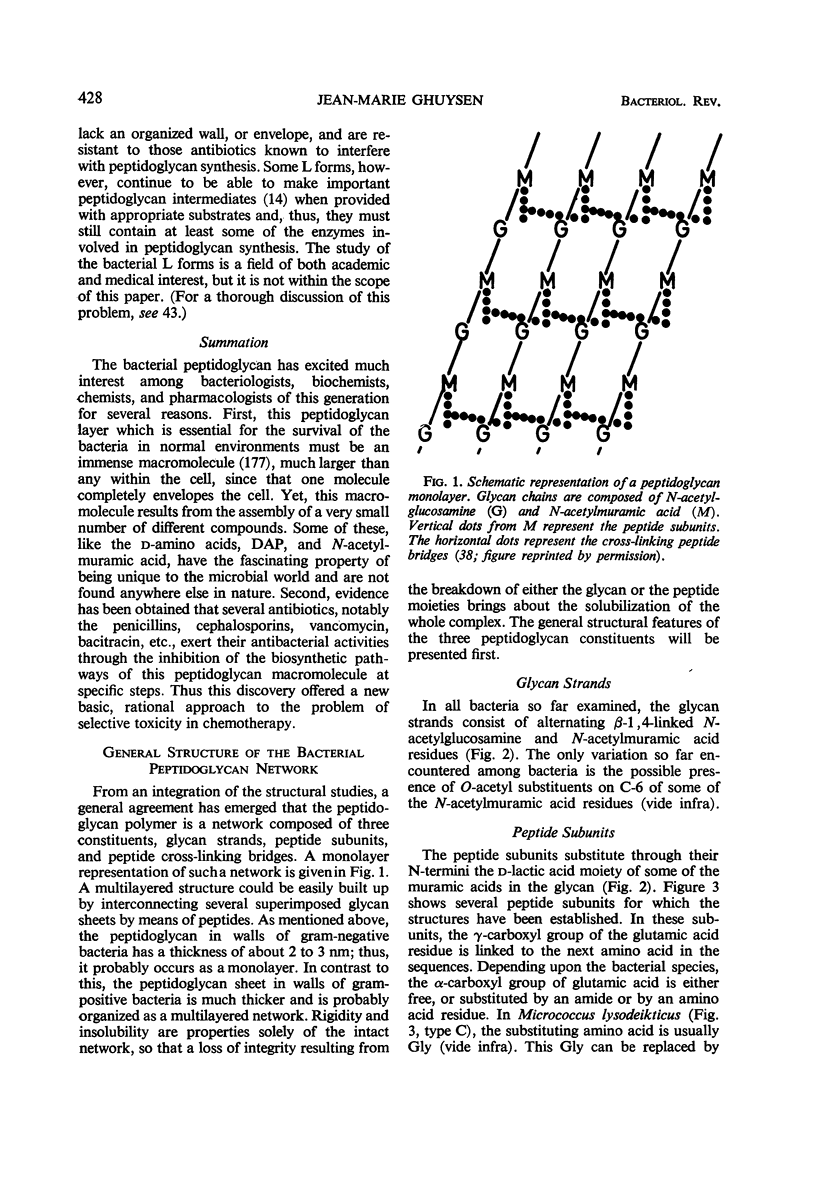
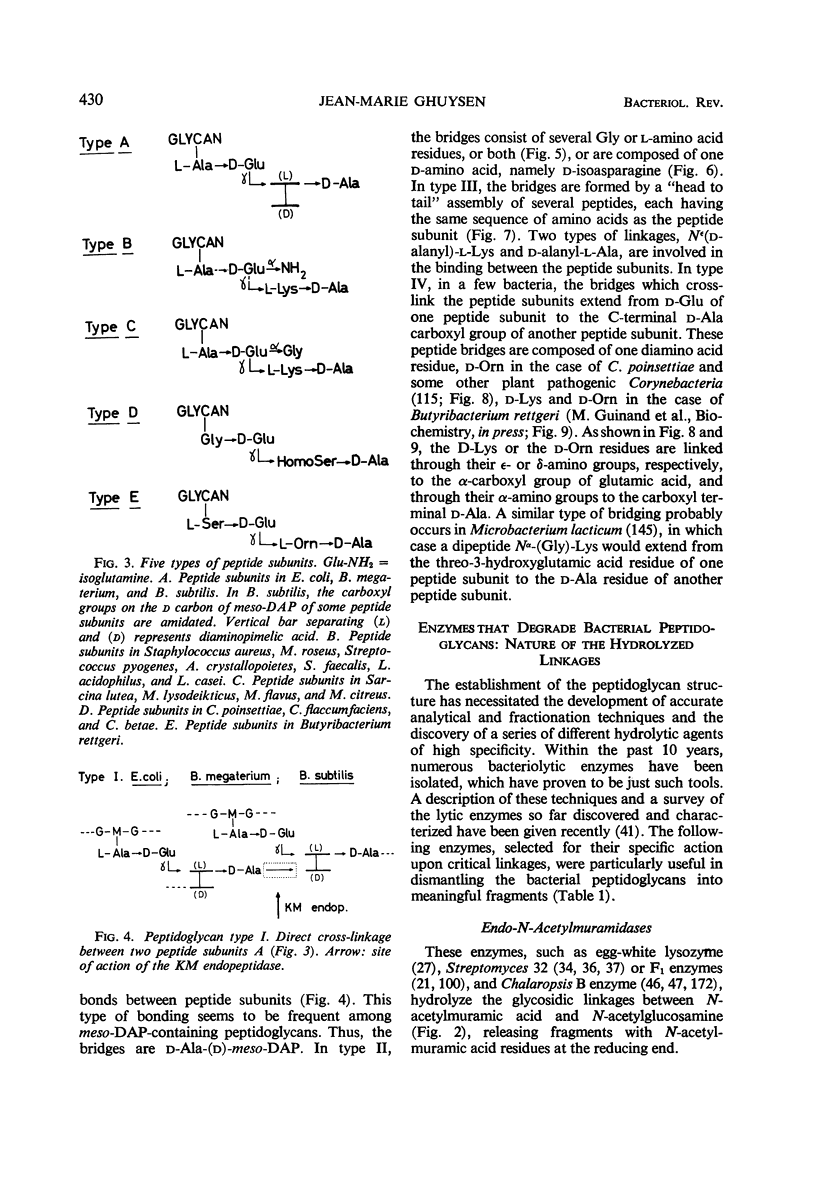
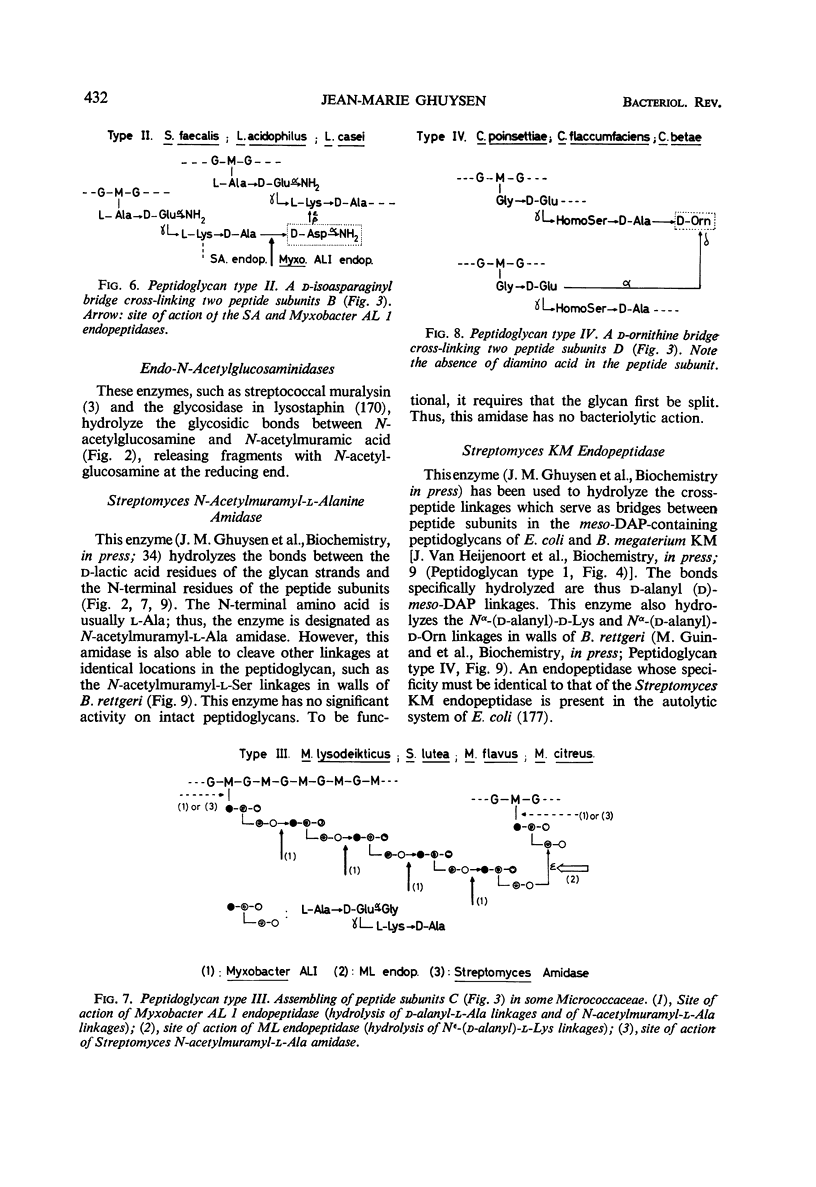
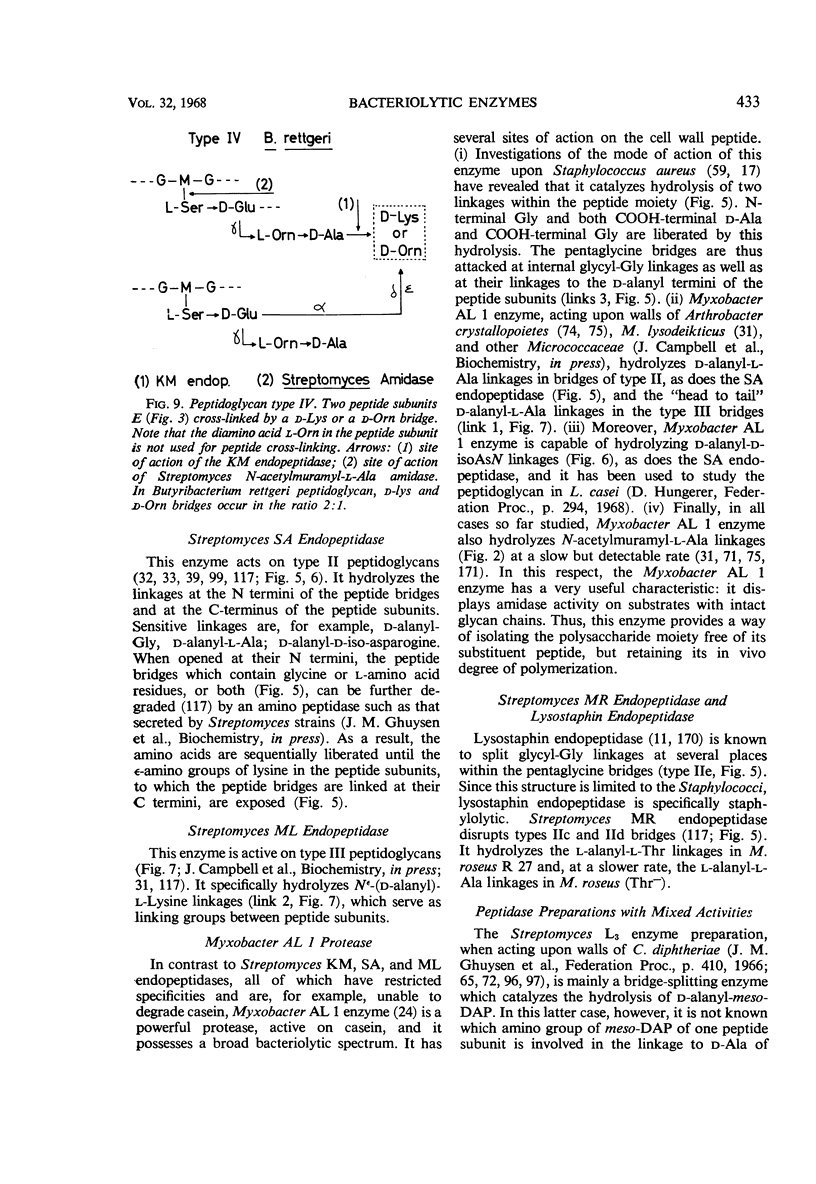
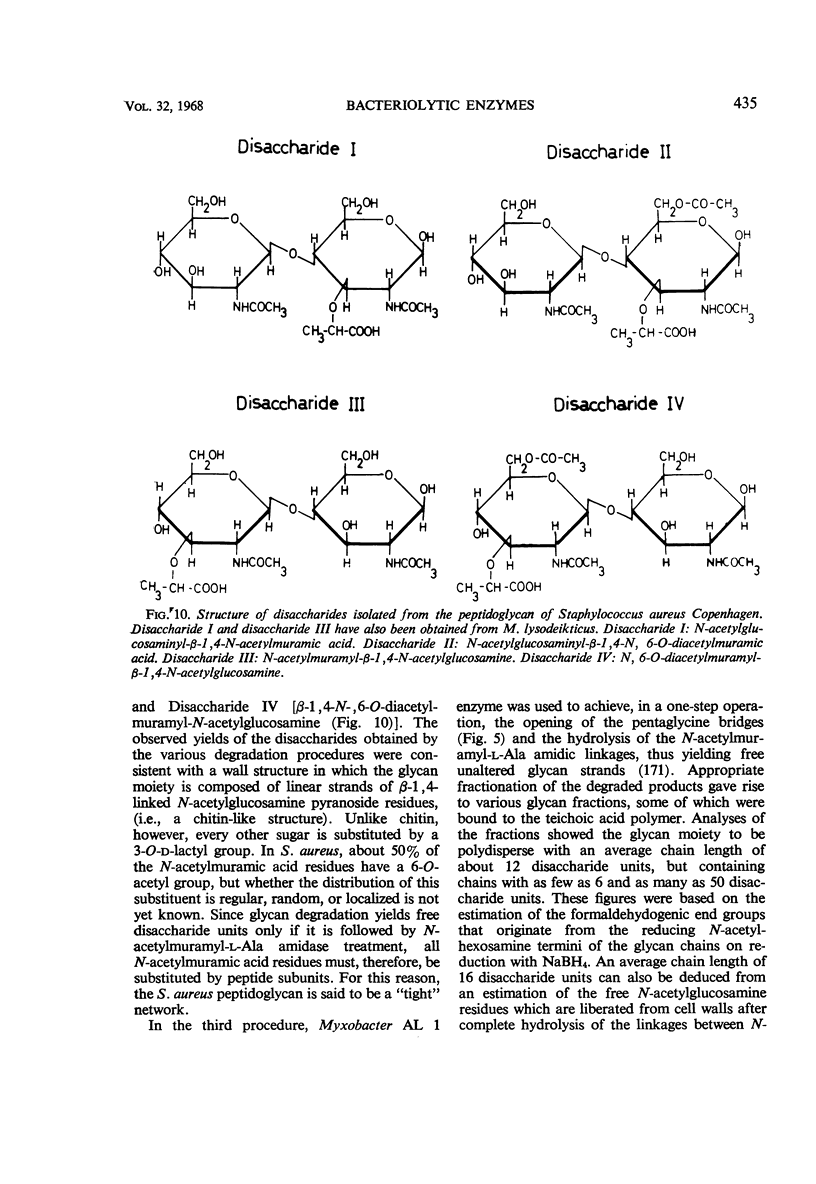
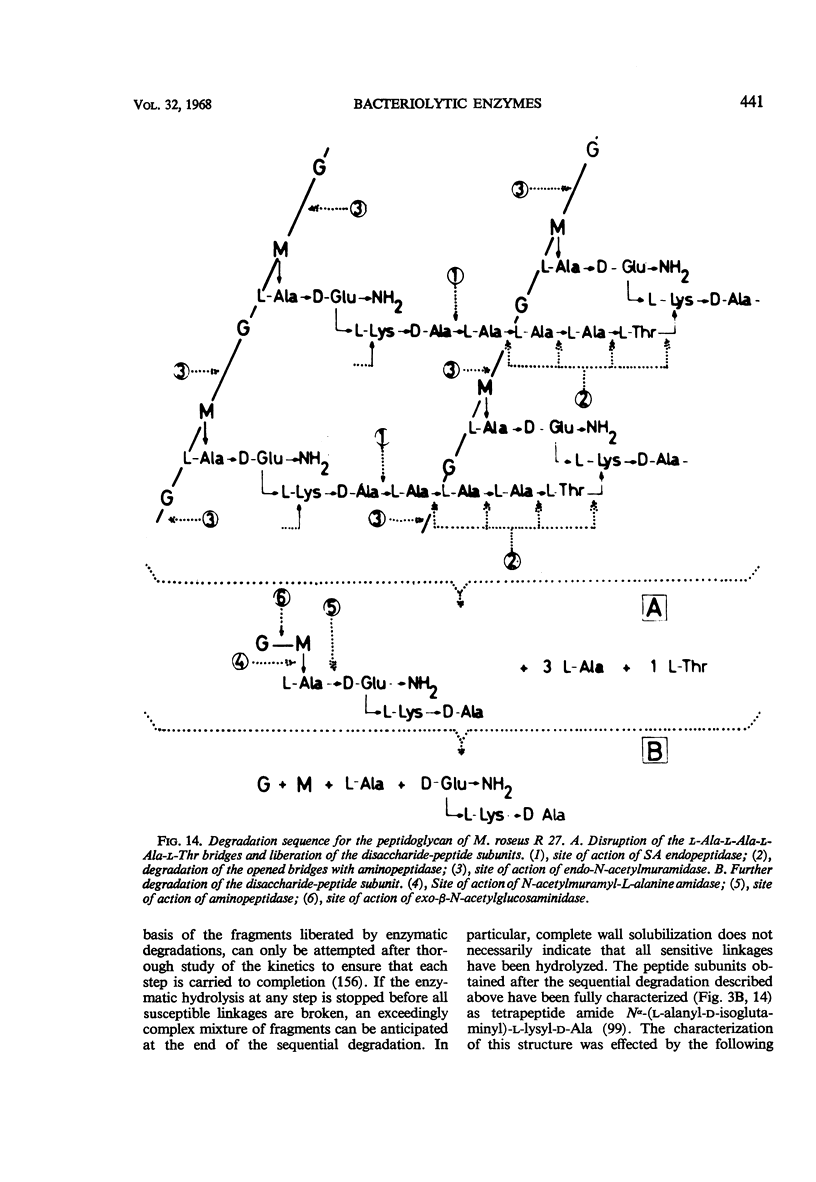
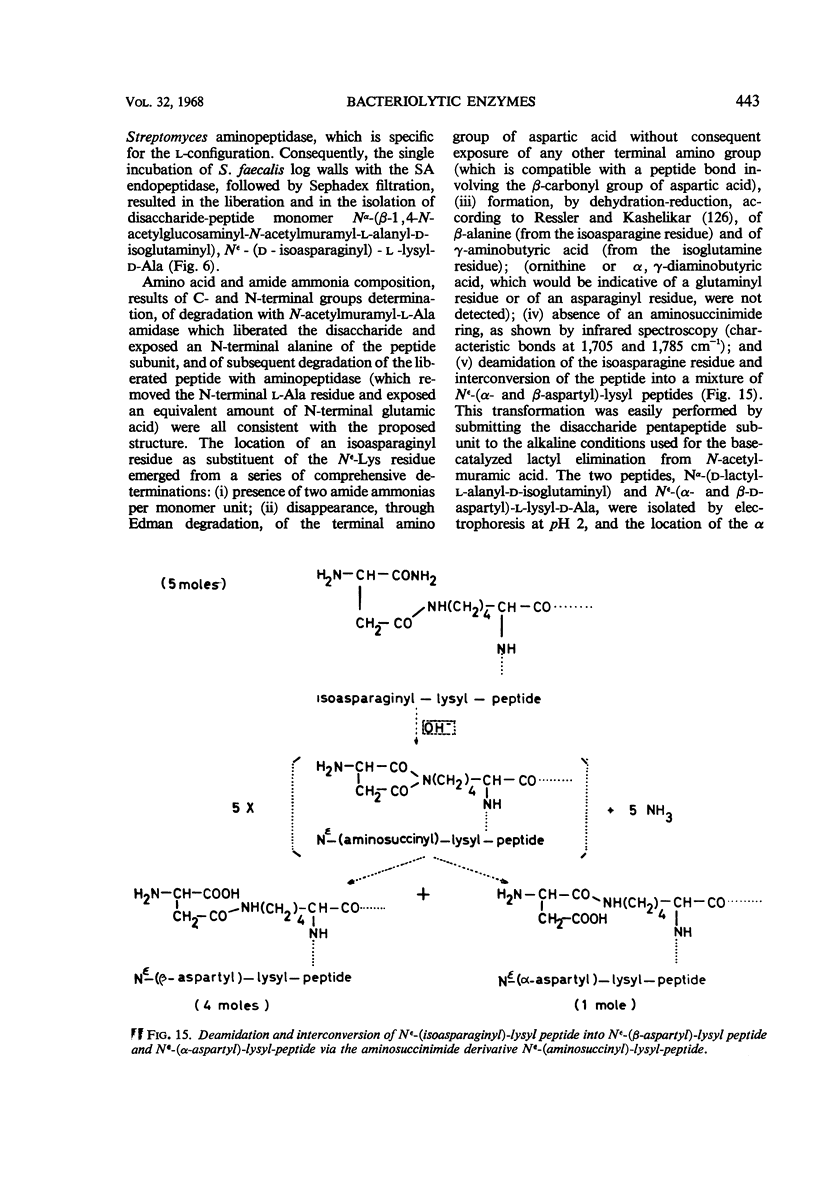
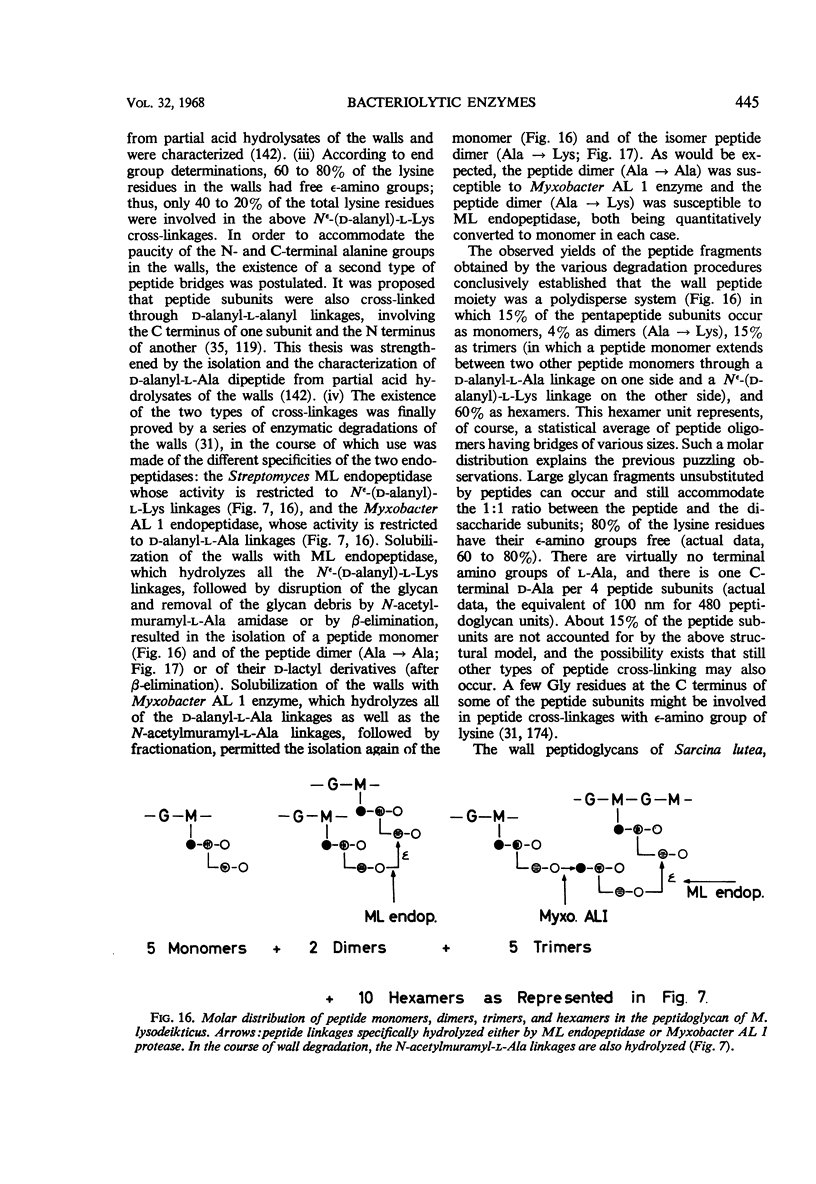
Full text
PDF






























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLSOP J WORK E. Cell walls of Propionibacterium species: fractionation and composition. Biochem J. 1963 Jun;87:512–519. doi: 10.1042/bj0870512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKULIS S. S., SMITH C., BOLTRALIK J. J., HEYMANN H. STRUCTURE OF STREPTOCOCCAL CELL WALLS. IV. PURIFICATION AND PROPERTIES OF STREPTOCOCCAL PHAGE MURALYSIN. J Biol Chem. 1964 Dec;239:4027–4033. [PubMed] [Google Scholar]
- BROWDER H. P., ZYGMUNT W. A., YOUNG J. R., TAVORMINA P. A. LYSOSTAPHIN: ENZYMATIC MODE OF ACTION. Biochem Biophys Res Commun. 1965 Apr 23;19:383–389. doi: 10.1016/0006-291x(65)90473-0. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Mair G. A., North A. C., Phillips D. C., Sarma V. R. On the conformation of the hen egg-white lysozyme molecule. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):365–377. doi: 10.1098/rspb.1967.0034. [DOI] [PubMed] [Google Scholar]
- Bricas E., Ghuysen J. M., Dezélée P. The cell wall peptidoglycan of Bacillus megaterium KM. I. Studies on the stereochemistry of alpha, alpha'-diaminopimelic acid. Biochemistry. 1967 Aug;6(8):2598–2607. doi: 10.1021/bi00860a043. [DOI] [PubMed] [Google Scholar]
- Bumsted R. M., Dahl J. L., Söll D., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. X. Further study of the glycyl transfer ribonucleic acids active in peptidoglycan synthesis in Staphylococcus aureus. J Biol Chem. 1968 Feb 25;243(4):779–782. [PubMed] [Google Scholar]
- Button D., Archibald A. R., Baddiley J. The linkage between teichoic acid and glycosaminopeptide in the walls of a strain of Staphylococcus lactis. Biochem J. 1966 May;99(2):11C–14C. doi: 10.1042/bj0990011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. J Gen Microbiol. 1956 Jul;14(3):583–600. doi: 10.1099/00221287-14-3-583. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. N., Ward J. B., Perkins H. R. Synthesis of mucopeptide by L-form membranes. Nature. 1967 Jun 24;214(5095):1311–1314. doi: 10.1038/2141311a0. [DOI] [PubMed] [Google Scholar]
- Chipman D. M., Grisaro V., Sharon N. The binding of oligosaccharides containing N-acetylglucosamine and N-acetylmuramic acid to lysozyme. The specificity of binding subsites. J Biol Chem. 1967 Oct 10;242(19):4388–4394. [PubMed] [Google Scholar]
- Chipman D. M., Pollock J. J., Sharon N. Lysozyme-catalyzed hydrolysis and transglycosylation reactions of bacterial cell wall oligosaccharides. J Biol Chem. 1968 Feb 10;243(3):487–496. [PubMed] [Google Scholar]
- Cole R. M. Symposium on the fine structure and replication of bacteria and their parts. 3. Bacterial cell-wall replication followed by immunofluorescence. Bacteriol Rev. 1965 Sep;29(3):326–344. doi: 10.1128/br.29.3.326-344.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. IX. Teichoic acid and phage adsorption. Biochemistry. 1968 Jun;7(6):2385–2389. doi: 10.1021/bi00846a048. [DOI] [PubMed] [Google Scholar]
- Cripps R. E., Work E. The accumulation of extracellular macromolecules by Staphylococcus aureus grown in the presence of sodium chloride and glucose. J Gen Microbiol. 1967 Oct;49(1):127–137. doi: 10.1099/00221287-49-1-127. [DOI] [PubMed] [Google Scholar]
- DIERICKX L., GHUYSEN J. M. [Purification of beta-(1-4)-N-acetylhexosaminidase secreted by Streptomyces albus G and active on the cell walls of Micrococcus lysodeikticus]. Biochim Biophys Acta. 1962 Mar 26;58:7–18. doi: 10.1016/0006-3002(62)90811-9. [DOI] [PubMed] [Google Scholar]
