Abstract
In the last decade, drastic changes in the understanding of the role of the olfactory bulb and piriform cortex in odor detection have taken place through awake behaving recording in rodents. It is clear that odor responses in mitral and granule cells are strikingly different in the olfactory bulb of anesthetized vs. awake animals. In addition, sniff recording has evidenced that mitral cell responses to odors during the sniff can convey information on the odor identity and sniff phase. Moreover, we review studies that show that the mitral cell conveys not only information on odor identity but also on whether the odor is rewarded or not (odor value). Finally, we discuss how the substantial increase in awake behaving recording raises questions for future studies.
Keywords: olfaction, awake behaving, anesthetized, sniff, olfactory bulb, piriform cortex
Introduction
One of the most substantial questions remaining in olfactory processing is how changes in neuronal activity encode information on sensory input and gate sensory decision-making. With a few interesting exceptions, in the past the majority of studies involved extracellular signal recording or imaging studies in the olfactory bulb (OB) of anesthetized animals (Rinberg and Gelperin, 2006, Pain et al., 2011). However, in recent years evidence has suggested that in awake animals odor coding is dramatically different depending on behavioral status. Indeed these recent studies have raised the question whether early in the olfactory system, in addition to information on odor stimulus, changes in activity of mitral and tufted cells (MTs) could contain information relevant to decision making. Thus, even though anesthetized preparations can be incredibly informative, it is critical to study neuronal responses in awake and behaving animals exposed to different behavioral paradigms. This scenario will truly uncover the neuronal-firing-pattern/behavioral-output relationship. In this chapter we discuss the interesting current attempts to break the olfactory code signal processing in awake preparations. We discuss how changes in neuronal activity are related to olfactory stimulus and how they can be affected by experience and sniffing of odors. We also describe the relevance of temporal coding in the transmission of information about the odor identity (what is the smell?) and odor value (is the odor rewarded?). We emphasize recent studies in the olfactory bulb and include related studies in other brain areas such as the piriform cortex (PC).
Odors induce substantial glomerular activity with differential timing of activation as input to the olfactory bulb
Information on odor quality and intensity is conveyed in the awake or anesthetized animal through changes in neuronal activity in the glomerular layer (GL) of the olfactory bulb (Wachowiak and Shipley, 2006). Of approximately one thousand olfactory receptors, olfactory sensory neurons (OSN) expressing the same receptor convey their axons to one or two glomeruli in the OB (Mombaerts, 2006, Mombaerts et al., 1996, Serizawa et al., 2000). While the majority of OSNs are narrowly tuned, some neurons are quite non-specific responding to many odors exhibiting an enormous combinatorial capacity (Malnic et al., 1999, Araneda and Firestein, 2006, Nara et al., 2011). In this arrangement, a multidimensional odor molecule will activate a determined set of OSN creating a spatial two-dimensional map downstream in the glomerular layer of the OB (Johnson and Leon, 2007, Mori et al., 2006). When odorant intensity is augmented activated glomeruli are generally recruited, but sometimes a subset of the glomeruli are turned off (Johnson and Leon, 2000, Schaefer et al., 2001, Spors and Grinvald, 2002, Wachowiak and Cohen, 2001, Fletcher et al., 2009). In addition to the spatial maps conveying information about odor identity and concentration, temporal dynamics of glomerular activation can also carry information about odor quality (Spors et al., 2006, Bathellier et al., 2010, Carey et al., 2009). Importantly, it has been recently demonstrated that mice can detect differences in glomerular activation timing during the sniff (Smear et al., 2011) and that this time code can be read out downstream by the PC (Haddad et al., 2013).
Odors induce substantial changes in mitral cell firing rate in the anesthetized animal
After information about the odor cue is represented in the GL it is transmitted to MTs whose changes in neuronal activity elicited by the glomerular input are modulated by local interneurons, such as periglomerular interneurons and granule cells (GC) (Wachowiak and Shipley, 2006, Jahr and Nicoll, 1982b, Isaacson and Strowbridge, 1998, Schoppa et al., 1998). Olfactory signals processed by these local circuits are modified and transferred to the piriform cortex and other subcortical regions (Shepherd et al., 2004, Nagayama et al., 2010, Wachowiak and Shipley, 2006, Linster and Cleland, 2009). Therefore, MT activity ultimately represents olfactory information in the OB. Based on work with anesthetized mice it was suggested that olfactory information is coded by overall changes in MT spike rate and decoded by upstream neurons, such as pyramidal neurons in the PC (Yokoi et al., 1995, Mori et al., 1999, Bathellier et al., 2008, Wellis et al., 1989,, Cang and Isaacson, 2003, Davison and Katz, 2007). It was found that olfactory input to MTs clearly affected the firing rate during the sniff in an odor quality-specific manner. These MTs in anesthetized animals tended to have a lack of firing in between sniffs that was reflected in low levels of spontaneous activity. Additionally, during the sniff cycle odors could enhance or suppress MT firing (Fig. 1) (Bathellier et al., 2008, Rinberg et al., 2006, Wellis et al., 1989, Yokoi et al., 1995, Cang and Isaacson, 2003). Therefore, in the anesthetized state, olfactory information is coded by complex temporal patterns of action potential firing strongly modulated by respiration.
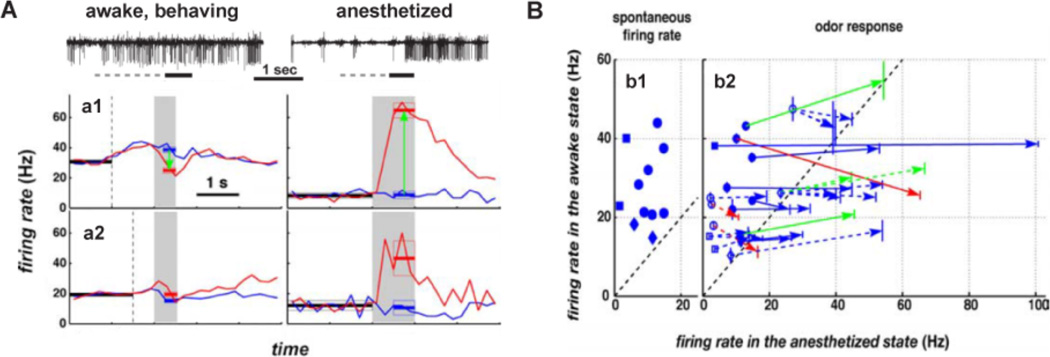
Figure 1.
