Abstract
Undifferentiated (embryonal) sarcoma of the liver (USL) is a rare malignant tumor most commonly seen in children aged 6 to 10 years. Previously believed to carry a poor prognosis, more recent reports indicate that treatment regimens combining surgical resection and adjuvant chemotherapy can yield long-term, disease-free survival. In this report, we review five pediatric patients with USL treated with a uniform approach of resection followed by adjuvant chemotherapy and radiation when indicated. All five patients are disease-free in their first remission at a median of 53 months.
Keywords: undifferentiated (embryonal) sarcoma, chemotherapy, tumors, liver
Introduction
Undifferentiated (embryonal) sarcoma of the liver (USL) is the third most common malignant hepatic tumor in children after hepatoblastoma and hepatocellular carcinoma, constituting 13% of all pediatric hepatic malignancies [1-2]. Most commonly occurring in children between the ages of 6 to 10 years, USL usually presents as a painful right upper quadrant mass accompanied by fever and weight loss [3-5]. In 1978, Stocker and Ishak established USL as a distinct clinical and pathologic entity, publishing a series of 31 cases with a median survival less than a year following surgical excision [1]. Historically, USL carried a very poor prognosis of 20-40% disease free survival [3,6-8]. More recent reports indicate that treatment regimens combining surgical resection, chemotherapy, and radiation when indicated are more likely to yield long-term, disease-free survival [9-14]. We present five patients with USL diagnosed and treated with multimodal therapy at Children's Hospital Colorado from 1993 to 2009. Colorado Multiple Institutional Review Board approval was obtained for chart review (protocol 07-0047). All of the treatment regimens contained a backbone of VAC (vincristine, actinomycin-D, cyclophosphamide) therapy, originally developed through the Intergroup Rhabdomyosarcoma Study IV (IRS) for treatment of intermediate-risk rhabdomyosarcoma [15-17]. All five patients are currently alive and disease-free 38-205 months from the time of diagnosis.
Cases
Patient 1 presented at 4 years of age with fever, malaise and a painful abdominal mass. Computed tomography (CT) imaging demonstrated a heterogeneously enhancing tumor arising from the left lateral segment of the liver. Laboratory tests were notable for α-fetoprotein (AFP) and human chorionic gonadotropin (β-HCG) levels within normal limits, a mildly elevated lactate dehydrogenase (LDH), and a low serum albumin. He had mild anemia, and a moderately elevated erythrocyte sedimentation rate. A metastatic evaluation including a bone scan and chest CT was negative. A left lobectomy with subtotal gastrectomy removed the 8 cm × 9 cm hepatic mass. Pathology demonstrated USL with a single pathologically positive margin at the gastric border. He was treated with a single course of doxorubicin (90mg/m2) and cisplatinum (90mg/m2), followed by ten courses of VAC. The patient has remained disease free for 16 years (204 months).
Patient 2 is a 12-year old girl who presented with right upper quadrant abdominal pain. An abdominal ultrasound confirmed a liver mass. AFP and β-HCG levels were normal. CT of the abdomen and pelvis revealed a large cystic mass in the right lobe of the liver measuring 14 cm × 13 cm × 18.5 cm. She underwent a right hepatic lobectomy, cholecystectomy, and excision of the cystic duct and right porto-hepatic lymph nodes. Pathology revealed USL with clear surgical margins. Chest CT and bone scans showed no evidence of metastatic disease. She was treated with seven cycles of VAC. Her course was complicated by post-operative bile collections requiring drainage, neutropenic coagulase-negative staphylococcal bacteremia and grade 3 peripheral neuropathy. These complications necessitated reductions in dosing of her cyclophosphamide after the first cycle (5 subsequent doses at 1.2g/m2) and vincristine (received 2 complete doses, and 4 doses reduced by 50%). Following completion of therapy, the patient required two subsequent exploratory laparotomies for lysis of adhesions. She remains healthy without evidence of disease 100 months off therapy.