- Diringer H. Uber die Bindung der D-Glutaminsäure im Murein von E. coli. Z Naturforsch B. 1968 Jun;23(6):883–884. [PubMed] [Google Scholar]
- Dorer F. E., Haley E. E., Buchanan D. L. Quantitative studies of urinary beta-aspartyl oligopeptides. Biochemistry. 1966 Oct;5(10):3236–3240. doi: 10.1021/bi00874a025. [DOI] [PubMed] [Google Scholar]
- Ensign J. C., Wolfe R. S. Characterization of a small proteolytic enzyme which lyses bacterial cell walls. J Bacteriol. 1966 Feb;91(2):524–534. doi: 10.1128/jb.91.2.524-534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P., Hancock R. The initial structural lesion of penicillin action in Bacillus megaterium. J Cell Biol. 1965 Aug;26(2):657–667. doi: 10.1083/jcb.26.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHUYSEN J. M., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. I. PREPARATION OF FRAGMENTS BY ENZYMATIC HYDROLYSIS. Biochemistry. 1963 Sep-Oct;2:1110–1119. doi: 10.1021/bi00905a035. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. II. SEPARATION AND STRUCTURE OF DISACCHARIDES. Biochemistry. 1963 Sep-Oct;2:1119–1125. doi: 10.1021/bi00905a036. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M., TIPPER D. J., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. IV. THE TEICHOIC ACID-GLYCOPEPTIDE COMPLEX. Biochemistry. 1965 Mar;4:474–485. doi: 10.1021/bi00879a016. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M. [Data on the structure of disaccharide-peptide complexes liberated from the wall of Micrococcus lysodeikticus by the action of beta(1-4)N-acetylhexosaminidases]. Biochim Biophys Acta. 1961 Mar 4;47:561–568. doi: 10.1016/0006-3002(61)90551-0. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Bricas E., Lache M., Leyh-Bouille M. Structure of the cell walls of Micrococcus lysodeikticus. 3. Isolation of a new peptide dimer, N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanyl-N-alpha-[L-alanyl-gamma-(alpha-D-glutamylglycine)]-L-lysyl-D-alanine. Biochemistry. 1968 Apr;7(4):1450–1460. doi: 10.1021/bi00844a030. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Bricas E., Leyh-Bouille M., Lache M., Shockman G. D. The peptide N alpha-(L-alanyl-D-isoglutaminyl)-N epsilon-(D-isoasparaginyl)-L-lysyl-D-alanine and the disaccharide N-acetylglucosaminyl-beta-1,4-N-acetylmuramic acid in cell wall peptidoglycan of Streptococcus faecalis strain ATCC 9790. Biochemistry. 1967 Aug;6(8):2607–2619. doi: 10.1021/bi00860a044. [DOI] [PubMed] [Google Scholar]
- Glaser L., Ionesco H., Schaeffer P. Teichoic acids as components of a specific phage receptor in Bacillus subtilis. Biochim Biophys Acta. 1966 Aug 24;124(2):415–417. doi: 10.1016/0304-4165(66)90211-x. [DOI] [PubMed] [Google Scholar]
- HASH J. H. PURIFICATION AND PROPERTIES OF STAPHYLOLYTIC ENZYMES FROM CHALAROPSIS SP. Arch Biochem Biophys. 1963 Sep;102:379–388. doi: 10.1016/0003-9861(63)90245-5. [DOI] [PubMed] [Google Scholar]
- HEYMANN H., MANNIELLO J. M., BARKULIS S. S. Structure of streptococcal cell walls. I. Methylation study of C-polysaccharide. J Biol Chem. 1963 Feb;238:502–509. [PubMed] [Google Scholar]
- HEYMANN H., MANNIELLO J. M., ZELEZNICK L. D., BARKULIS S. S. STRUCTURE OF STREPTOCOCCAL CELL WALLS. II. GROUP A BIOSE AND GROUP A TRIOSE FROM C-POLYSACCHARIDE. J Biol Chem. 1964 May;239:1656–1663. [PubMed] [Google Scholar]
- HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid. II. Their distribution in the bacterial order Actinomycetales and in certain Eubacteriales. Biochem J. 1957 Mar;65(3):441–447. doi: 10.1042/bj0650441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley E. E., Corcoran B. J., Dorer F. E., Buchanan D. L. Beta-aspartyl peptides in enzymatic hydrolysates of protein. Biochemistry. 1966 Oct;5(10):3229–3235. doi: 10.1021/bi00874a024. [DOI] [PubMed] [Google Scholar]