Differential response of MT cells to odors in awake vs. anesthetized animals. A. Mitral cells exhibited increased spontaneous activity and weaker responses to odors in the awake state. Top, raw traces of MTs of extracellular recordings in awake, behaving and anesthetized mice. The solid horizontal bar indicate the time of odorant exposure (amyl acetate) and the dash lines represent the time the animal spent in the port before odor delivery. Bottom, average firing rate responses of MTs to odorants in the awake and the anesthetized states. The gray area represents the time of odorant exposure and the vertical dashed line shows the average time a mouse poked its nose into the odor port. The horizontal thick lines indicate the average firing rate ± S.E., during spontaneous activity (black), null response (blue) and response to the odor (red). a1, comparison of the firing rate of a MT in response to amyl acetate (red) and citral (blue). a2, comparison of the firing rate of a different MT in reponse to 2-nonanone (red) and amyl acetate (blue). Notice that an increase in average firing rate started when the animal poked its nose onto the odor port. B. Summary of all recordings. b1, spontaneous firing rate for single-unit recordings in the awake state versus spontaneous firing rate in the anesthetized state during the first 10 min after anesthesia. Error bars are smaller than the symbols. b2, odorant responses for single units (filled symbols and solid lines) and multiunit recordings (open symbols and dashed lines). Each arrow starts at the null responses for a given cell and ends at the response to an odorant. Vertical lines at the origin and the end of each arrow are S.E. for firing rate estimations in the awake state. For blue arrows, there are no statistically significant differences between the null response and odorant response. Green and red arrows indicate recordings for which there is a statistically significant excitatory (green) or inhibitory (red) odorant response. Symbols in b1 and b2 indicate the type of behavioral paradigm used in the recording: circle, go/no go paradigm; square, two alternative forced choice paradigm; diamond, BUZ paradigm. In the BUZ paradigm the mouse needed to keep its nose in the odor port and be exposed to an odorant until an audio signal sounded. Modified from Rinberg et al (Rinberg et al., 2006).
Odor-induced firing of mitral cells is high in anesthetized animals and substantially decreased in awake animals because of increased inhibition by granule cells
As opposed to MT responses in anesthetized preparations, in the awake animal odors elicit spared increases in firing rate (Cury and Uchida, 2010, Spors et al., 2012, Smear et al., 2011, Rinberg et al., 2006, Doucette et al., 2011, Doucette and Restrepo, 2008, Gschwend et al., 2012) (Fig. 1). What can be influencing MT responses in the awake animal? The OB regulates MT neuronal activity and the transmission of information through inhibitory neurons by either the glomerular interneurons or GCs. Indeed, dendrodendritic inhibition by GCs onto MT lateral dendrites modulates the output to other brain regions (Isaacson and Strowbridge, 1998, Lowe, 2003, Schoppa et al., 1998, Jahr and Nicoll, 1982a) contributing to the processing of olfactory information, such as olfactory discrimination (Imamura et al., 1992, Yokoi et al., 1995, Doucette et al., 2011). Interestingly, Kato and co-workers, have shown that under anesthesia there was virtually no GC spontaneous activity compared to substantial activity in the awake animal (Kato et al., 2012) (Fig. 2). Furthermore, GC responses were weaker and induced by fewer odorants when compared to the awake state (Kato et al., 2012). Therefore, the augmentation in MTs of odor-responsiveness under anesthesia results, at least partially, from a dramatic decrease in M-TGC dedrodendritic inhibition.
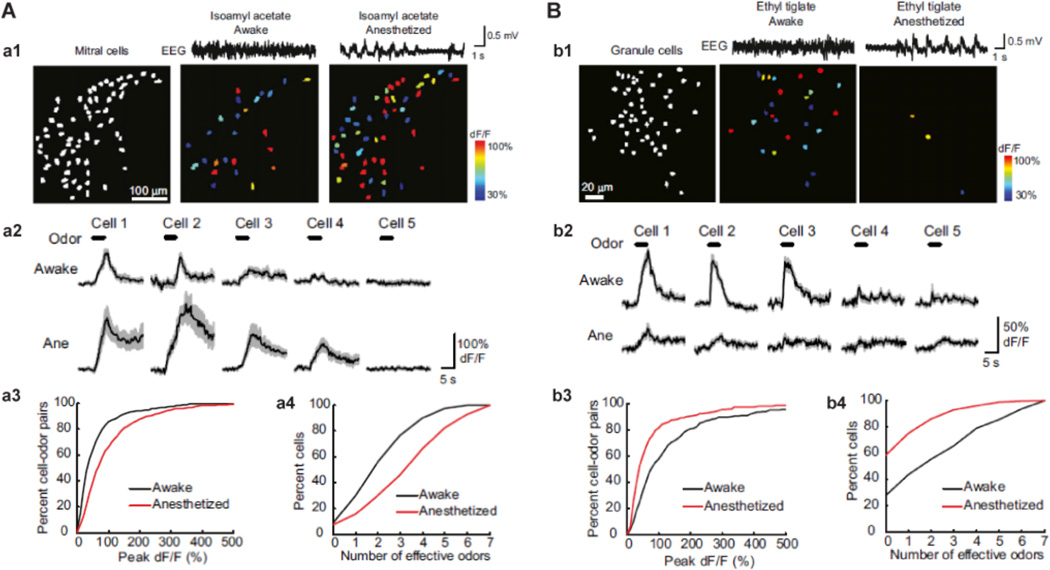
Figure 2.
Calcium imaging with GCaMP3 of neuronal responses in the awake and anesthetized brain states. A. Mitral cell odor responses in the awake state are sparse compared to the anesthetized animals. a1, mitral cell odor-evoked activity maps from a mouse recorded before and after the induction of anesthesia. Mitral cell ensemble response is weaker in the awake state (middle) compared to the anesthetized state (right). The left panel shows all imaged mitral cells in white. The top traces show example electroencephalography (EEG) traces. a2, average (solid lines) and S.E.M. (shading) of odor-evoked GCaMP3 responses from five mitral cells in (a1) during awake and anesthetized states show an increase in odor-evoked activity after the induction of anesthesia. a3, cumulative frequency plot of the peak response amplitude for all mitral cell-odor pairs that were responsive in both the awake and anesthetized animal. Anesthesia causes a rightward shift (i.e., larger responses) in the anesthetized state. a4, summary data shown in cumulative frequency plot of odor tuning (the number of odors eliciting responses out of the seven tested odors) for mitral cells in the awake state compared to after induction of anesthesia. Anesthesia causes a rightward shift (i.e., broader tuning) in the anesthetized state. B. Granule cell activity is enhanced during wakefulness. b1, granule cell odor-evoked activity maps (pseudocoloring represents odor-evoked GCaMP3 dF/F response averaged across seven trials) from a mouse recorded in both the awake and anesthetized state. Granule cell ensembles respond more strongly to the same odor in the awake state (middle) compared to under anesthesia (right). The left panel shows all imaged granule cells in white. Top traces are example EEG traces in both states. b2, average (solid lines) and S.E.M. (shading) of odor-evoked GCaMP3 responses from five granule cells in (b1) during awake and anesthetized states show a decrease in odor-evoked activity after the induction of anesthesia. b3, cumulative frequency plot of the peak response amplitude of all granule cell-odor pairs that were responsive in both the awake and anesthetized state shows a leftward shift (i.e., smaller responses) in the anesthetized state. b4, summary data shown in cumulative frequency plot of odor tuning for granule cells in the awake state compared to after induction of anesthesia. Anesthesia causes a leftward shift (i.e., narrower tuning). Modified from (Kato et al., 2012).
