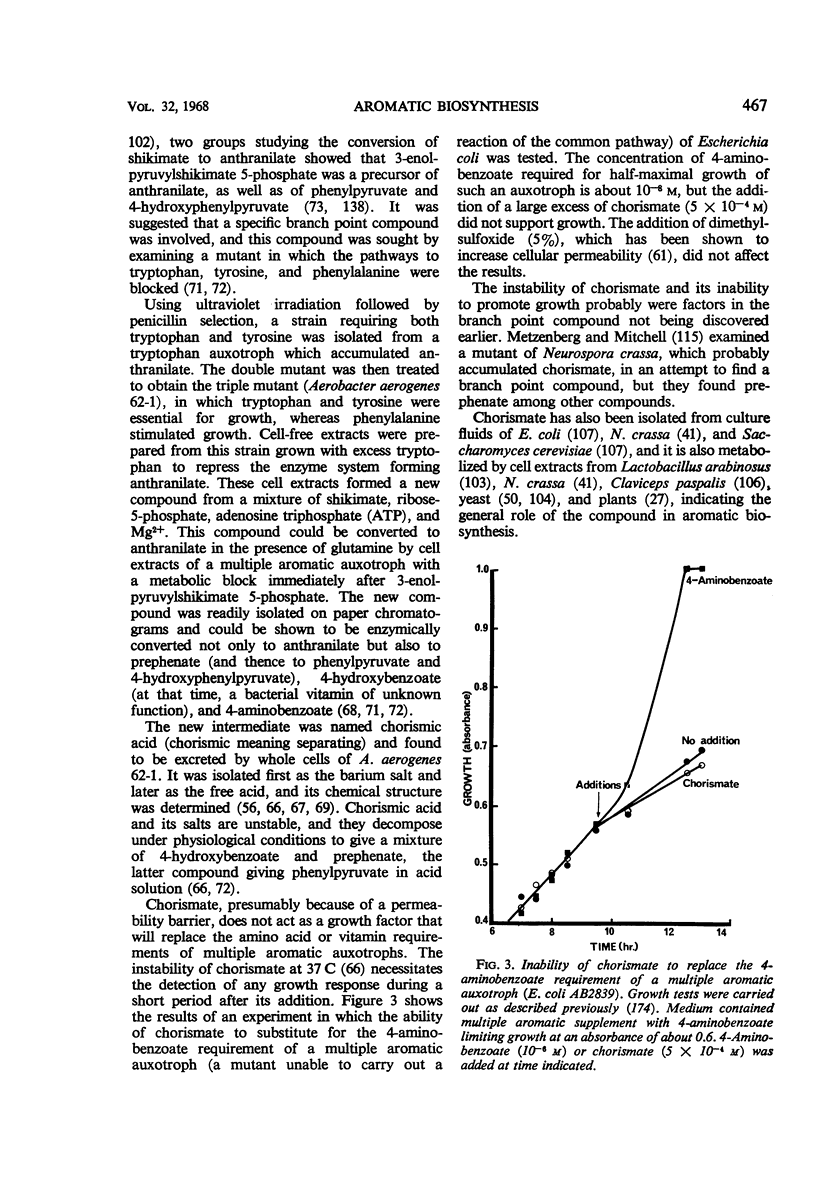
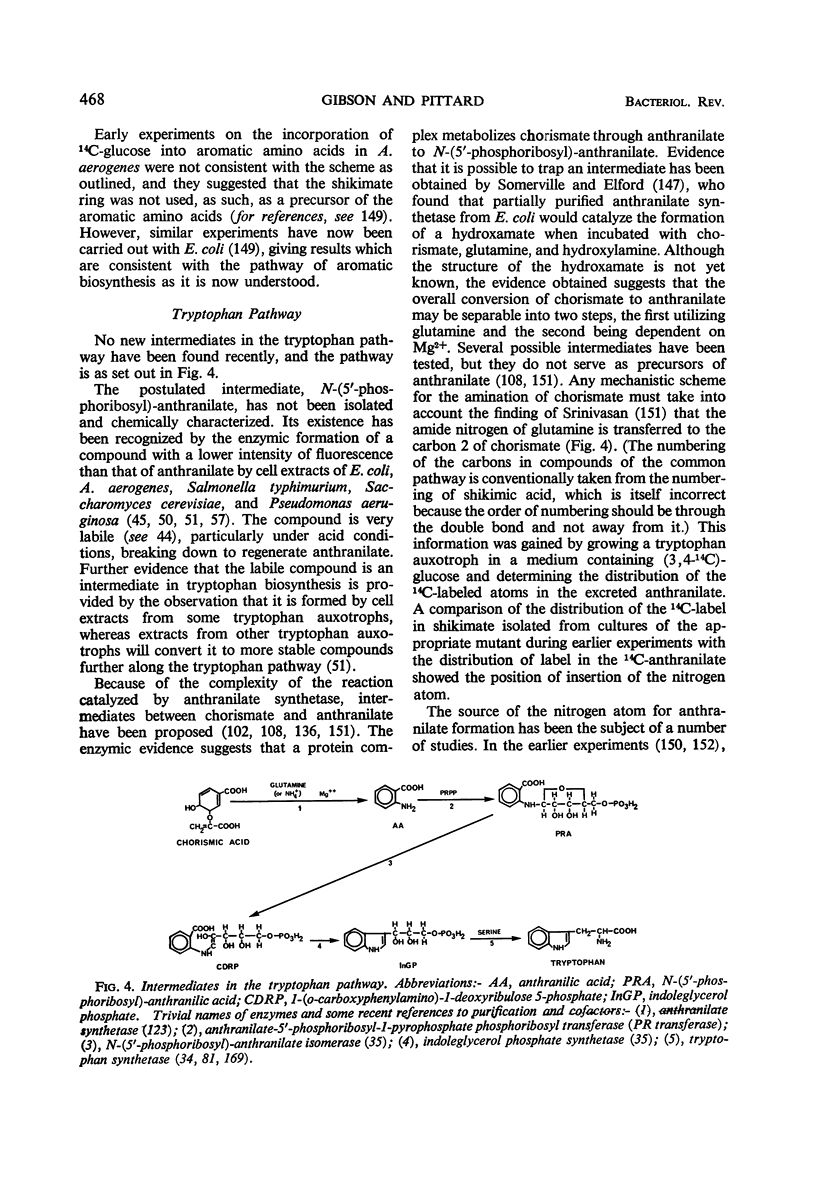
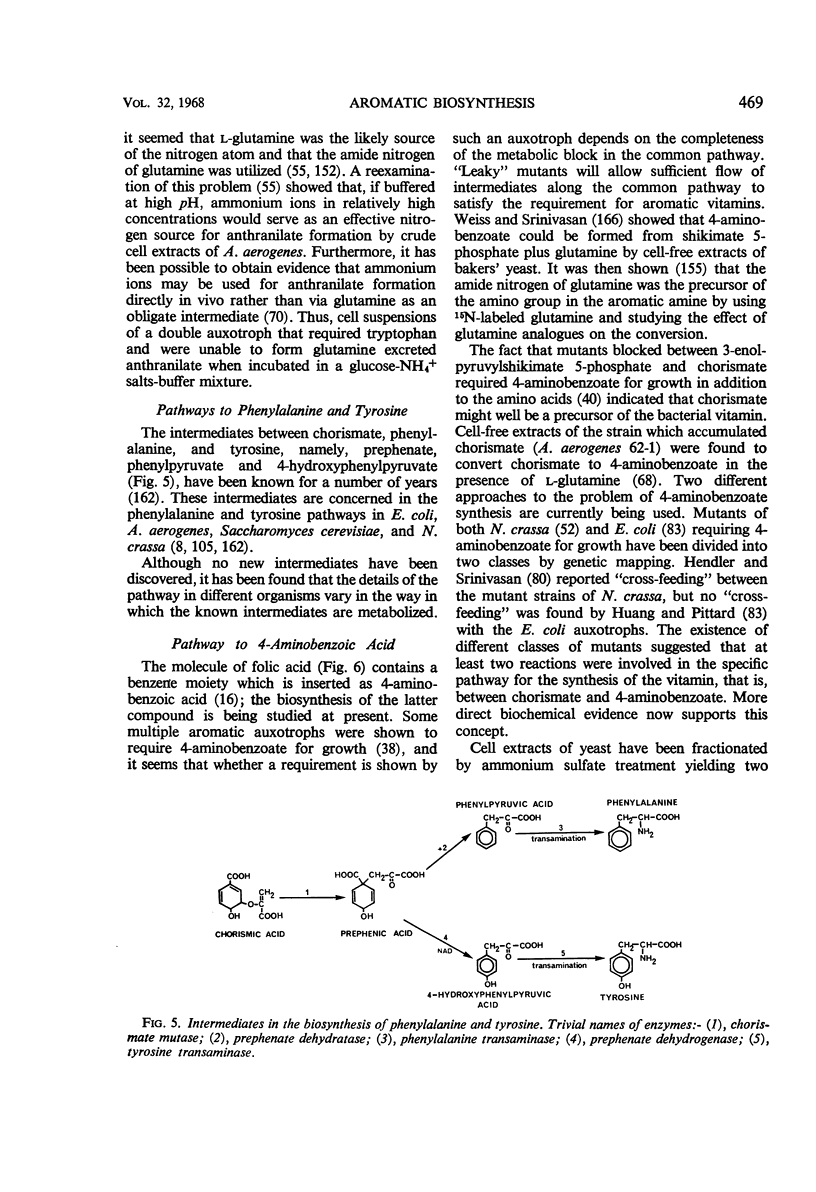
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAGNOSTOPOULOS C., CRAWFORD I. P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Mar 15;47:378–390. doi: 10.1073/pnas.47.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARCAMONE F., CHAIN E. B., FERRETTI A., PENNELLA P. Formation of 2,3-dihydroxybenzoic acid in fermentation liquors during the submerged culture production of lysergic acid alpha-hydroxyethylamide by Claviceps paspali Stevens and Hall. Nature. 1961 Nov 11;192:552–553. doi: 10.1038/192552a0. [DOI] [PubMed] [Google Scholar]
- AZERAD R., BLEILER-HILL R., LEDERER E. BIOSYNTHESIS OF A VITAMIN K2 BY CELL-FREE EXTRACTS OF MYCOBACTERIUM PHLEI. Biochem Biophys Res Commun. 1965 Apr 9;19:194–197. doi: 10.1016/0006-291x(65)90503-6. [DOI] [PubMed] [Google Scholar]
- Azerad R., Bleiler-Hill R., Catala F., Samuel O., Lederer E. Biosynthesis of dihydromenaquinone-9 by Mycobacterium phlei. Biochem Biophys Res Commun. 1967 Apr 20;27(2):253–257. doi: 10.1016/s0006-291x(67)80070-6. [DOI] [PubMed] [Google Scholar]
- Azerad R., Cyrot M. O., Lederer E. Structure of the dihydromenaquinone-9 of Mycobacterium phlei. Biochem Biophys Res Commun. 1967 Apr 20;27(2):249–252. doi: 10.1016/s0006-291x(67)80069-x. [DOI] [PubMed] [Google Scholar]
- BROWN K. D., DOY C. H. END-PRODUCT REGULATION OF THE GENERAL AROMATIC-PATHWAY IN ESCHERICHIA COLI W. Biochim Biophys Acta. 1963 Sep 3;77:170–172. doi: 10.1016/0006-3002(63)90489-x. [DOI] [PubMed] [Google Scholar]
- Baker T. I., Crawford I. P. Anthranilate synthetase. Partial purification and some kinetic studies on the enzyme from Escherichia coli. J Biol Chem. 1966 Dec 10;241(23):5577–5584. [PubMed] [Google Scholar]
- Baker T. I. Phenylalanine-Tyrosine Biosynthesis in NEUROSPORA CRASSA. Genetics. 1968 Mar;58(3):351–359. doi: 10.1093/genetics/58.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. I. Tryptophan: a feedback activator for chorismate mutase from Neurospora. Biochemistry. 1966 Aug;5(8):2654–2657. doi: 10.1021/bi00872a025. [DOI] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. The functional organization of the tryptophan gene cluster in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1966 Jul;56(1):111–118. doi: 10.1073/pnas.56.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N., Goodwin J., Fales H. In vivo and in vitro formation of 2,3-dihydroxybenzoylserine by Escherichia coli K12. Biochem Biophys Res Commun. 1966 Nov 22;25(4):454–461. doi: 10.1016/0006-291x(66)90227-0. [DOI] [PubMed] [Google Scholar]
- Brot N., Goodwin J. Regulation of 2,3-dihydroxybenzoylserine synthetase by iron. J Biol Chem. 1968 Feb 10;243(3):510–513. [PubMed] [Google Scholar]
- Brown K. D., Doy C. H. Control of three isoenzymic 7-phospho-2-oxo-3-deoxy-Darabino-heptonate-D-erythrose-4-phosphate lyases of Escherichia coli W and derived mutants by repressive and "inductive" effects of the aromatic amino acids. Biochim Biophys Acta. 1966 Apr 12;118(1):157–172. doi: 10.1016/s0926-6593(66)80153-4. [DOI] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- COX G. B., GIBSON F. BIOSYNTHESIS OF VITAMIN K AND UBIQUINONE. RELATION TO THE SHIKIMIC ACID PATHWAY IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Oct 9;93:204–206. doi: 10.1016/0304-4165(64)90285-5. [DOI] [PubMed] [Google Scholar]
- Campbell I. M., Coscia C. J., Kelsey M., Bentley R. Origin of the aromatic nucleus in bacterial menaquinones. Biochem Biophys Res Commun. 1967 Jul 10;28(1):25–29. doi: 10.1016/0006-291x(67)90400-7. [DOI] [PubMed] [Google Scholar]