Patient 3 is a 13-year old female who presented with a one-month history of right upper quadrant pain. She was taking isoniazid for a positive purified protein derivative (PPD) test and her liver function tests were mildly elevated. An ultrasound demonstrated a 14 cm × 14 cm mass, confirmed on abdominal CT. A biopsy was obtained, and initial pathologic review indicated the mass to be a hepatic cystadenoma. Following resection of the right lobe, pathology on the mass demonstrated USL. At the time of resection, the patient had an enlarging mass involving the left nasal ala. Biopsy of the nasal mass demonstrated metastatic undifferentiated sarcoma (Figure 1). A bone scan and CT of the abdomen and chest showed no further evidence of metastatic disease. She received fourteen cycles of VAC with the addition of 66 Gy of focal radiation to her left nasal ala. Subsequent resection of the nasal tumor showed dense fibrocollagenous tissue with rare atypical cells but no evidence of disease. Her course was complicated by grade 3 peripheral neuropathy, requiring 25-50% reduction of eight doses of vincristine. She is 53 months off therapy without recurrence.
Figure 1.
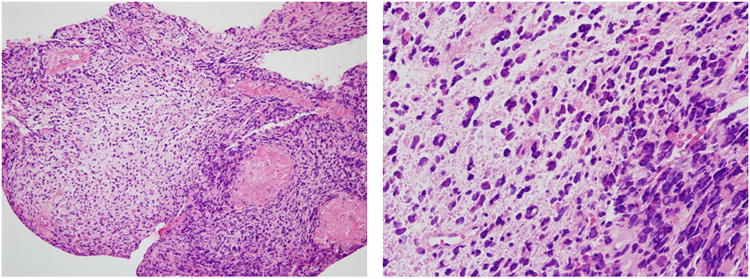
Biopsy of the nasal mass shows a primitive malignant neoplasm with round and spindled hyperchromatic cells in a loose myxoid matrix, typical of undifferentiated embryonal sarcoma of the liver. The primary liver tumor cells (not shown) showed no expression of epithelial or skeletal muscle markers (pan-cytokeratin, muscle specific actin, desmin). (Hematoxylin and eosin, 25 × (a) and 100× (b) original magnification).
Patient 4 is a 13-year old female who was diagnosed following a month of non-specific intermittent abdominal pain. A CT scan showed an 18 cm × 19 cm hepatic mass in the right lobe of the liver which was not amenable to primary resection. CT scan of her chest was negative for metastatic disease. The patient underwent an open wedge biopsy diagnostic of USL. She received seven cycles of VAC. After two cycles, her tumor had decreased in size and she was able to undergo a right lobectomy. Her treatment was complicated by a biloma requiring drain placement and a Stenotrophomonas biloma infection. She is now 40 months off therapy without evidence of recurrence.
Patient 5 is a previously healthy 6 year old male at the time of diagnosis. He presented with a one day history of epigastric pain and abdominal distention. A CT of the abdomen defined a 10 cm × 11 cm liver mass. Laboratory evaluations, including AFP and β-HCG levels, were normal. CT scans of his head, neck and chest and bone marrow biopsies showed no evidence of metastatic disease. A biopsy of the liver mass showed USL, and the patient underwent a left hepatic lobectomy. At resection, the tumor capsule was found have been ruptured by the tumor and was confirmed pathologically. There was no peritoneal studding noted. Chemotherapy was delayed due to a persistent biloma which required cholecystectomy and bile duct ligation. The patient was subsequently treated similarly to IRSG D9803 regimen B, with fourteen cycles of chemotherapy using VAC (total of 11 cycles) alternating with vincristine, topotecan, and cyclophosphamide (VTC) (total of 3 cycles). Due to rupture of the tumor capsule, he received 19.5 Gy of whole abdominal radiation. Complications included culture-negative neutropenic sepsis with typhlitis, pancreatitis, candida fungemia, cardiopulmonary arrest of unclear etiology following recovery from sepsis with acute respiratory distress syndrome (ARDS), and prolonged intubation. Other complications included cavernous transformation of the portal vein and grade IV lower extremity neuropathy which required cessation of further dosing of vincristine (following 3 cycles). With physical therapy, he regained full function of his lower extremities following completion of chemotherapy. He is now 38 months off therapy and remains disease-free.