There is a lack of overall odor-induced changes in firing rate in mitral cells in a subset of awake animals
As stated above, sensory information processing, is strikingly different in the awake state. Animals under anesthesia do not actively regulate stimulus sampling through changes in sniffing, a behavior that has been well documented in awake rodents and involves an increase in sniffing frequency while the animal is actively discriminating an odor (Wachowiak, 2010, Doucette et al., 2011, Gire et al., 2013, Carey et al., 2009). Moreover, under anesthesia projections to the OB from other brain regions are suppressed strongly affecting neural dynamics (Rinberg and Gelperin, 2006). In addition, the OB receives extensive centrifugal fibers from other brain regions including feedback projections from the accessory olfactory nucleus and piriform cortex and neuromodulatory afferents (Matsutani, 2010) all of which are known to play an important role in olfactory mediated tasks. For instance, cholinergic and GABAergic release from the basal forebrain is known to modulate MT and GC excitability and to facilitate olfactory discrimination (Ma and Luo, 2012, Smith and Araneda, 2010, Devore and Linster, 2012, Nunez-Parra et al., 2013). On the same token, recent work suggests that feedback projection from the PC and accessory olfactory nucleus regulate olfactory processing in the bulb affecting the individual’s behavioral output (Balu et al., 2007, Boyd et al., 2012, Markopoulos et al., 2012). Therefore, the disruption onto the centrifugal fibers by anesthetics can have an enormous impact on the activity of bulbar neurons and a dramatic impact on how odorant-related information is encoded.
Indeed, extracellular recordings of MTs showed that their spontaneous activity was larger in the awake state compared to the anesthetized state (Rinberg et al., 2006). Moreover, neuronal responses to olfactory stimulation through changes in firing rate were sparser during wakefulness: MTs lost their sensitivity to odorants after the transition from anesthetized to awake state. Interestingly, some MTs exhibited weaker responses or even reverted signs when the animal recovered from anesthesia (Fig. 1). These results are in agreement with one of the first studies performed in awake freely moving animals, in which MT activity was recorded in rats trained to discriminate between two odors (Kay and Laurent, 1999). They observed that very few MTs (only 11%) responded with changes in firing rate after the presentation of the odor stimulus. Importantly, studies in several experiments showed that odors did not or elicit weak changes in MT firing rate (Rinberg et al., 2006, Gschwend et al., 2012). In addition, in an odor go-no go odor learning experiment MT differential responses by changes in firing rate were nearly absent when the animal starts learning but then it increases substantially to 56% (Doucette et al., 2011). Moreover, recent calcium imaging studies performed in the MT layer of head-fix animals corroborated these findings. After odor exposure, MTs greatly enhanced their odor-evoked responses and the density of odor representations (the number of MTs responding to a particular odor) in the transition from awake to anesthetized (Kato et al., 2012). Importantly they showed that the reason why in the awake animal the MTs are not responsive is likely due to the fact that the activity of the granule cell in anesthetized mice is absent and is much larger in an awake animal.
Importantly, in mice undergoing go-no go learning the responses of MTs to rewarded and non-rewarded odors are extremely different. Thus Fig. 3 shows that synchronized firing between two different neurons is increased by exposure to the rewarded odor, and decreased in firing elicited by exposure to the unrewarded odor. Importantly, as shown in Fig. 3B if the odor that was a rewarded is made an unrewarded odor the response is reversed. This evidence strongly suggests that the positive response elicited by the odor does not reflect its identity, but rather whether that odor is rewarded as opposed to unrewarded. As a result this shows that synchronized firing in these MTs carries information on odor quality (is the odor rewarded or not) as opposed to odor identity (what is this smell?)(Doucette et al., 2011).
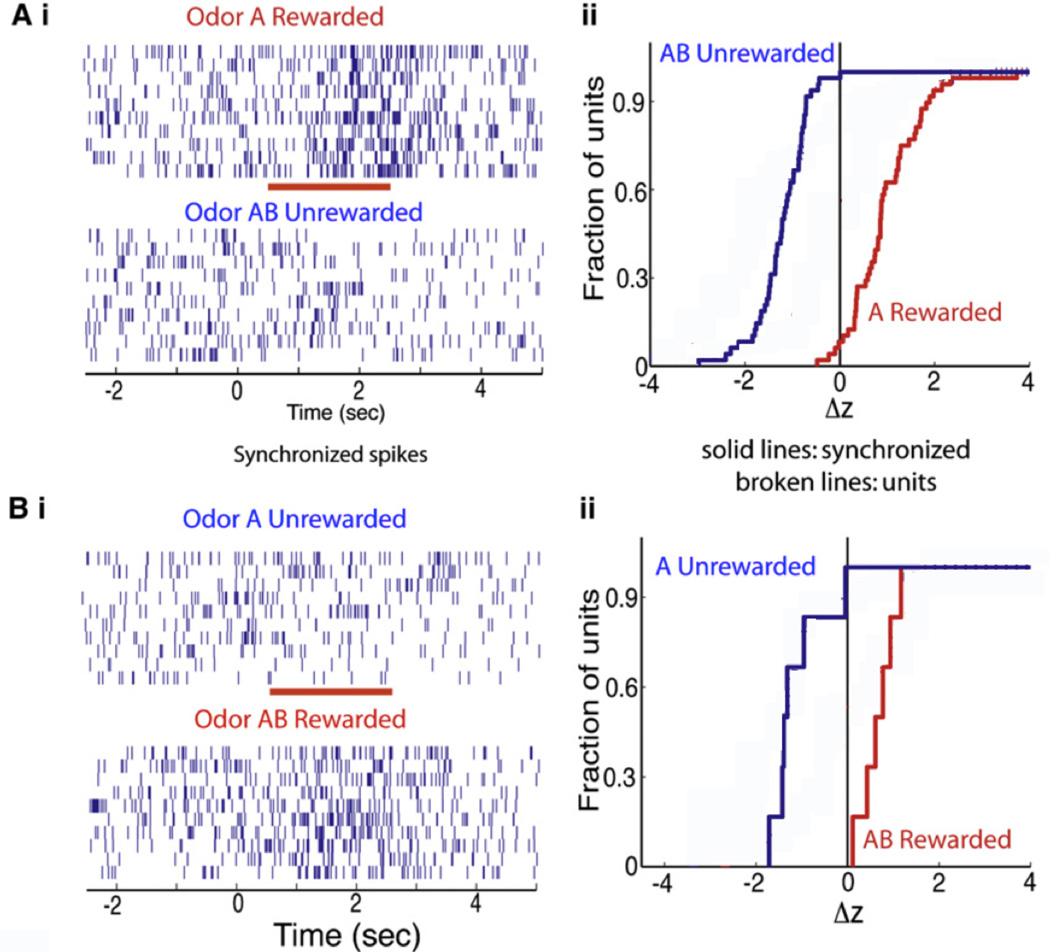
Figure 3.