- Carlton B. C. Fine-structure mapping by transformation in the tryptophan region of Bacillus subtilis. J Bacteriol. 1966 May;91(5):1795–1803. doi: 10.1128/jb.91.5.1795-1803.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin E. R., Hassall C. H., Pratt B. C. Biosynthesis of phenols. XIV. Isolation of some shikimic acid-derived metabolites from mutant strains of Streptomyces rimosus unable to produce oxytetracycline. Biochim Biophys Acta. 1968 Feb 1;156(1):109–118. [PubMed] [Google Scholar]
- Coats J. H., Nester E. W. Regulation reversal mutation: characterization of end product-activated mutants of Bacillus subtilis. J Biol Chem. 1967 Nov 10;242(21):4948–4955. [PubMed] [Google Scholar]
- Cotton R. G., Gibson F. The biosynthesis of phenylalanine and tyrosine in the pea (Pisum sativum): chorismate mutase. Biochim Biophys Acta. 1968 Feb 1;156(1):187–189. doi: 10.1016/0304-4165(68)90118-9. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Gibson F. The biosynthesis of tyrosine in Aerobacter aerogenes. Evidence for a suunit structure of the protein converting chorismate into 4-hydroxyphenylpyruvate. Biochim Biophys Acta. 1968 Jun 26;160(2):188–195. doi: 10.1016/0005-2795(68)90086-x. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Gibson F. The biosynthesis of tyrosine in Aerobacter aerogenes: partial purification of the T protein. Biochim Biophys Acta. 1967 Oct 23;147(2):222–237. doi: 10.1016/0005-2795(67)90401-1. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. 2,3-Dihydroxybenzoic acid, a new growth factor for multiple aromatic auxotrophs. J Bacteriol. 1967 Jan;93(1):502–503. doi: 10.1128/jb.93.1.502-503.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Pittard J. Mutant strains of Escherichia coli K-12 unable to form ubiquinone. J Bacteriol. 1968 May;95(5):1591–1598. doi: 10.1128/jb.95.5.1591-1598.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. The role of shikimic acid in the biosynthesis of vitamin K2. Biochem J. 1966 Jul;100(1):1–6. doi: 10.1042/bj1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. ON THE SEPARATION OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI INTO TWO PROTEIN COMPONENTS. Proc Natl Acad Sci U S A. 1958 Dec 15;44(12):1161–1170. doi: 10.1073/pnas.44.12.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATTA P., GEST H. ALTERNATIVE PATTERNS OF END-PRODUCT CONTROL IN BIOSYNTHESIS OF AMINO-ACIDS OF THE ASPARTIC FAMILY. Nature. 1964 Sep 19;203:1259–1261. doi: 10.1038/2031259a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Aromatic biosynthesis. VII. Accumulation of two derivatives of shikimic acid by bacterial mutants. J Bacteriol. 1953 Aug;66(2):129–136. doi: 10.1128/jb.66.2.129-136.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D. p-Hydroxybenzoic acid; a new bacterial vitamin. Nature. 1950 Dec 30;166(4235):1120–1121. doi: 10.1038/1661120b0. [DOI] [PubMed] [Google Scholar]
- DEMOSS J. A. THE CONVERSION OF SHIKIMIC ACID TO ANTHRANILIC ACID BY EXTRACTS OF NEUROSPORA CRASSA. J Biol Chem. 1965 Mar;240:1231–1235. [PubMed] [Google Scholar]
- DOY C. H. Lability of N-o-carboxyphenylribosylamine as a factor in the study of tryptophan biosynthesis. Nature. 1961 Feb 11;189:461–463. doi: 10.1038/189461a0. [DOI] [PubMed] [Google Scholar]
- DOY C. H., RIVERA A., Jr, SRINIVASAN P. R. Evidence for the enzymatic synthesis of N-(5'-phosphoribosyl) anthranilic acid, a new intermediate in tryptophan biosynthesis. Biochem Biophys Res Commun. 1961 Jan 25;4:83–88. doi: 10.1016/0006-291x(61)90261-3. [DOI] [PubMed] [Google Scholar]
- DOY C. H. THE BIOCHEMICAL DIFFERENCE BETWEEN CERTAIN PHENOTYPICALLY SIMILAR, BUT GENOTYPICALLY DIFFERENT, TRYPTOPHAN AUXOTROPHS OF PSEUDOMONAS AERUGINOSA. Biochim Biophys Acta. 1964 Jul 15;90:180–183. doi: 10.1016/0304-4165(64)90136-9. [DOI] [PubMed] [Google Scholar]