Discussion
Undifferentiated sarcoma of the liver was first defined as a unique clinicopathologic entity by Stocker and Ishak in 1978, who presented a series of 31 USL cases in which 80% of patients (25 out of 31) died within one year of follow-up despite surgical resection [1]. Leuschner et al reviewed clinical outcomes from 1950 to 1988 and found a 37.5% disease free survival rate at an average of 3 years [8]. More recent reports demonstrate improved survival rates with implementation of multimodal therapy typically used for sarcomas, including primary resection, neo-adjuvant or adjuvant chemotherapy, and radiation in selected cases [3,6,9-10,12-14,18-22]. The Soft Tissue Sarcoma Italian and German Cooperative Groups published results from a cohort of 17 USL patients that showed a 70% long term survival rate when multimodal therapy was employed[10]. A variety of chemotherapeutic regimens were used in these case reports, including vincristine, actinomycin-D, cyclophosphamide, doxorubicin, cisplatinum, carboplatinum, ifosfamide, and etoposide. A review of the literature found 26 patients who were treated with VAC or chemotherapy with a modified VAC backbone. Of the 10 patients treated with VAC alone, 4 (40%) showed no evidence of disease 14 mo – 8 years from diagnosis [7,12,18,23-24]. Additionally, 11 of 16 (68%) patients treated with a chemotherapy regimen incorporating a VAC backbone were reported as alive and without evidence of disease, 1 who had tumor spill at surgery and another with lung metastasis at diagnosis. Four patients treated with VAC based regimen died of progressive or recurrent disease and 1 died from complications of chemotherapy [10-11,18,25].
While these reports have suggested an important role for adjuvant chemotherapy in the treatment of USL, the optimal combination of agents and length of therapy is not known. Alkylating agents, with or without anthracyclines, appear to be an important component of the treatment. We chose to adopt an adjuvant chemotherapy protocol similar to the standard arm of IRSG D9803 using vincristine, actinomycin, and cyclophosphamide (VAC) given in 3 week cycles [15]. When given in combination with tumor resection and radiation therapy when indicated, this regimen at our institution conferred excellent outcomes. All five patients are alive without evidence of disease at a median of 53 months (38, 40, 53, 100, and 204 months). The positive outcome was particularly notable in the two patients with high-risk disease (one with metastatic disease at presentation and the other with tumor rupture), given that such patients historically have been shown to have a poorer prognosis despite aggressive multimodal therapy [23-26]. Several of our patients had surgical complications, associated with the extent of resection and location of the tumors. While significant acute toxicities were experienced, none of the patients have shown significant late effects to date. Our single institution experience suggests that VAC therapy in conjunction with best possible surgical resection is sufficient to provide a high likelihood of cure for USL, indicating that exposure to other agents with significant late effects, such as anthracyclines, topoisomerase inhibitors, and platinum compounds, can be avoided. Due to the rarity of this tumor, large case series and/or prospective randomized trials are unlikely to occur in the near future, making treatment decisions difficult. Our findings indicate that VAC therapy, together with surgical resection and radiation therapy when indicated, provides a plausible treatment strategy for patients with USL.
Table 1.