Odor responses of spikes synchronized between two multiunits spike trains are shown for one block of the session wherein the mice learned to differentiate between odors A (rewarded) and AB (unrewarded) (Fig. 3A) and one for a second session wherein the odors were reversed for reward (AB rewarded, A unrewarded) (Fig. 3B). Ai. Synchronized spike trains for divergent odor responses, the red bar is the when the odor is applied. Aii Z-score cumulative histogram for 48 odor-divergent synchronized spike trains. Bi and Bii. Synchronized spike trains (Bi) and z-score cumulative histogram (Bii) for odor reversal session including 6 multiunit synchronized pairs (solid lines, n = 6) (odor A, blue-unrewarded; and odor AB, red-rewarded) are shown. Modified from (Doucette et al., 2011).
Neural representation of input to olfactory bulb can be shaped by sniff
Olfactory sampling can be actively modified by changes in respiration frequency. This active control on incoming sensory information allows animals to generate critical context-dependent odorant representations. Interestingly, activation of OSNs and concomitant input transmission to the GL can occur during the sniff even in the absence of an odor, suggesting that sniff itself could somehow activate OSNs. It has been proposed that this sniff-mediated effect is likely mediated by mechanical receptors located in the OSNs, activated by the sniff air pressure (Grosmaitre et al., 2007), although it could also be caused by stimuli present in the regular air. Consistent with these findings, studies using fluorescent calcium sensitive dyes expressed presynaptically in OSN terminals found that in the absence of odor stimulation about 50% of glomerular activation was driven by sniff (Carey et al., 2009). When OSNs are activated by odorants delivered by sniff, the number of glomeruli showing inhalation (sniff) locked response patterns increases considerably. Interestingly, this type of odor evoked, sniff locked response pattern is dependent of sniff frequency: while low frequency sampling (<4Hz) evokes sniff locked responses that return rapidly to baseline, high frequency sniffing (4–8 Hz) evokes sustained responses showing no clear coupling to inhalation. The attenuation of OSN inputs during high sniff frequency sampling might be useful to selectively suppress OSN activation by background odors during exploratory sniffing (Wachowiak, 2011). This OSN decoupling phenomenon could be mediated by low-level processes and may not depend on centrifugal modulation by behavioral state (Verhagen et al., 2007). Therefore, sniff can shape activity pattern of OSN input to glomeruli, both at a baseline levels and during odor evoked responses (Carey et al., 2009, Verhagen et al., 2007).
Another interesting point that can be addressed by imaging studies performed in awake animals is a simultaneous comparison between neural responses and behavioral choices (Verhagen et al., 2007, Wesson et al., 2008b). In awake head-fixed animals engaged in an olfactory discrimination task, the behavioral output shows that the time required to correctly discriminate between different odorants can be as short as 140 msec, a period of time that lies within a fraction of the sniff cycle. However, OSN inputs to the bulb arrives about 100–150 msec after inhalation begins, leaving only 50–100 msec for central processing and response initiation. Yet, odor discrimination can occur before the full development of OSN activation and input arrival to the OB (Wesson et al., 2008a) indicating that although sniff could shape receptor input to the glomeruli, no significant role is played by sniff when olfactory discrimination is performed quickly (Wesson et al., 2009). These results are in contrast with other studies performed in freely-moving animals that found that odor information coded within the sniff is critical for odor discrimination (Kepecs et al., 2007). It is worth to mention that these studies used different behavioral tasks (2-choice discrimination vs. passive discrimination) and states (free-moving vs head-fixed; (Wesson et al., 2009) vs. (Kepecs et al., 2007), respectively) that can account for the differences observed.
Overall, at the very early glomerular input level to the OB, sniff dramatically affects the neural activity input, including patterns of spontaneous and odor evoked glomerular activity, response intensity, and even spatial patterns of activation. However, the contribution of the change of neural activities on behavior output is debated and more work is necessary in the future.
Sniff locked mitral and tufted cells firing provides odor information
The MT cells are the main output from the OB to higher olfactory centers, such as PC and anterior olfactory nuclei. They convey odor information that has been represented and processed by local neural circuits in OB. The fact that spontaneous activity without odor stimulation and/or odor evoked responses of most MT cells showed highly specific phase locking to sniffing cycle (Kepecs et al., 2006, Wachowiak, 2011), indicates profound and dramatic effects of sniff on the output neural activity in the OB.
The sniff phase locked firing of MT cells has been a common feature found in anesthetized animals (Buonviso et al., 2003, Gervais et al., 2007, Grosmaitre et al., 2007), and recently was also directly observed in awake animals (Cury and Uchida, 2010, Fukunaga et al., 2012, Patterson et al., 2013, Shusterman et al., 2011). The firing occurs at almost the same phase of each sniff cycle, yielding a specific distribution of spikes within the sniff cycle (Fig. 4). The specific distribution phases of different MT cells are largely variable, with some cells firing at the beginning of inhalation, some at the beginning of exhalation and some in between. A very recent in vivo study performed in awake mice has shown that MT cells lock their activity to distinct phases of sniff cycle, with tufted cell firing earlier, and mitral cells later. (Fukunaga et al., 2012). This phase shift is suggested to result from inhibition in the OB that selectively delays mitral cell activity. These results indicate that mitral and tufted cells might be encoding different type of information about the odors, idea that is supported by the fact that mitral and tufted cells project differentially to the anterior PC and olfactory tubercle, respectively (Igarashi et al., 2012, Nagayama et al., 2010).