- DeMoss J. A., Wegman J. An enzyme aggregate in the tryptophan pathway of Neurospora crassa. Proc Natl Acad Sci U S A. 1965 Jul;54(1):241–247. doi: 10.1073/pnas.54.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doy C. H. Aromatic biosynthesis in yeast. II. Feedback inhibition and repression of 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthase. Biochim Biophys Acta. 1968 Jan 8;151(1):293–295. doi: 10.1016/0005-2744(68)90190-3. [DOI] [PubMed] [Google Scholar]
- Doy C. H., Brown K. D. Control of aromatic biosynthesis: the multiplicity of 7-phospho-2-oxo-3-deoxy-D-arabino-heptonate D-erythrose-4-phosphate-lyase (pyruvate-phosphorylating) in Escherichia coli W. Biochim Biophys Acta. 1965 Jul 8;104(2):377–389. doi: 10.1016/0304-4165(65)90343-0. [DOI] [PubMed] [Google Scholar]
- Doy C. H., Cooper J. M. Aromatic biosynthesis in yeast. I. The synthesis of tryptophan and the regulation of this pathway. Biochim Biophys Acta. 1966 Oct 31;127(2):302–316. [PubMed] [Google Scholar]
- Doy C. H. Tryptophan as an inhibitor of 3-deoxy-arabino-heptulosonate 7-phosphate synthetase. Biochem Biophys Res Commun. 1967 Jan 23;26(2):187–192. doi: 10.1016/0006-291x(67)90232-x. [DOI] [PubMed] [Google Scholar]
- Dunphy P. J., Gutnick D. L., Phillips P. G., Brodie A. F. A new natural naphthoquinone in Mycobacterium phlei. Cis-dihydromenaquinone-9, structure and function. J Biol Chem. 1968 Jan 25;243(2):398–407. [PubMed] [Google Scholar]
- EDWARDS J. M., GIBSON F., JACKMAN L. M., SHANNON J. S. THE SOURCE OF THE NITROGEN ATOM FOR THE BIOSYNTHESIS OF ANTHRANILIC ACID. Biochim Biophys Acta. 1964 Oct 9;93:78–84. doi: 10.1016/0304-4165(64)90262-4. [DOI] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EZEKIEL D. H. FALSE FEEDBACK INHIBITION OF AROMATIC AMINO ACID BIOSYNTHESIS BY BETA-2-THIENYLALANINE. Biochim Biophys Acta. 1965 Jan 11;95:54–62. doi: 10.1016/0005-2787(65)90210-8. [DOI] [PubMed] [Google Scholar]
- FEWSTER J. A. Phosphorylation of shikimic acid by ultrasonic extracts of micro-organisms. Biochem J. 1962 Nov;85:388–393. doi: 10.1042/bj0850388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRADEJAS R. G., RAVEL J. M., SHIVE W. The control of shikimic acid synthesis by tyrosine and phenylalamine. Biochem Biophys Res Commun. 1961 Jul 26;5:320–323. doi: 10.1016/0006-291x(61)90171-1. [DOI] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Effects of Dimethylsulfoxide on the Lactose Operon in Escherichia coli. J Bacteriol. 1966 Aug;92(2):353–357. doi: 10.1128/jb.92.2.353-357.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis P., Daves G. D., Jr, Folkers K. Isolation of ubiquinone-5, new member of ubiquinone group. Biochem Biophys Res Commun. 1966 Jul 20;24(2):252–256. doi: 10.1016/0006-291x(66)90728-5. [DOI] [PubMed] [Google Scholar]
- Friis P., Nilsson J. L., Daves G. D., Jr, Folkers K. New multiprenylquinones in the biosynthesis of ubiquinone. Biochem Biophys Res Commun. 1967 Aug 7;28(3):324–327. doi: 10.1016/0006-291x(67)90312-9. [DOI] [PubMed] [Google Scholar]
- GIBSON F., GIBSON M., COX G. B. THE BIOSYNTHESIS OF P-AMINOBENZOIC ACID FROM CHORISMIC ACID. Biochim Biophys Acta. 1964 Mar 16;82:637–638. doi: 10.1016/0304-4165(64)90465-9. [DOI] [PubMed] [Google Scholar]
- GIBSON F., JACKMAN L. M. Structure of chorismic acid, a new intermediate in aromatic biosynthesis. Nature. 1963 Apr 27;198:388–389. doi: 10.1038/198388a0. [DOI] [PubMed] [Google Scholar]
- GIBSON M. I., GIBSON F. A new intermediate in aromatic biosynthesis. Biochim Biophys Acta. 1962 Nov 19;65:160–163. doi: 10.1016/0006-3002(62)90166-x. [DOI] [PubMed] [Google Scholar]
- GORINI L., LORD R. Nécessité des orthodiphénols pour la croissance de Coccus P (Sarcina sp.). Biochim Biophys Acta. 1956 Jan;19(1):84–90. doi: 10.1016/0006-3002(56)90388-2. [DOI] [PubMed] [Google Scholar]