- Hall E. A., Knox K. W. Properties of the polysaccharide and mucopeptide components of the cell wall of Lactobacillus casei. Biochem J. 1965 Aug;96(2):310–318. doi: 10.1042/bj0960310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hash J. H., Rothlauf M. V. The N,O-diacetylmuramidase of Chalaropsis species. I. Purification and crystallization. J Biol Chem. 1967 Dec 10;242(23):5586–5590. [PubMed] [Google Scholar]
- Heymann H., Manniello J. M., Barkulis S. S. Structure of streptococcal cell walls. V. Phosphate esters in the walls of group A Streptococcus pyogenes. Biochem Biophys Res Commun. 1967 Feb 21;26(4):486–491. doi: 10.1016/0006-291x(67)90574-8. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofschneider P. H., Martin H. H. Diversity of surface layers in L-forms of Proteus mirabilis. J Gen Microbiol. 1968 Apr;51(1):23–33. doi: 10.1099/00221287-51-1-23. [DOI] [PubMed] [Google Scholar]
- Hughes R. C. The cell wall of Bacillus licheniformis N.C.T.C. 6346. Isolation of low-molecular-weight fragments from the soluble mucopeptide. Biochem J. 1968 Jan;106(1):49–59. doi: 10.1042/bj1060049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKAWA M., SNELL E. E. Cell wall composition of lactic acid bacteria. J Biol Chem. 1960 May;235:1376–1382. [PubMed] [Google Scholar]
- IKAWA M. THE CONFIGURATION OF ASPARTIC ACID IN CELL WALLS OF LACTIC ACID BACTERIA AND FACTORS AFFECTING THE RACEMIZATION OF ASPARTIC ACID. Biochemistry. 1964 Apr;3:594–597. doi: 10.1021/bi00892a021. [DOI] [PubMed] [Google Scholar]
- ITO E., STROMINGER J. L. ENZYMATIC SYNTHESIS OF THE PEPTIDE IN BACTERIAL URIDINE NUCLEOTIDES. III. PURIFICATION AND PROPERTIES OF L-LYSIN-ADDING ENZYME. J Biol Chem. 1964 Jan;239:210–214. [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEANLOZ R. W., SHARON N., FLOWERS H. M. THE CHEMICAL STRUCTURE OF A DISACCHARIDE ISOLATED FROM MICROCOCCUS LYSODEIKTICUS CELL WALL. Biochem Biophys Res Commun. 1963 Sep 10;13:20–25. doi: 10.1016/0006-291x(63)90155-4. [DOI] [PubMed] [Google Scholar]
- JUSIC D., ROY C., WATSON R. W. SEQUENCE STUDIES ON BACTERIAL CELL WALL PEPTIDES. Can J Biochem. 1964 Nov;42:1553–1559. doi: 10.1139/o64-166. [DOI] [PubMed] [Google Scholar]
- KOTANI S., KATO K., MATSUBARA T., MORI Y., CHIMORI M., KISHIDA H. CHANGES IN SUSCEPTIBILITY OF STAPHYLOCOCCUS AUREUS AND CORYNEBACTERIUM DIPHTHERIAE CELL WALLS TO EGG WHITE LYSOZYME, THE L3- AND L-11-ENZYMES CAUSED BY TRICHLOROACETIC ACID TREATMENT. Biken J. 1964 Jan;6:317–320. [PubMed] [Google Scholar]
- KRAUSE R. M. SYMPOSIUM ON RELATIONSHIP OF STRUCTURE OF MICROORGANISMS TO THEIR IMMUNOLOGICAL PROPERTIES. IV. ANTIGENIC AND BIOCHEMICAL COMPOSITION OF HEMOLYTIC STREPTOCOCCAL CELL WALLS. Bacteriol Rev. 1963 Dec;27:369–380. doi: 10.1128/br.27.4.369-380.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler W., Plapp R., Holzapfel W. Die Aminosäuresequenz des serinhaltigen Mureins von Lactobacillus viridescens und Leuconostoc. Biochim Biophys Acta. 1967 Oct 23;147(2):252–261. [PubMed] [Google Scholar]
- Kanetsuna F. Chemical analyses of mycobacterial cell walls. Biochim Biophys Acta. 1968 Apr 16;158(1):130–143. doi: 10.1016/0304-4165(68)90080-9. [DOI] [PubMed] [Google Scholar]
- Kato K., Strominger J. L., Kotani S. Structure of the cell wall of Corynebacterium diphtheriae. I. Mechanism of hydrolysis by the L-3 enzyme and the structure of the peptide. Biochemistry. 1968 Aug;7(8):2762–2773. doi: 10.1021/bi00848a010. [DOI] [PubMed] [Google Scholar]