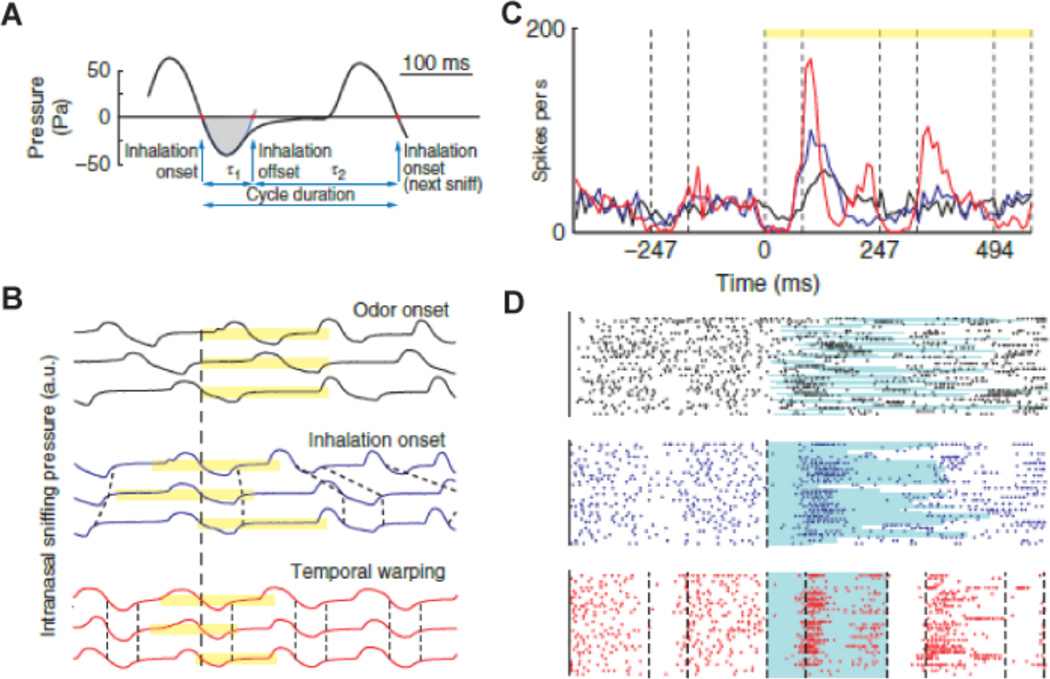
Figure 4.
Odor responses are locked to the sniff cycle. A. Pressure waveform of a typical breathing cycle. Red dots indicate the inhalation onsets and offsets. The blue line is the parabolic fit to the first minimum after the inhalation onset. The sniff offset was defined as the second zero crossing of the parabolic fit. The gray shaded area marks the inhalation interval. B. Intranasal pressure signal for three trials: aligned by odor onset (black), by the first inhalation after odor onset (blue) and temporally warped, so that each inhalation interval and the reminder of the sniff duration are equal to the average values (red). Yellow bars indicate the stimulus duration. Dashed lines indicate the beginning and the end of the inhalation intervals. (C,D) Peristimulus time histogram (PSTH) and raster plots for a mitral/tufted cells in response to an odor stimulus: synchronized by odor onset (black), inhalation onset (blue) and temporally warped (red, see Susterman et al for method used for temporally wraping(Smear et al., 2011)). The light blue lines underlying the raster plots indicate the duration of the first sniff after odor onset. Vertical dashed lines indicate the beginning and end of inhalation intervals. Yellow bars indicate the odor stimulus duration. Modified from (Shusterman et al., 2011).
Similar to glomerular activation, the sniff locked firing pattern of MT cells in awake animals is usually observed when the sniff frequency is below 5 Hz, a frequency slight higher that the ~2 Hz sniffing frequency that animals exhibit while awake but not engaged in an odor behavioral tasks. However, the sniff frequency during specific behavioral states can be as high as 12–15 Hz (Wesson et al., 2008b). To what extent can the OSN firing be locked to each sniff cycle during high frequency sampling has been partially studied in awake animals. Such study can give important insights on how self-controlled sniff shapes temporal pattern input onto MT cells.
Based on the tight relationship between sniff cycle and neural activity in OB, some odor information should be then encoded on a single sniff timescale (Wachowiak, 2011) and that a single sniff is required to generate a perceptual “snapshot” of the olfactory stimulus (Wesson et al., 2008a). Data supporting this hypothesis has been collected in recent studies where it has been found that the first inhalation of an odorant evoked reliable and cell-specific spike patterns within the time course of a single rapid sniff (Cury and Uchida, 2010, Shusterman et al., 2011). Cury and Uchida, found that these subsniff patterned responses convey significantly more information in their fine-scale fluctuations (20–40 msec timescale) as compared with the total spike count from the entire respiration cycle. and that the initial portions of these patterns are highly conserved between rapid sniffing and slow breathing odor response (Cury and Uchida, 2010). A study performed by Shusterman and co-workers also showed that odorants evoked precisely first sniff-locked activity in MT cells with a trial-to-trial response jitter of approximately 12 msec. Since sniff cycles are so diverse in awake animals (varying in duration, amplitude and waveform), they showed that studying MTs responses to odor by aligning spike generation to odor onset, could miss essential information on odor coding. Therefore, they aligned MTs responses to inhalation onset (as opposed to odor onset) and also normalized MT responses so that each sniff cycle will have the same standard duration (sniff-wrapped analysis, Fig. 5). They found that analysis of these data uncovered temporal structures that were not evident before. Moreover, they found that MT exhibit inhibitory or excitatory responses after odor stimulation, with MT responses varying in latency of activation (Shusterman et al., 2011). Taken together, this evidence suggests that high temporal precision of inhalation-coupled MT activity generates a robust temporal neural code in awake behaving animals.
As discussed above, odor-evoked changes in MT activity during wakefulness are sparse and weak (Koulakov and Rinberg, 2011, Rinberg et al., 2006, Gelperin and Ghatpande, 2009). The theory of sparse coding in the OB raises the question of how odor information, such as odor identity, is encoded by the neural activity in the OB. One proposed hypothesis states that the temporal encoding strategy, in which one single intact sniff cycle functions as a base for spike distribution, is extremely useful and important for MT cells to transmit information about the odor. Indeed, recordings in awake head-fixed mice, it has been found that although most MT cells are rate invariant over the complete breathing cycle, this neuronal population expresses fine temporal changes in firing within the sniff that contain sufficient information to be used to discriminate between different odorants (Gschwend et al., 2012). This kind of subsniff temporal coding could then convey information about the odorant identity. However whether this coding strategy is used in awake behaving animals need to be clarified in future studies.
Few studies have explored the role played by respiration in regions others than the OB. These studies have shown that higher olfactory centers, such as piriform cortex (Gire et al., 2013, Miura et al., 2012, Zhan and Luo, 2010) and anterior olfactory nuclei (Kikuta et al., 2010, Kikuta et al., 2008) also exhibit spontaneous and/or odor evoked sniff-locked firing in both anesthetized and awake states (Litaudon et al., 2008, Miura et al., 2012, Gire et al., 2013, Zhan and Luo, 2010). In the future, the comparison of how sniff shapes the neural representation between the OB and other olfactory centers is crucial and important to understand neural coding in the central olfactory system.
Sniff and local field potentials (LFPs)
While spikes encode information transmitted by single neurons, local field potential (LFP) recordings provide reliable information on synchronized activity of large groups of neurons (Buzsaki and Watson, 2012). Importantly, LFP oscillation in the OB transfers information on MT synchronization that is particularly important for olfactory mediated tasks, such as olfactory discrimination (Kay, 2005, Beshel et al., 2007, Lagier et al., 2007, Doucette et al., 2011).