- GROSS S. R. The enzymatic conversion of 5-dehydroshikimic acid to protocatechuic acid. J Biol Chem. 1958 Nov;233(5):1146–1151. [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Pittard J., Reich E. Ammonium ions as the source of nitrogen for tryptophan biosynthesis in whole cells of Escherichia coli. Biochim Biophys Acta. 1967 Apr 25;136(3):573–576. doi: 10.1016/0304-4165(67)90020-7. [DOI] [PubMed] [Google Scholar]
- Gibson M. I., Gibson F. Preliminary studies on the isolation and metabolism of an intermediate in aromatic biosynthesis: chorismic acid. Biochem J. 1964 Feb;90(2):248–256. doi: 10.1042/bj0900248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles N. H., Case M. E., Partridge C. W., Ahmed S. I. A gene cluster in Nuerospora crassa coding for an aggregate of five aromatic synthetic enzymes. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1453–1460. doi: 10.1073/pnas.58.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles N. H., Partridge C. W., Ahmed S. I., Case M. E. The occurrence of two dehydroquinases in Neurospora crassa, one constitutive and one inducible. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1930–1937. doi: 10.1073/pnas.58.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollub E., Zalkin H., Sprinson D. B. Correlation of genes and enzymes, and studies on regulation of the aromatic pathway in Salmonella. J Biol Chem. 1967 Nov 25;242(22):5323–5328. [PubMed] [Google Scholar]
- Gross S R, Fein A. Linkage and Function in Neurospora. Genetics. 1960 Jul;45(7):885–904. doi: 10.1093/genetics/45.7.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNING U., HELINSKI D. R., CHAO F. C., YANOFSKY C. The A protein of the tryptophan synthetase of Escherichia coli. Purification, crystallization, and composition studies. J Biol Chem. 1962 May;237:1523–1530. [PubMed] [Google Scholar]
- Hendler S., Srinivasan P. R. An intermediate in the conversion of chorismate to p-aminobenzoate. Biochim Biophys Acta. 1967 Aug 29;141(3):656–658. doi: 10.1016/0304-4165(67)90200-0. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Ito K., Hamada K., Yura T. A new regulatory gene for the tryptophan operon of Escherichia coli. Biochem Biophys Res Commun. 1967 Mar 9;26(5):522–527. doi: 10.1016/0006-291x(67)90095-2. [DOI] [PubMed] [Google Scholar]
- Huang M., Pittard J. Genetic analysis of mutant strains of Escherichia coli requiring p-aminobenzoic acid for growth. J Bacteriol. 1967 Jun;93(6):1938–1942. doi: 10.1128/jb.93.6.1938-1942.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter R., DeMoss J. A. Organization of the tryptophan pathway: a phylogenetic study of the fungi. J Bacteriol. 1967 Dec;94(6):1896–1907. doi: 10.1128/jb.94.6.1896-1907.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto S., Senoh S. Two new metabolites, 2- nonaprenylphenol and 2-nonaprenyl-3-methyl-6-methoxy-1, 4-benzoquinone, from Pseudomonas ovalis. Vox Sang. 1967 Jun;12(6):1237–1240. doi: 10.1016/s0040-4039(00)90675-0. [DOI] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. The nature of the anthranilic acid synthetase complex of Escherichia coli. J Biol Chem. 1966 Sep 10;241(17):4112–4114. [PubMed] [Google Scholar]
- JENSEN R. A., NESTER E. W. THE REGULATORY SIGNIFICANCE OF INTERMEDIARY METABOLITES: CONTROL OF AROMATIC ACID BIOSYNTHESIS BY FEEDBACK INHIBITION IN BACILLUS SUBTILIS. J Mol Biol. 1965 Jun;12:468–481. doi: 10.1016/s0022-2836(65)80270-4. [DOI] [PubMed] [Google Scholar]
- Jackman L. M., O'Brien I. G., Cox G. B., Gibson F. Methionine as the source of methyl groups for ubiquinone and vitamin K: a study using nuclear magnetic resonance and mass spectrometry. Biochim Biophys Acta. 1967 Jun 13;141(1):1–7. doi: 10.1016/0304-4165(67)90239-5. [DOI] [PubMed] [Google Scholar]