- Katz W., Matsuhashi M., Dietrich C. P., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. IV. Incorporation of glycine in Micrococcus lysodeikticus. J Biol Chem. 1967 Jul 10;242(13):3207–3217. [PubMed] [Google Scholar]
- Katz W., Strominger J. L. Structure of the cell wall of Micrococcus lysodeikticus. II. Study of the structure of the peptides produced after lysis with the Myxobacterium enzyme. Biochemistry. 1967 Mar;6(3):930–937. doi: 10.1021/bi00855a037. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Hall E. A. The linkage between the polysaccharide and mucopeptide components of the cell wall of Lactobacillus casei. Biochem J. 1965 Aug;96(2):302–309. doi: 10.1042/bj0960302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Ensign J. C. Isolation and chemical structure of the peptidoglycan of Spirillum serpens cell walls. J Bacteriol. 1968 Jan;95(1):201–210. doi: 10.1128/jb.95.1.201-210.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. I. Cell wall composition and polysaccharides of the peptidoglycan. J Bacteriol. 1967 Sep;94(3):734–740. doi: 10.1128/jb.94.3.734-740.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C., Tipper D. J., Strominger J. L. Sphere-rod morphogenesis in Arthrobacter crystallopoietes. II. Peptides of the cell wall peptidoglycan. J Bacteriol. 1967 Sep;94(3):741–750. doi: 10.1128/jb.94.3.741-750.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka I. Growth and division of protoplasts of Bacillus megaterium and inhibition of division by penicillin. J Bacteriol. 1967 Oct;94(4):884–888. doi: 10.1128/jb.94.4.884-888.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARK C., BRADLEY D., LARK K. G. FURTHER STUDIES ON THE INCORPORATION OF D-METHIONINE INTO THE BACTERIAL CELL WALL; ITS INCORPORATION INTO THE R-LAYER AND THE STRUCTURAL CONSEQUENCES. Biochim Biophys Acta. 1963 Oct 29;78:278–288. doi: 10.1016/0006-3002(63)91638-x. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J. Mechanism of action of penicillin. J Bacteriol. 1957 Jan;73(1):144–144. doi: 10.1128/jb.73.1.144-144.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechevalier H. A., Lechevalier M. P. Biology of actinomycetes. Annu Rev Microbiol. 1967;21:71–100. doi: 10.1146/annurev.mi.21.100167.000443. [DOI] [PubMed] [Google Scholar]
- Lefrancier P., Bricas E. Synthèse de la subunité peptidique du peptidoglycane de la paroi de trois bactéries gram-positif et de peptides de structure analogue. Bull Soc Chim Biol (Paris) 1967 Nov 10;49(10):1257–1271. [PubMed] [Google Scholar]
- Leyh-Bouille M., Ghuysen J. M., Tipper D. J., Stominger J. L. Structure of the cell wall of Micrococcus lysodeikticus. I. Study of the structure of the glycan. Biochemistry. 1966 Oct;5(10):3079–3090. doi: 10.1021/bi00874a001. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C. Muramic acid phosphate as a component of the mucopeptide of Gram-positive bacteria. J Biol Chem. 1967 Feb 10;242(3):471–476. [PubMed] [Google Scholar]
- MARTIN H. H. COMPOSITION OF THE MUCOPOLYMER IN CELL WALLS OF THE UNSTABLE AND STABLE L-FORM OF PROTEUS MIRABILIS. J Gen Microbiol. 1964 Sep;36:441–450. doi: 10.1099/00221287-36-3-441. [DOI] [PubMed] [Google Scholar]
- MCCARTY M., MORSE S. I. CELL WALL ANTIGENS OF GRAM-POSITIVE BACTERIA. Adv Immunol. 1964;27:249–286. doi: 10.1016/s0065-2776(08)60709-9. [DOI] [PubMed] [Google Scholar]
- MCQUILLEN K. Bacterial protoplasts: growth and division of protoplasts of Bacillus megaterium. Biochim Biophys Acta. 1955 Nov;18(3):458–461. doi: 10.1016/0006-3002(55)90129-3. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Martin H. H. Biochemistry of bacterial cell walls. Annu Rev Biochem. 1966;35:457–484. doi: 10.1146/annurev.bi.35.070166.002325. [DOI] [PubMed] [Google Scholar]