When an animal is engaged in exploring behavior and actively sniffing, LFP recording of the OB exhibits a pronounced theta oscillation band (4–12 Hz) (Kay et al., 2009). Indeed, the theta LFP oscillations and sniffs are somewhat related and it has been proposed that the sniff cycle could elicit the theta oscillation in the bulb. This hypothesis is supported by findings that OSNs can function both as chemical and mechanical sensors generating sniff-related output to the glomeruli even in the absence of odor presentation, and because the knockout of cycle nucleotide gated channel that blocks transduction in OSNs elicits inactivation of the theta LFP in the OB (Grosmaitre et al., 2007). In awake behaving animals, however, this strong correlation of sniff and theta LFP varies depending on the behavioral context. For instance, in an experiment involving rats tracking odor trails, the correlation between sniff and theta oscillation was largely variable (Khan et al., 2012). Interestingly, in a go-no go odor discrimination task, it was shown that odor learning was highly correlated with the coherence of the LFP in the OB and higher olfactory centers such as the dorsal hippocampus (Kay, 2005). Future studies should determine the theta oscillation/sniff relationship in go no-go and alternative forced choice discrimination tasks and whether variations in sniff/LFP relate to animal performance.
The gamma oscillation from the OB (40–90 Hz) usually appears at a specific phase of the sniff cycle, (Cenier et al., 2009, Manabe and Mori, 2013, Rosero and Aylwin, 2011). Considering the important role of gamma oscillation during olfactory perception and cognition, and olfaction related learning and memory (Kay et al., 2009, Beshel et al., 2007, Martin et al., 2004), the modulation gamma oscillation during sniff is likely crucial for neural transmission of information to higher olfactory brain areas. In a recent study, it has been found that high-frequency sniffing augmented the power of gamma oscillation, while gamma oscillation was not modified at the lower frequency respiratory rate (Rosero and Aylwin, 2011, Manabe and Mori, 2013). However, high-frequency sniffing prolonged the overall response to odorants and increased the frequency of oscillation, indicating that high-frequency sniffing reduces the adaptation to continuous odorant stimulation. Therefore, the modulation of sniff frequency on gamma oscillation may facilitate odorant memory formation for subsequent odorant identification.
The sniff coupled gamma oscillations from mitral vs. tufted cells are quite different at the temporal domain. The gamma oscillations recorded from tufted cells are fast and at early phase of the sniff, while the gamma oscillations from mitral cells are slow and at late phase of the sniff (Manabe and Mori, 2013). The shift of gamma oscillations probably corresponds to the difference in activation timing of tufted cells and mitral cells. This kind of sniff coupled gamma oscillation from different cell types is also dependent on brain states: both fast and slow gamma oscillations can be induced during awake state, while both oscillations failed to induce during slow wave and rapid-eye-movement sleep. Interestingly the axons from mitral and tufted cells differ largely in their main targeting of piriform cortex as opposed to the olfactory tubercle (Nagayama et al., 2010).
In general, sniff can shape gamma oscillations at a single cycle, and modulate the intensity and temporal domain by change the sniff frequencies. Whether neural oscillations are related to behavioral output is an important direction for future studies.
Sniff and olfactory afterimage
During behavior, animals can percept the odorant within a single sniff, and the responses of some MT cells are robust within the first sniff cycle (Cury and Uchida, 2010, Shusterman et al., 2011). However, in awake mice some MT cells showed persistent responses to odors after the first sniff (Patterson et al., 2013, Doucette et al., 2011, Doucette and Restrepo, 2008) and responses after the first sniff were related to behavioral responses (Doucette et al., 2011, Doucette and Restrepo, 2008). During the odor stimulation, the odor representation for some cells shifts significantly after the first sniff and not via simple attenuation. A subset of cells responded in an odor-specific manner after odor cessation, a phenomenon named olfactory afterimage (Patterson et al., 2013). Therefore, sniffs of responses during and after odor stimulation can carry information of odorants. The first sniff and the following sniffs probably provide different aspects of information for the odorant, such as identity, concentration, and even odor value.
Conclusion
The awake behaving recording from mice has mushroomed in the last decade resulting in a substantial increase of the understanding of the involvement of the olfactory bulb in conveying information on the fact that odor identity and odor value are both present in MT activity. Likely the information on odor identity is conveyed by the sniff-locked MT activity, while the information on odor value is conveyed by odor-induced changes in average MT rate. However, in future work it is key to perform awake behaving recording and relate changes in MT activity to different behaviors. In addition, in future work with these behavioral experiments and experiments when the animal is not performing specific behavior it is important to record not only the activity of areas of the brain such as olfactory bulb or piriform cortex but also simultaneously record from upstream areas such as prefrontal cortex. Future studies will provide exciting improved understanding of odor signal processing and why the olfactory information is conveyed through direct output of the information from the piriform cortex to prefrontal cortex bypassing the thalamus.
Acknowledgements
Funded by NIH/NIDCD grants R01 DC00566 and P30 DC04657, and National Natural Science Foundation of China (NSFC, 31100799).