- Jeffries L., Cawthorne M. A., Harris M., Diplock A. T., Green J., Price S. A. Distribution of menaquinones in aerobic Micrococcaceae. Nature. 1967 Jul 15;215(5098):257–259. doi: 10.1038/215257a0. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S. Comparative regulation of isoenzymic 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetases in microorganisms. J Bacteriol. 1968 Jan;95(1):188–196. doi: 10.1128/jb.95.1.188-196.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Nester E. W. Regulatory enzymes of aromatic amino acid biosynthesis in Bacillus subtilis. I. Purification and properties of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase. J Biol Chem. 1966 Jul 25;241(14):3365–3372. [PubMed] [Google Scholar]
- LESTER G. Repression and inhibition of indole-synthesizing activity in Neurospora crassa. J Bacteriol. 1961 Aug;82:215–223. doi: 10.1128/jb.82.2.215-223.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER R. L., WHITE D. C., SMITH S. L. THE 2-DESMETHYL VITAMIN K2'S. A NEW GROUP OF NAPHTHOQUINONES ISOLATED FROM HEMOPHILUS PARAINFLUENZAE. Biochemistry. 1964 Jul;3:949–954. doi: 10.1021/bi00895a018. [DOI] [PubMed] [Google Scholar]
- LEVIN J. G., SPRINSON D. B. THE ENZYMATIC FORMATION AND ISOLATION OF 3-ENOLPYRUVYLSHIKIMATE 5-PHOSPHATE. J Biol Chem. 1964 Apr;239:1142–1150. [PubMed] [Google Scholar]
- LEVIN J. G., SPRINSON D. B. The formation of 3-enolpyruvyl shikimate 5-phosphate in extracts of Escherichia coli. Biochem Biophys Res Commun. 1960 Aug;3:157–163. doi: 10.1016/0006-291x(60)90214-x. [DOI] [PubMed] [Google Scholar]
- Lederer E. The origin and function of some methyl groups in branched-chain fatty acids, plant sterols and quinones. Biochem J. 1964 Dec;93(3):449–468. doi: 10.1042/bj0930449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistner E., Schmitt J. H., Zenk M. H. Alpha-naphthol: a precursor of vitamin K2. Biochem Biophys Res Commun. 1967 Sep 27;28(6):845–850. doi: 10.1016/0006-291x(67)90054-x. [DOI] [PubMed] [Google Scholar]
- Lingens F., Goebel W., Gimminger H. Uber die Biosynthese aromatischer Aminosäuren in Lactobacillus arabinosus. Naturwissenschaften. 1967 Feb;54(4):91–92. doi: 10.1007/BF00608772. [DOI] [PubMed] [Google Scholar]
- Lingens F., Goebel W., Uesseler H. Regulation der Biosynthese der aromatischen Aminosäuren in Claviceps paspali. Eur J Biochem. 1967 Nov;2(4):442–447. doi: 10.1111/j.1432-1033.1967.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Lingens F., Goebel W., Uesseler H. Regulation der Biosynthese der aromatischen Aminosäuren in Saccharomyces cerevisiae. 2. Repression, Induktion und Aktivierung. Eur J Biochem. 1967 May;1(3):363–374. doi: 10.1111/j.1432-1033.1967.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Lingens F., Müller G. Uber die Akkumulation von Chorisminsäure bei Mutaten und Wildstammen von Escherichia coli und Saccharomyces cerevisiae. Z Naturforsch B. 1967 Sep;22(9):991–991. [PubMed] [Google Scholar]
- Lingens F., Sprössler B., Goebel W. Zur Biosynthese der Anthranilsäure in Saccharomyces cerevisiae. Biochim Biophys Acta. 1966 May 26;121(1):164–166. [PubMed] [Google Scholar]
- Lorence J. H., Nester E. W. Multiple molecular forms of chorismate mutase in Bacillus subtillis. Biochemistry. 1967 May;6(5):1541–1553. doi: 10.1021/bi00857a041. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. II. DOMINANCE OF REPRESSIBILITY IN DIPLOIDS. J Mol Biol. 1964 Mar;8:365–370. doi: 10.1016/s0022-2836(64)80200-x. [DOI] [PubMed] [Google Scholar]
- MATSUSHIRO A., KIDA S., ITO J., SATO K., IMAMOTO F. The regulatory mechanism of enzyme synthesis in the tryptophan biosynthetic pathway of Escherichia coli K-12. Biochem Biophys Res Commun. 1962 Oct 17;9:204–207. doi: 10.1016/0006-291x(62)90058-x. [DOI] [PubMed] [Google Scholar]