- Martin H. H. Murein structure in cell walls of normal bacteria and L-forms of Proteus mirabilis and the site of action of penicillin. Folia Microbiol (Praha) 1967;12(3):234–239. doi: 10.1007/BF02868737. [DOI] [PubMed] [Google Scholar]
- Miller I., Plapp R., Kandler O. Isolierung und Identifizierung eines serinhaltigen UDP-muramyl-tripeptides aus Butyribacterium rettgeri. Z Naturforsch B. 1968 Feb;23(2):217–220. [PubMed] [Google Scholar]
- Miller I., Plapp R., Kandler O. The amino acid sequence of the serine containing murein of Butybacterium Rettgeri. Biochem Biophys Res Commun. 1966 Nov 22;25(4):415–420. doi: 10.1016/0006-291x(66)90221-x. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Sharon N. Isolation and characterization of the disaccharide N-acetyl-glucosaminyl-beta(1 bound to 4)-N-acetylmuramic acid and two tripeptide derivatives of this disaccharide from lysozyme digests of Bacillus licheniformis ATCC 9945 cell walls. J Biol Chem. 1968 May 10;243(9):2279–2287. [PubMed] [Google Scholar]
- Mirelman D., Sharon N. Isolation and characterization of two disaccharide-peptides from lysozyme digests of Micrococcus lysodeikticus cell walls. Biochem Biophys Res Commun. 1966 Jul 20;24(2):237–243. doi: 10.1016/0006-291x(66)90726-1. [DOI] [PubMed] [Google Scholar]
- Montague M. D., Moulds J. D. The phosphorus content of cell walls of Micrococcus lysodeikticus. Biochim Biophys Acta. 1967 Jul 3;135(3):565–567. doi: 10.1016/0005-2736(67)90047-8. [DOI] [PubMed] [Google Scholar]
- Munoz E., Ghuysen J. M., Heymann H. Cell walls of Streptococcus pyogenes, type 14. C polysaccharide-peptidoglycan and G polysaccharide-peptidoglycan complexes. Biochemistry. 1967 Dec;6(12):3659–3670. doi: 10.1021/bi00864a007. [DOI] [PubMed] [Google Scholar]
- NEUHAUS F. C., LYNCH J. L. THE ENZYMATIC SYNTHESIS OF D-ALANYL-D-ALANINE. 3. ON THE INHIBITION OF D-ALANYL-D-ALANINE SYNTHETASE BY THE ANTIBIOTIC D-CYCLOSERINE. Biochemistry. 1964 Apr;3:471–480. doi: 10.1021/bi00892a001. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Ishimoto N., Anderson J. S., Strominger J. L. Enzymatic synthesis and immunochemistry of alpha- and beta-N-acetylglucosaminylribitol linkages in teichoic acids from several strains of Staphylococcus aureus. J Biol Chem. 1966 Feb 10;241(3):651–658. [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyomporn B., Dahl J. L., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. IX. Purification and properties of glycyl transfer ribonucleic acid synthetase from Staphylococcus aureus. J Biol Chem. 1968 Feb 25;243(4):773–778. [PubMed] [Google Scholar]
- PELZER H. MUCOPEPTIDHYDROLASEN IN ESCHERICHIA COLI B. I. NACHWEIS UND WIRKUNGSSPEZIFITAET. Z Naturforsch B. 1963 Nov;18:950–956. [PubMed] [Google Scholar]
- PERKINS H. R. A polymer containing glucose and aminohexuronic acid isolated from the cell walls of micrococcus lysodeikticus. Biochem J. 1963 Mar;86:475–483. doi: 10.1042/bj0860475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R. THE ACTION OF HOT FORMAMIDE ON BACTERIAL CELL WALLS. Biochem J. 1965 Jun;95:876–882. doi: 10.1042/bj0950876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R. The structure of a disaccharide liberated by lysozyme from the cell walls of Micrococcus lysodeikticus. Biochem J. 1960 Jan;74:182–186. doi: 10.1042/bj0740182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRASAD A. L., LITWACK G. GROWTH AND BIOCHEMICAL CHARACTERISTICS OF MICROCOCCUS LYSODEIKTICUS, SENSITIVE OR RESISTANT TO LYSOZYME. Biochemistry. 1965 Mar;4:496–501. doi: 10.1021/bi00879a018. [DOI] [PubMed] [Google Scholar]
- Perkins H. R. 2,6-Diamino-3-hydroxypimelic acid in microbial cell wall mucopeptide. Nature. 1965 Nov 27;208(5013):872–873. doi: 10.1038/208872a0. [DOI] [PubMed] [Google Scholar]