References
- Araneda RC, Firestein S. Adrenergic enhancement of inhibitory transmission in the accessory olfactory bulb. J.Neurosci. 2006;26:3292–3298. doi: 10.1523/JNEUROSCI.4768-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu R, Pressler RT, Strowbridge BW. Multiple modes of synaptic excitation of olfactory bulb granule cells. J.Neurosci. 2007;27:5621–5632. doi: 10.1523/JNEUROSCI.4630-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron. 2008;57:586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Bathellier B, Gschwend O, Carleton A. Temporal Coding in Olfaction. In: Menini A, editor. The neurobiology of olfaction. Boca Raton, FL: CRC Press/Taylor & Francis; 2010. [PubMed] [Google Scholar]
- Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci. 2007;27:8358–8365. doi: 10.1523/JNEUROSCI.1199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AM, Sturgill JF, Poo C, Isaacson JS. Cortical feedback control of olfactory bulb circuits. Neuron. 2012;76:1161–1174. doi: 10.1016/j.neuron.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonviso N, Amat C, Litaudon P, Roux S, Royet JP, Farget V, Sicard G. Rhythm sequence through the olfactory bulb layers during the time window of a respiratory cycle. Eur.J.Neurosci. 2003;17:1811–1819. doi: 10.1046/j.1460-9568.2003.02619.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Watson BO. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin Neurosci. 2012;14:345–367. doi: 10.31887/DCNS.2012.14.4/gbuzsaki. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Isaacson JS. In vivo whole-cell recording of odor-evoked synaptic transmission in the rat olfactory bulb. J Neurosci. 2003;23:4108–4116. doi: 10.1523/JNEUROSCI.23-10-04108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Verhagen JV, Wesson DW, Pirez N, Wachowiak M. Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats. J Neurophysiol. 2009;101:1073–1088. doi: 10.1152/jn.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenier T, David F, Litaudon P, Garcia S, Amat C, Buonviso N. Respiration-gated formation of gamma and beta neural assemblies in the mammalian olfactory bulb. Eur J Neurosci. 2009;29:921–930. doi: 10.1111/j.1460-9568.2009.06651.x. [DOI] [PubMed] [Google Scholar]
- Cury KM, Uchida N. Robust odor coding via inhalation-coupled transient activity in the mammalian olfactory bulb. Neuron. 2010;68:570–585. doi: 10.1016/j.neuron.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Davison IG, Katz LC. Sparse and selective odor coding by mitral/tufted neurons in the main olfactory bulb. J.Neurosci. 2007;27:2091–2101. doi: 10.1523/JNEUROSCI.3779-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore S, Linster C. Noradrenergic and cholinergic modulation of olfactory bulb sensory processing. Front Behav Neurosci. 2012;6:52. doi: 10.3389/fnbeh.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette W, Gire DH, Whitesell J, Carmean V, Lucero MT, Restrepo D. Associative Cortex Features in the First Olfactory Brain Relay Station. Neuron. 2011;69:1176–1187. doi: 10.1016/j.neuron.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette W, Restrepo D. Profound context-dependent plasticity of mitral cell responses in olfactory bulb. Plos Biol. 2008;6:e258. doi: 10.1371/journal.pbio.0060258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Masurkar AV, Xing J, Imamura F, Xiong W, Nagayama S, Mutoh H, Greer CA, Knopfel T, Chen WR. Optical imaging of postsynaptic odor representation in the glomerular layer of the mouse olfactory bulb. J Neurophysiol. 2009;102:817–830. doi: 10.1152/jn.00020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga I, Berning M, Kollo M, Schmaltz A, Schaefer AT. Two distinct channels of olfactory bulb output. Neuron. 2012;75:320–329. doi: 10.1016/j.neuron.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Gelperin A, Ghatpande A. Neural basis of olfactory perception. Ann N Y Acad Sci. 2009;1170:277–285. doi: 10.1111/j.1749-6632.2009.04110.x. [DOI] [PubMed] [Google Scholar]
- Gervais R, Buonviso N, Martin C, Ravel N. What do electrophysiological studies tell us about processing at the olfactory bulb level? J.Physiol Paris. 2007;101:40–45. doi: 10.1016/j.jphysparis.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Gire DH, Whitesell JD, Doucette W, Restrepo D. Information for decision-making and stimulus identification is multiplexed in sensory cortex. Nat Neurosci. 2013;16:991–993. doi: 10.1038/nn.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci. 2007;10:348–354. doi: 10.1038/nn1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwend O, Beroud J, Carleton A. Encoding odorant identity by spiking packets of rate-invariant neurons in awake mice. Plos one. 2012;7:e30155. doi: 10.1371/journal.pone.0030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Lanjuin A, Madisen L, Zeng H, Murthy VN, Uchida N. Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nat Neurosci. 2013;16:949–957. doi: 10.1038/nn.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM, Ieki N, An M, Yamaguchi Y, Nagayama S, Kobayakawa K, Kobayakawa R, Tanifuji M, Sakano H, Chen WR, Mori K. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J Neurosci. 2012;32:7970–7985. doi: 10.1523/JNEUROSCI.0154-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Mataga N, Mori K. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. I. Aliphatic compounds. J Neurophysiol. 1992;68:1986–2002. doi: 10.1152/jn.1992.68.6.1986. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Nicoll RA. An intracellular analysis of dendrodendritic inhibition in the turtle in vitro olfactory bulb. J Physiol. 1982a;326:213–234. doi: 10.1113/jphysiol.1982.sp014187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Nicoll RA. Noradrenergic modulation of dendrodendritic inhibition in the olfactory bulb. Nature. 1982b;297:227–229. doi: 10.1038/297227a0. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J.Comp Neurol. 2000;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Chemotopic odorant coding in a mammalian olfactory system. J.Comp Neurol. 2007;503:1–34. doi: 10.1002/cne.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Chu MW, Isaacson JS, Komiyama T. Dynamic sensory representations in the olfactory bulb: modulation by wakefulness and experience. Neuron. 2012;76:962–975. doi: 10.1016/j.neuron.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM. Theta oscillations and sensorimotor performance. Proc Natl Acad Sci U S A. 2005;102:3863–3868. doi: 10.1073/pnas.0407920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Brea J, Martin C, Rojas-Libano D, Kopell N. Olfactory oscillations: the what, how and what for. Trends Neurosci. 2009 doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat.Neurosci. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem.Senses. 2006;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. Rapid and precise control of sniffing during olfactory discrimination in rats. J Neurophysiol. 2007;98:205–213. doi: 10.1152/jn.00071.2007. [DOI] [PubMed] [Google Scholar]
- Khan AG, Sarangi M, Bhalla US. Rats track odour trails accurately using a multi-layered strategy with near-optimal sampling. Nat Commun. 2012;3:703. doi: 10.1038/ncomms1712. [DOI] [PubMed] [Google Scholar]
- Kikuta S, Kashiwadani H, Mori K. Compensatory rapid switching of binasal inputs in the olfactory cortex. J Neurosci. 2008;28:11989–11997. doi: 10.1523/JNEUROSCI.3106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta S, Sato K, Kashiwadani H, Tsunoda K, Yamasoba T, Mori K. From the Cover: Neurons in the anterior olfactory nucleus pars externa detect right or left localization of odor sources. Proc Natl Acad Sci U S A. 2010;107:12363–12368. doi: 10.1073/pnas.1003999107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulakov AA, Rinberg D. Sparse incomplete representations: a potential role of olfactory granule cells. Neuron. 2011;72:124–136. doi: 10.1016/j.neuron.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier S, Panzanelli P, Russo RE, Nissant A, Bathellier B, Sassoe-Pognetto M, Fritschy JM, Lledo PM. Gabaergic inhibition at dendrodendritic synapses tunes gamma oscillations in the olfactory bulb. Proc Natl Acad Sci U S A. 2007;104:7259–7264. doi: 10.1073/pnas.0701846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Cleland TA. Glomerular microcircuits in the olfactory bulb. Neural Netw. 2009;22:1169–1173. doi: 10.1016/j.neunet.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litaudon P, Garcia S, Buonviso N. Strong coupling between pyramidal cell activity and network oscillations in the olfactory cortex. Neuroscience. 2008;156:781–787. doi: 10.1016/j.neuroscience.2008.07.077. [DOI] [PubMed] [Google Scholar]