- MATSUSHIRO A., SATO K., ITO J., KIDA S., IMAMOTO F. ON THE TRANSCRIPTION OF THE TRYTOPHAN OPERON IN ESCHERICHIA COLI. I. THE TRYPTOPHAN OPERATOR. J Mol Biol. 1965 Jan;11:54–63. doi: 10.1016/s0022-2836(65)80170-x. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L., MITCHELL H. K. The biosynthesis of aromatic compounds by Neurospora crassa. Biochem J. 1958 Jan;68(1):168–172. doi: 10.1042/bj0680168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. E. BIOSYNTHESIS OF THE BENZOQUINONE RING OF UBIQUINONE IN TETRAHYMENA PYRIFORMIS. Biochem Biophys Res Commun. 1965 Apr 23;19:335–339. doi: 10.1016/0006-291x(65)90464-x. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- Meuris P., Lacroute F., Slonimski P. P. Etude systematique de mutants inhibes par leurs propres metabolites chez la levure Saccharomyces cerevisiae. I. Obtention et caracterisation des differentes classes de mutants. Genetics. 1967 May;56(1):149–161. doi: 10.1093/genetics/56.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuris P. Mise en évidence et séparation génétique de deux PODH aldolases soumises à la rétroinihibition chez S. cerevisiae. C R Acad Sci Hebd Seances Acad Sci D. 1967 Feb 27;264(9):1197–1199. [PubMed] [Google Scholar]
- Morell H., Clark M. J., Knowles P. F., Sprinson D. B. The enzymic synthesis of chorismic and prephenic acids from 3-enolpyruvylshikimic acid 5-phosphate. J Biol Chem. 1967 Jan 10;242(1):82–90. [PubMed] [Google Scholar]
- Morell H., Sprinson D. B. Shikimate kinase isoenzymes in Salmonella typhimurium. J Biol Chem. 1968 Feb 10;243(3):676–677. [PubMed] [Google Scholar]
- Nass G. Regulation of histidine biosynthetic enzymes in a mutant of Escherichia coli with an altered histidyl-tRNA synthetase. Mol Gen Genet. 1967;100(2):216–224. doi: 10.1007/BF00333608. [DOI] [PubMed] [Google Scholar]
- Nasser D., Nester E. W. Aromatic amino acid biosynthesis: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1967 Nov;94(5):1706–1714. doi: 10.1128/jb.94.5.1706-1714.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Lorence J. H., Nasser D. S. An enzyme aggregate involved in the biosynthesis of aromatic amino acids in Bacillus subtilis. Its possible function in feedback regulation. Biochemistry. 1967 May;6(5):1553–1563. doi: 10.1021/bi00857a042. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Demerec M., Eisenstark A. Genetic analysis of aromatic mutants of Salmonella typhimurium. Genetics. 1967 Jun;56(2):341–351. doi: 10.1093/genetics/56.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSEN R. K., SMITH J. L., DAVES G. D., MOORE H. W., FOLKERS K., PARSON W. W., RUDNEY H. 2-DECAPRENYLPHENOL, BIOSYNTHETIC PRECURSOR OF UBIQUINONE-10. J Am Chem Soc. 1965 May 20;87:2298–2300. doi: 10.1021/ja01088a045. [DOI] [PubMed] [Google Scholar]
- Olsen R. K., Daves G. D., Jr, Moore H. W., Folkers K., Parson W. W., Rudney H. 2-multiprenylphenols and 2-decaprenyl-6-methoxyphenol, biosynthetic precursors of ubiquinones. J Am Chem Soc. 1966 Dec 20;88(24):5919–5923. doi: 10.1021/ja00976a036. [DOI] [PubMed] [Google Scholar]
- PARSON W. W., RUDNEY H. THE BIOSYNTHESIS OF UBIQUINONE AND RHODOQUINONE FROM P-HYDROXYBENZOATE AND P-HYDROXYBENZALDEHYDE IN RHODOSPIRILLUM RUBRUM. J Biol Chem. 1965 Apr;240:1855–1863. [PubMed] [Google Scholar]
- PITTARD A. J., GIBSON F., DOY C. H. A possible relationship between the formation of o-dihydric phenols and tryptophan biosynthesis by Aerobacter aerogens. Biochim Biophys Acta. 1962 Feb 26;57:290–298. doi: 10.1016/0006-3002(62)91122-8. [DOI] [PubMed] [Google Scholar]
- PITTARD A. J., GIBSON F., DOY C. H. Phenolic compounds accumulated by washed cell suspensions of a tryptophan auxotroph of Aerobacter aerogenes. Biochim Biophys Acta. 1961 May 27;49:485–494. doi: 10.1016/0006-3002(61)90245-1. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A., Luft J. H., Love D. S., Krebs E. G. Crystallization and properties of rabbit skeletal muscle phosphofructokinase. J Biol Chem. 1966 Oct 25;241(20):4625–4637. [PubMed] [Google Scholar]