- Perkins H. R. The use of photolysis of dinitrophenyl-peptides in structural studies on the cell-wall mucopeptide of Corynebacterium poinsettiae. Biochem J. 1967 Feb;102(2):29C–32C. doi: 10.1042/bj1020029c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. F., Munoz E., Ghuysen J. M. Peptide cross-links in bacterial cell wall peptidoglycans studied with specific endopeptidases from Streptomyces albus G. Biochemistry. 1966 Aug;5(8):2764–2776. doi: 10.1021/bi00872a037. [DOI] [PubMed] [Google Scholar]
- Petit J. F., Strominger J. L., Söll D. Biosynthesis of the peptidoglycan of bacterial cell walls. VII. Incorporation of serine and glycine into interpeptide bridges in Staphylococcus epidermidis. J Biol Chem. 1968 Feb 25;243(4):757–767. [PubMed] [Google Scholar]
- Pickering B. T. Components of the cell wall of Clostridium welchii (type A). Biochem J. 1966 Aug;100(2):430–440. doi: 10.1042/bj1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., Boone C. J. Comparative cell wall analyses of morphological forms within the genus Actinomyces. J Bacteriol. 1967 Oct;94(4):875–883. doi: 10.1128/jb.94.4.875-883.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plapp R., Kandler O. Die Aminosäuresequenz des Asparaginsäure enthaltenden Mureins von Lactobacillus coryniformis und Lactobacillus cellobiosus. Z Naturforsch B. 1967 Oct;22(10):1062–1067. [PubMed] [Google Scholar]
- Plapp R., Kandler O. Identification of L-ornithine and delta-aminosuccinyl ornithine in cell wall hydrolysates of Lactobacillus cellobiosus. Nature. 1967 Feb 25;213(5078):803–804. doi: 10.1038/213803a0. [DOI] [PubMed] [Google Scholar]
- Plapp R., Kandler O. Isolation of an ornithine-containing cell wall precursor of Lactobacillus cellobiosus. Biochem Biophys Res Commun. 1967 Jul 21;28(2):141–145. doi: 10.1016/0006-291x(67)90420-2. [DOI] [PubMed] [Google Scholar]
- Plapp R., Schleifer K. H., Kandler O. The amino acid sequence of the mureins of lactic acid bacteria. Folia Microbiol (Praha) 1967;12(3):205–213. doi: 10.1007/BF02868733. [DOI] [PubMed] [Google Scholar]
- ROGERS H. J., GARRETT A. J. THE INTERRELATIONSHIP BETWEEN MUCOPEPTIDE AND RIBITOL TEICHOIC ACID FORMATION AS SHOWN BY THE EFFECT OF INHIBITORS. Biochem J. 1965 Jul;96:231–243. doi: 10.1042/bj0960231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL D. W. Studies on the photochemical behavior of 2,4-dinitrophenyl derivatives of some amino acids and peptides. Biochem J. 1963 Apr;87:1–4. doi: 10.1042/bj0870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. S., Petit J. F., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Specificity in the utilization of L-alanyl transfer ribonucleic acid for interpeptide bridge synthesis in Arthrobacter crystallopoietes. J Biol Chem. 1968 Feb 25;243(4):768–772. [PubMed] [Google Scholar]
- Roberts W. S., Strominger J. L., Söll D. Biosynthesis of the peptidoglycan of bacterial cell walls. VI. Incorporation of L-threonine into interpeptide bridges in Micrococcus roseus. J Biol Chem. 1968 Feb 25;243(4):749–756. [PubMed] [Google Scholar]
- SALTON M. R., GHUYSEN J. M. Acetylhexosamine compounds enzymically released from Micrococcus lysodeikticus cell walls. III. The structure of DI- and tetra-saccharides released from cell walls by lysozyme and Streptomyces F1 enzyme. Biochim Biophys Acta. 1960 Dec 4;45:355–363. doi: 10.1016/0006-3002(60)91458-x. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. IV. The composition of the cell walls of some Gram-positive and Gram-negative bacteria. Biochim Biophys Acta. 1953 Apr;10(4):512–523. doi: 10.1016/0006-3002(53)90296-0. [DOI] [PubMed] [Google Scholar]