All patients were treated with VAC (vincristine, actinomycin-D, cyclophosphamide) as per the Children's Oncology Group study D9803 protocol [15]. M: male, F: female, doxo: doxorubicin, cddp: cisplatinum, VTC: vincristine, topotecan, cyclophosphamide, AWED: Alive without evidence of disease
| Patient | Age at diagnosis (years) | Gender | Initial surgery | Chemotherapy | Radiation | Current Status | Time from therapy |
|---|---|---|---|---|---|---|---|
| 1 | 4 | M | Left lateral segment hepatectomy and partial gastrectomy | doxo, CDDP × 1 cycle VAC × 10 cycles |
No | AWED | 204 months |
| 2 | 12 | F | Right hepatic lobectomy, cholecystectomy, excision of the cystic duct and right portohepatic lymph nodes | VAC × 7 cycles | No | AWED | 100 months |
| 3 | 13 | F | Right hepatic lobectomy, nasal biopsy | VAC × 14 cycles | 66 Gy to nasal ala | AWED | 53 months |
| 4 | 13 | F | Right hepatic lobectomy | VAC × 7 cycles | No | AWED | 40 months |
| 5 | 6 | M | Left hepatic lobectomy | VAC × 11 cycles VTC × 3 cycles |
19.5 Gy to whole abdomen | AWED | 38 months |
Acknowledgments
MM is a recipient of the National Institutes of Health K12 CA086913-08.
References
- 1.Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: Report of 31 cases. Cancer. 1978;42(1):336–348. doi: 10.1002/1097-0142(197807)42:1<336::aid-cncr2820420151>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg AG, Finegold MJ. Primary hepatic tumors of childhood. Hum Pathol. 1983;14(6):512–537. doi: 10.1016/s0046-8177(83)80005-7. [DOI] [PubMed] [Google Scholar]
- 3.Smithson WA, Telander RL, Carney JA. Mesenchymoma of the liver in childhood: Five-year survival after combined-modality treatment. J Pediatr Surg. 1982;17(1):70–72. doi: 10.1016/s0022-3468(82)80331-x. [DOI] [PubMed] [Google Scholar]
- 4.Stocker JT. An approach to handling pediatric liver tumors. Am J Clin Pathol. 1998;109(4 Suppl 1):S67–72. [PubMed] [Google Scholar]
- 5.Stocker JT. Hepatic tumors in children. Clin Liver Dis. 2001;5(1):259–281. viii–ix. doi: 10.1016/s1089-3261(05)70163-x. [DOI] [PubMed] [Google Scholar]
- 6.Harris MB, Shen S, Weiner MA, et al. Treatment of primary undifferentiated sarcoma of the liver with surgery and chemotherapy. Cancer. 1984;54(12):2859–2862. doi: 10.1002/1097-0142(19841215)54:12<2859::aid-cncr2820541208>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Horowitz ME, Etcubanas E, Webber BL, et al. Hepatic undifferentiated (embryonal) sarcoma and rhabdomyosarcoma in children. Results of therapy. Cancer. 1987;59(3):396–402. doi: 10.1002/1097-0142(19870201)59:3<396::aid-cncr2820590307>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Leuschner I, Schmidt D, Harms D. Undifferentiated sarcoma of the liver in childhood: Morphology, flow cytometry, and literature review. Hum Pathol. 1990;21(1):68–76. doi: 10.1016/0046-8177(90)90077-i. [DOI] [PubMed] [Google Scholar]
- 9.Baron PW, Majlessipour F, Bedros AA, et al. Undifferentiated embryonal sarcoma of the liver successfully treated with chemotherapy and liver resection. J Gastrointest Surg. 2007;11(1):73–75. doi: 10.1007/s11605-006-0044-4. [DOI] [PubMed] [Google Scholar]
- 10.Bisogno G, Pilz T, Perilongo G, et al. Undifferentiated sarcoma of the liver in childhood: A curable disease. Cancer. 2002;94(1):252–257. doi: 10.1002/cncr.10191. doi:10.1002/cncr.10191 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Kim DY, Kim KH, Jung SE, et al. Undifferentiated (embryonal) sarcoma of the liver: Combination treatment by surgery and chemotherapy. J Pediatr Surg. 2002;37(10):1419–1423. doi: 10.1053/jpsu.2002.35404. doi:S002234680200115X [pii] [DOI] [PubMed] [Google Scholar]