- Peters W. J., Warren R. A. Itoic acid synthesis in Bacillus subtilis. J Bacteriol. 1968 Feb;95(2):360–366. doi: 10.1128/jb.95.2.360-366.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polglase W. J., Pun W. T., Withaar J. Lipoquinones of Escherichia coli. Biochim Biophys Acta. 1966 May 5;118(2):425–426. doi: 10.1016/s0926-6593(66)80053-x. [DOI] [PubMed] [Google Scholar]
- RATLEDGE C. RELATIONSHIP BETWEEN THE PRODUCTS OF AROMATIC BIOSYNTHESIS IN MYCOBACTERIUM SMEGMATIS AND AEROBACTER AEROGENES. Nature. 1964 Jul 25;203:428–429. doi: 10.1038/203428a0. [DOI] [PubMed] [Google Scholar]
- RIVERA A., Jr, SRINIVASAN P. R. 3-Enolpyruvylshikimate 5-phosphate, an intermediate in the biosynthesis of anthranillate. Proc Natl Acad Sci U S A. 1962 May 15;48:864–867. doi: 10.1073/pnas.48.5.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDNEY H., PARSON W. W. THE CONVERSION OF P-HYDROXYBENZALDEHYDE TO THE BENZOQUINONE RING OF UBIQUINONE IN RHODOSPIRILLUM RUBRUM. J Biol Chem. 1963 Sep;238:3137–3138. [PubMed] [Google Scholar]
- Rao P. V., Moore K., Towers G. H. The conversion of tryptophan to 2,3-dihydroxybenzoic acid and catechol by Aspergillus niger. Biochem Biophys Res Commun. 1967 Sep 27;28(6):1008–1012. [PubMed] [Google Scholar]
- Ravel J. M., White M. N., Shive W. Activation of tyrosine analogs in relation to enzyme repression. Biochem Biophys Res Commun. 1965 Jul 26;20(3):352–359. doi: 10.1016/0006-291x(65)90372-4. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. REQUIREMENT OF DIHYDROXYPHENOLS FOR THE GROWTH OF MICROCOCCUS LYSODEIKTICUS IN SYNTHETIC MEDIA. Biochim Biophys Acta. 1964 May 11;86:421–422. doi: 10.1016/0304-4165(64)90078-9. [DOI] [PubMed] [Google Scholar]
- SANDERSON K. E., DEMEREC M. THE LINKAGE MAP OF SALMONELLA TYPHIMURIUM. Genetics. 1965 Jun;51:897–913. doi: 10.1093/genetics/51.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLESINGER S., MAGASANIK B. EFFECT OF ALPHA-METHYLHISTIDINE ON THE CONTROL OF HISTIDINE SYNTHESIS. J Mol Biol. 1964 Sep;9:670–682. doi: 10.1016/s0022-2836(64)80174-1. [DOI] [PubMed] [Google Scholar]
- SILBERT D. F., JORGENSEN S. E., LIN E. C. Repression of transaminase A by tyrosine in Escherichia coli. Biochim Biophys Acta. 1963 Jun 11;73:232–240. doi: 10.1016/0006-3002(63)90307-x. [DOI] [PubMed] [Google Scholar]
- SMITH L. C., RAVEL J. M., LAX S. R., SHIVE W. THE EFFECTS OF PHENYLALANINE AND TYROSINE ANALOGS ON THE SYNTHESIS AND ACTIVITY OF 3-DEOXY-D-ARABINO-HEPTULOSONIC ACID 7-PHOSPHATE SYNTHETASES. Arch Biochem Biophys. 1964 May;105:424–430. doi: 10.1016/0003-9861(64)90026-8. [DOI] [PubMed] [Google Scholar]
- SMITH L. C., RAVEL J. M., LAX S. R., SHIVE W. The control of 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthesis by phenylalanine and tyrosine. J Biol Chem. 1962 Nov;237:3566–3570. [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. STUDIES ON THE REGULATION OF TRYPTOPHAN BIOSYNTHESIS IN ESCHERICHIA COLI. J Mol Biol. 1965 Apr;11:747–759. doi: 10.1016/s0022-2836(65)80032-8. [DOI] [PubMed] [Google Scholar]
- SRINIVASAN P. R., RIVERA A., Jr THE ENZYMATIC SYNTHESIS OF ANTHRANILATE FROM SHIKIMATE 5-PHOSPHATE AND L-GLUTAMINE. Biochemistry. 1963 Sep-Oct;2:1059–1062. doi: 10.1021/bi00905a025. [DOI] [PubMed] [Google Scholar]
- SRINIVASAN P. R., ROTHSCHILD J., SPRINSON D. B. THE ENZYMIC CONVERSION OF 3-DEOXY-D-ARABINO-HEPTULOSONIC ACID 7-PHOSPHATE TO 5-DEHYDROQUINATE. J Biol Chem. 1963 Oct;238:3176–3182. [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- SRINIVASAN P. R., WEISS B. The biosynthesis of p-aminobenzoic acid: studies on the origin of the amino group. Biochim Biophys Acta. 1961 Aug 19;51:597–599. doi: 10.1016/0006-3002(61)90623-0. [DOI] [PubMed] [Google Scholar]