- SCHOCHER A. J., BAYLEY S. T., WATSON R. W. Composition of purified mucopeptide from the wall of Aerobacter cloacae. Can J Microbiol. 1962 Feb;8:89–98. doi: 10.1139/m62-012. [DOI] [PubMed] [Google Scholar]
- SWALLOW D. L., ABRAHAM E. P. Formation of epsilon-(aminosuccinyl)-lysine from epsilon-aspartyl-lysine from bacitracin A, and from the cell of lactobacilli. Biochem J. 1958 Nov;70(3):364–373. doi: 10.1042/bj0700364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R. Structure and function of bacterial cell membranes. Annu Rev Microbiol. 1967;21:417–442. doi: 10.1146/annurev.mi.21.100167.002221. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Micrococcus lysodeikticus: a new type of cross-linkage of the murein. Biochem Biophys Res Commun. 1967 Sep 27;28(6):965–972. doi: 10.1016/0006-291x(67)90074-5. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Zur chemischen Zusammensetzung der Zellwand der Streptokokken. I. Die Aminosäuresequenz des Mureins von Str. thermophilus und Str. faecalis. Arch Mikrobiol. 1967 Jul 6;57(4):335–364. [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Zur chemischen Zusammensetzung der Zellwand der Streptokokken. II. Die Aminosäuresequenz des Mureins von Str. lactis und cremoris. Arch Mikrobiol. 1967 Jul 6;57(4):365–381. [PubMed] [Google Scholar]
- Schleifer K. H., Plapp R., Kandler O. Die Aminosäuresequenz des Mureins von Microbacterium lacticum. Biochim Biophys Acta. 1968 Apr 9;154(3):573–582. [PubMed] [Google Scholar]
- Schleifer K. H., Plapp R., Kandler O. Identification of threo-3-hydroxyglutamic acid in the cell wall of Microbacterium lacticum. Biochem Biophys Res Commun. 1967 Aug 23;28(4):566–570. doi: 10.1016/0006-291x(67)90351-8. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Plapp R., Kandler O. The amino acid sequence of a glycine-containing cell wall precursor of Microbacterium lacticum. Biochem Biophys Res Commun. 1967 Feb 21;26(4):492–496. doi: 10.1016/0006-291x(67)90575-x. [DOI] [PubMed] [Google Scholar]
- Sharon N., Osawa T., Flowers H. M., Jeanloz R. W. Isolation and study of the chemical structure of a disaccharide from Micrococcus lysodeikticus cell walls. J Biol Chem. 1966 Jan 10;241(1):223–230. [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. Replacement of Lysine by Hydroxylysine and Its Effects on Cell Lysis in Streptococcus faecalis. J Bacteriol. 1965 Sep;90(3):575–588. doi: 10.1128/jb.90.3.575-588.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XI. Formation of the isoglutamine amide group in the cell walls of Staphylococcus aureus. J Biol Chem. 1968 Feb 25;243(4):783–790. [PubMed] [Google Scholar]
- Stolp H., Starr M. P. Bacteriolysis. Annu Rev Microbiol. 1965;19:79–104. doi: 10.1146/annurev.mi.19.100165.000455. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Tipper D. J. Bacterial cell wall synthesis and structure in relation to the mechanism of action of penicillins and other antibacterial agents. Am J Med. 1965 Nov;39(5):708–721. doi: 10.1016/0002-9343(65)90093-8. [DOI] [PubMed] [Google Scholar]
- Szaniszlo P. J., Gooder H. Cell wall composition in relation to the taxonomy of some Actinoplanaceae. J Bacteriol. 1967 Dec;94(6):2037–2047. doi: 10.1128/jb.94.6.2037-2047.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEBE I. EXTENT OF CROSS LINKAGE IN THE MUREIN SACCULUS OF ESCHERICHIA COLI B CELL WALL. Biochim Biophys Acta. 1965 Mar 1;101:124–126. doi: 10.1016/0926-6534(65)90038-2. [DOI] [PubMed] [Google Scholar]
- TIPPER D. J., STROMINGER J. L., GHUYSEN J. M. STAPHYLOLYTIC ENZYME FROM CHALAROPSIS: MECHANISM OF ACTION. Science. 1964 Nov 6;146(3645):781–782. doi: 10.1126/science.146.3645.781. [DOI] [PubMed] [Google Scholar]
- Tinelli R. Etude de l'autolyse des parois de Listeria monocytogenes. C R Acad Sci Hebd Seances Acad Sci D. 1965 Nov 15;261(20):4265–4267. [PubMed] [Google Scholar]