- Lowe G. Electrical signaling in the olfactory bulb. Curr.Opin.Neurobiol. 2003;13:476–481. doi: 10.1016/s0959-4388(03)00092-8. [DOI] [PubMed] [Google Scholar]
- Ma M, Luo M. Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J Neurosci. 2012;32:10105–10116. doi: 10.1523/JNEUROSCI.0058-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Manabe H, Mori K. Sniff rhythm-paced fast and slow gamma-oscillations in the olfactory bulb: relation to tufted and mitral cells and behavioral states. J Neurophysiol. 2013;110:1593–1599. doi: 10.1152/jn.00379.2013. [DOI] [PubMed] [Google Scholar]
- Markopoulos F, Rokni D, Gire DH, Murthy VN. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 2012;76:1175–1188. doi: 10.1016/j.neuron.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Gervais R, Chabaud P, Messaoudi B, Ravel N. Learning-induced modulation of oscillatory activities in the mammalian olfactory system: the role of the centrifugal fibres. J Physiol Paris. 2004;98:467–478. doi: 10.1016/j.jphysparis.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Matsutani S. Trajectory and terminal distribution of single centrifugal axons from olfactory cortical areas in the rat olfactory bulb. Neuroscience. 2010;169:436–448. doi: 10.1016/j.neuroscience.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Miura K, Mainen ZF, Uchida N. Odor representations in olfactory cortex: distributed rate coding and decorrelated population activity. Neuron. 2012;74:1087–1098. doi: 10.1016/j.neuron.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P. Axonal wiring in the mouse olfactory system. Annu Rev Cell Dev Biol. 2006;22:713–737. doi: 10.1146/annurev.cellbio.21.012804.093915. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the Mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Enerva A, Fletcher ML, Masurkar AV, Igarashi KM, Mori K, Chen WR. Differential axonal projection of mitral and tufted cells in the mouse main olfactory system. Front Neural Circuits. 2010;4:1–7. doi: 10.3389/fncir.2010.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara K, Saraiva LR, Ye X, Buck LB. A large-scale analysis of odor coding in the olfactory epithelium. J Neurosci. 2011;31:9179–9191. doi: 10.1523/JNEUROSCI.1282-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Parra A, Maurer RK, Krahe K, Smith RS, Araneda RC. Disruption of centrifugal inhibition to olfactory bulb granule cells impairs olfactory discrimination. Proc Natl Acad Sci U S A. 2013;110:14777–14782. doi: 10.1073/pnas.1310686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain F, L'Heureux B, Gurden H. Visualizing odor representation in the brain: a review of imaging techniques for the mapping of sensory activity in the olfactory glomeruli. Cell Mol Life Sci. 2011;68:2689–2709. doi: 10.1007/s00018-011-0708-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MA, Lagier S, Carleton A. Odor representations in the olfactory bulb evolve after the first breath and persist as an odor afterimage. Proc Natl Acad Sci U S A. 2013;110:E3340–E3349. doi: 10.1073/pnas.1303873110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Gelperin A. Olfactory neuronal dynamics in behaving animals. Semin Cell Dev Biol. 2006;17:454–461. doi: 10.1016/j.semcdb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J.Neurosci. 2006;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosero MA, Aylwin ML. Sniffing shapes the dynamics of olfactory bulb gamma oscillations in awake behaving rats. Eur J Neurosci. 2011;34:787–799. doi: 10.1111/j.1460-9568.2011.07800.x. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Young DA, Restrepo D. Olfactory fingerprints for major histocompatibility complex-determined body odors. J Neurosci. 2001;21:2481–2487. doi: 10.1523/JNEUROSCI.21-07-02481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE, Kinzie JM, Sahara Y, Segerson TP, Westbrook GL. Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors. J.Neurosci. 1998;18:6790–6802. doi: 10.1523/JNEUROSCI.18-17-06790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa S, Ishii T, Nakatani H, Tsuboi A, Nagawa F, Asano M, Sudo K, Sakagami J, Sakano H, Ijiri T, Matsuda Y, Suzuki M, Yamamori T, Iwakura Y. Mutually exclusive expression of odorant receptor transgenes. Nat Neurosci. 2000;3:687–693. doi: 10.1038/76641. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Greer CA. Olfactory bulb. In: Shepherd GM, editor. The Synaptic Organization of the Brain. New York: Oxford University Press; 2004. [Google Scholar]
- Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle. Nature neuroscience. 2011;14:1039–1044. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- Smear M, Shusterman R, O'Connor R, Bozza T, Rinberg D. Perception of sniff phase in mouse olfaction. Nature. 2011;479:397–400. doi: 10.1038/nature10521. [DOI] [PubMed] [Google Scholar]
- Smith RS, Araneda RC. Cholinergic modulation of neuronal excitability in the accessory olfactory bulb. J Neurophysiol. 2010;104:2963–2974. doi: 10.1152/jn.00446.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spors H, Albeanu DF, Murthy VN, Rinberg D, Uchida N, Wachowiak M, Friedrich RW. Illuminating vertebrate olfactory processing. J Neurosci. 2012;32:14102–14108. doi: 10.1523/JNEUROSCI.3328-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spors H, Grinvald A. Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron. 2002;34:301–315. doi: 10.1016/s0896-6273(02)00644-x. [DOI] [PubMed] [Google Scholar]
- Spors H, Wachowiak M, Cohen LB, Friedrich RW. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J.Neurosci. 2006;26:1247–1259. doi: 10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat.Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Wachowiak M. Active Sensing in Olfaction. Chapter 12. Boca Raton FL: CRC Press; 2010. [PubMed] [Google Scholar]
- Wachowiak M. All in a sniff: olfaction as a model for active sensing. Neuron. 2011;71:962–973. doi: 10.1016/j.neuron.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 79.Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin.Cell Dev.Biol. 2006;17:411–423. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Wellis DP, Scott JW, Harrison TA. Discrimination among odorants by single neurons of the rat olfactory bulb. J Neurophysiol. 1989;61:1161–1177. doi: 10.1152/jn.1989.61.6.1161. [DOI] [PubMed] [Google Scholar]
- 81.Wesson DW, Carey RM, Verhagen JV, Wachowiak M. Rapid encoding and perception of novel odors in the rat. Plos.Biol. 2008a;6:e82. doi: 10.1371/journal.pbio.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wesson DW, Donahou TN, Johnson MO, Wachowiak M. Sniffing behavior of mice during performance in odor-guided tasks. Chem.Senses. 2008b;33:581–596. doi: 10.1093/chemse/bjn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wesson DW, Verhagen JV, Wachowiak M. Why sniff fast? The relationship between sniff frequency, odor discrimination, and receptor neuron activation in the rat. J.Neurophysiol. 2009;101:1089–1102. doi: 10.1152/jn.90981.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc.Natl.Acad.Sci.U.S.A. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhan C, Luo M. Diverse patterns of odor representation by neurons in the anterior piriform cortex of awake mice. J.Neurosci. 2010;30:16662–16672. doi: 10.1523/JNEUROSCI.4400-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]