- Scholes P. B., King H. K. Isolation of a naphthaquinone with partly hydrogenated side chain from Corynebacterium diphtheriae. Biochem J. 1965 Dec;97(3):766–768. doi: 10.1042/bj0970766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville R. L., Elford R. Hydroxamate formation by anthranilate synthetase of Escherichia coli K12. Biochem Biophys Res Commun. 1967 Aug 7;28(3):437–444. doi: 10.1016/0006-291x(67)90331-2. [DOI] [PubMed] [Google Scholar]
- Sprecher M., Srinivasan P. R., Sprinson D. B., Davis B. D. The biosynthesis of tyrosine from labeled glucose in Escherichia coli. Biochemistry. 1965 Dec;4(12):2855–2860. doi: 10.1021/bi00888a042. [DOI] [PubMed] [Google Scholar]
- Srinivasan P. R. The biosynthesis of anthranilate from [3,4-'+C]glucose in Escherichia coli. Biochemistry. 1965 Dec;4(12):2860–2865. doi: 10.1021/bi00888a043. [DOI] [PubMed] [Google Scholar]
- TANIUCHI H., HATANAKA M., KUNO S., HAYAISHI O., NAKAJIMA M., KURIHARA N. ENZYMATIC FORMATION OF CATECHOL FROM ANTHRANILIC ACID. J Biol Chem. 1964 Jul;239:2204–2211. [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINS J. H., BARNES J. H. Effects of phencyclidine on the radiosensitivity of mice. Nature. 1962 Sep 22;195:1173–1175. [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Chromatography of 3-deoxy-D-arabinoheptulosonic acid-7-phosphate synthetase (trp) on diethylaminoethyl cellulose: a correction. J Bacteriol. 1967 Oct;94(4):1279–1280. doi: 10.1128/jb.94.4.1279-1280.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Genetic and biochemical analysis of the isoenzymes concerned in the first reaction of aromatic biosynthesis in Escherichia coli. J Bacteriol. 1967 Jan;93(1):237–244. doi: 10.1128/jb.93.1.237-244.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Srinivasan P. R. THE BIOSYNTHESIS OF p-AMINOBENZOIC ACID. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1491–1494. doi: 10.1073/pnas.45.10.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R., Goodwin T. W. Incorporation of [G-14cC]shikimate and [U-14C]para-hydroxybenzoate into phytoquinones and chromanols. Biochem Biophys Res Commun. 1966 Jun 21;23(6):849–853. doi: 10.1016/0006-291x(66)90565-1. [DOI] [PubMed] [Google Scholar]
- White P. J., Woods D. D. The synthesis of p-aminobenzoic acid and folic acid by staphylococci sensitive and resistant to sulphonamides. J Gen Microbiol. 1965 Aug;40(2):243–253. doi: 10.1099/00221287-40-2-243. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Crawford I. P. Purification and properties of the B component of Escherichia coli tryptophan synthetase. J Biol Chem. 1965 Dec;240(12):4801–4808. [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The tryptophan synthetase system. Bacteriol Rev. 1960 Jun;24(2):221–245. doi: 10.1128/br.24.2.221-245.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Gene structure and protein structure. Harvey Lect. 1967;61:145–168. [PubMed] [Google Scholar]
- Young I. G., Cox G. B., Gibson F. 2,3-Dihydroxybenzoate as a bacterial growth factor and its route of biosynthesis. Biochim Biophys Acta. 1967 Jul 25;141(2):319–331. doi: 10.1016/0304-4165(67)90106-7. [DOI] [PubMed] [Google Scholar]
- Young I. G., Jackman I. M., Gibson F. 2,3-dihydro-2,3-dihydroxybenzoic acid: an intermediate in the biosynthesis of 2,3-dihydroxybenzoic acid. Biochim Biophys Acta. 1967 Oct 9;148(1):313–315. doi: 10.1016/0304-4165(67)90311-x. [DOI] [PubMed] [Google Scholar]
- Zenk M. H., Leistner E. On the mode of incorporation of shikimic acid into 2-hydroxy-1,4-naphthoquinone (lawsone). Z Naturforsch B. 1967 Apr;22(4):460–460. doi: 10.1515/znb-1967-0425. [DOI] [PubMed] [Google Scholar]