- Tinelli R. Mise en évidence d'enzymes autolytiques dans les parois de différentes Sporulales. C R Acad Sci Hebd Seances Acad Sci D. 1968 Apr 22;266(17):1792–1795. [PubMed] [Google Scholar]
- Tipper D. J., Katz W., Strominger J. L., Ghuysen J. M. Substituents on the alpha-carboxyl group of D-glutamic acid in the peptidoglycan of several bacterial cell walls. Biochemistry. 1967 Mar;6(3):921–929. doi: 10.1021/bi00855a036. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L., Ensign J. C. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. VII. Mode of action of the bacteriolytic peptidase from Myxobacter and the isolation of intact cell wall polysaccharides. Biochemistry. 1967 Mar;6(3):906–920. doi: 10.1021/bi00855a035. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Isolation of 4-O-beta-N-acetylmuramyl-N-acetylglucosamine and 4-O-beta-N, 6-O-diacetylmuramyl-N-acetylglucosamine and the structure of the cell wall polysaccharide of Staphylococcus aureus. Biochem Biophys Res Commun. 1966 Jan 4;22(1):48–56. doi: 10.1016/0006-291x(66)90601-2. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. S., Whitaker D. R., Jurásek L., Gillespie D. C. Lytic enzymes of Sorangium sp. Action of the alpha- and beta-lytic proteases on two bacterial mucopeptides. Can J Biochem. 1965 Dec;43(12):1971–1983. doi: 10.1139/o65-220. [DOI] [PubMed] [Google Scholar]
- Veerkamp J. H., Lambert R., Saito Y. The composition of the cell wall of Lactobacillius bifidus var. pennsylvanicus. Arch Biochem Biophys. 1965 Oct;112(1):120–125. doi: 10.1016/0003-9861(65)90019-6. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. The isolation of protoplasts from Bacillus megaterium by controlled treatment with lysozyme. J Bacteriol. 1953 Dec;66(6):688–695. doi: 10.1128/jb.66.6.688-695.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Weiss N., Plapp R., Kandler O. Die Aminosäuresequenz des DAP-haltigen Mureins von Lactobacillus plantarum und Lactobacillus inulinus. Arch Mikrobiol. 1967;58(4):313–323. [PubMed] [Google Scholar]
- Welsch M. Bactériolyse et parois cellulaires bactériennes. Pathol Microbiol (Basel) 1966;29(5):571–601. [PubMed] [Google Scholar]
- White P. J., Kelly B., Suffling A., Work E. Variation of activity of bacterial diaminopimelate decarboxylase under different conditions of growth. Biochem J. 1964 Jun;91(3):600–610. doi: 10.1042/bj0910600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. G., Grula E. A. A major attachment site for D-serine in the cell wall mucopeptide of Micrococcus lysodeikticus. Biochim Biophys Acta. 1968 Apr 16;158(1):124–129. doi: 10.1016/0304-4165(68)90079-2. [DOI] [PubMed] [Google Scholar]
- Work E., Griffiths H. Morphology and chemistry of cell walls of Micrococcus radiodurans. J Bacteriol. 1968 Feb;95(2):641–657. doi: 10.1128/jb.95.2.641-657.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAGUCHI T. COMPARISON OF THE CELL-WALL COMPOSITION OF MORPHOLOGICALLY DISTINCT ACTINOMYCETES. J Bacteriol. 1965 Feb;89:444–453. doi: 10.1128/jb.89.2.444-453.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Arias L. Biosynthesis of the N-acyl-galactosaminase in cell walls of Bacillus subtilis. J Bacteriol. 1967 Nov;94(5):1783–1784. doi: 10.1128/jb.94.5.1783-1784.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Competence in Bacillus subtilis transformation system. Nature. 1967 Feb 25;213(5078):773–775. doi: 10.1038/213773a0. [DOI] [PubMed] [Google Scholar]
- Young F. E. Fractionation and partial characterization of the products of autolysis of cell walls of Bacillus subtilis. J Bacteriol. 1966 Oct;92(4):839–846. doi: 10.1128/jb.92.4.839-846.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]