- 12.Walker NI, Horn MJ, Strong RW, et al. Undifferentiated (embryonal) sarcoma of the liver. Pathologic findings and long-term survival after complete surgical resection. Cancer. 1992;69(1):52–59. doi: 10.1002/1097-0142(19920101)69:1<52::aid-cncr2820690111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Ware R, Friedman HS, Filston HC, et al. Childhood hepatic mesenchymoma: Successful treatment with surgery and multiple-agent chemotherapy. Med Pediatr Oncol. 1988;16(1):62–65. doi: 10.1002/mpo.2950160114. [DOI] [PubMed] [Google Scholar]
- 14.Webber EM, Morrison KB, Pritchard SL, et al. Undifferentiated embryonal sarcoma of the liver: Results of clinical management in one center. J Pediatr Surg. 1999;34(11):1641–1644. doi: 10.1016/s0022-3468(99)90634-6. doi:S0022-3468(99)90634-6 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's oncology group study d9803. J Clin Oncol. 2009;27(31):5182–5188. doi: 10.1200/JCO.2009.22.3768. doi:JCO.2009.22.3768[pii] 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crist W, Gehan EA, Ragab AH, et al. The third intergroup rhabdomyosarcoma study. J Clin Oncol. 1995;13(3):610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 17.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-iv: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19(12):3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 18.Babin-Boilletot A, Flamant F, Terrier-Lacombe MJ, et al. Primitive malignant nonepithelial hepatic tumors in children. Med Pediatr Oncol. 1993;21(9):634–639. doi: 10.1002/mpo.2950210905. [DOI] [PubMed] [Google Scholar]
- 19.Dower NA, Smith LJ, Lees G, et al. Experience with aggressive therapy in three children with unresectable malignant liver tumors. Med Pediatr Oncol. 2000;34(2):132–135. doi: 10.1002/(sici)1096-911x(200002)34:2<132::aid-mpo11>3.0.co;2-h. doi:10.1002/(SICI)1096-911X(200002)34:2<132∷AID-MPO11>3.0.CO;2-H [pii] [DOI] [PubMed] [Google Scholar]
- 20.Upadhyaya M, McKiernan P, Hobin D, et al. Primary hepatic sarcomas in children--a single-center experience over 19 years. J Pediatr Surg. 2010;45(11):2124–2128. doi: 10.1016/j.jpedsurg.2010.07.016. doi:S0022-3468(10)00576-2 [pii] 10.1016/j.jpedsurg.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Urban CE, Mache CJ, Schwinger W, et al. Undifferentiated (embryonal) sarcoma of the liver in childhood. Successful combined-modality therapy in four patients. Cancer. 1993;72(8):2511–2516. doi: 10.1002/1097-0142(19931015)72:8<2511::aid-cncr2820720833>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.Wei ZG, Tang LF, Chen ZM, et al. Childhood undifferentiated embryonal liver sarcoma: Clinical features and immunohistochemistry analysis. J Pediatr Surg. 2008;43(10):1912–1919. doi: 10.1016/j.jpedsurg.2008.06.016. doi:S0022-3468(08)00554-X [pii] 10.1016/j.jpedsurg.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Newman KD, Schisgall R, Reaman G, et al. Malignant mesenchymoma of the liver in children. J Pediatr Surg. 1989;24(8):781–783. doi: 10.1016/s0022-3468(89)80536-6. doi:S0022346889002824 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Pachera S, Nishio H, Takahashi Y, et al. Undifferentiated embryonal sarcoma of the liver: Case report and literature survey. J Hepatobiliary Pancreat Surg. 2008;15(5):536–544. doi: 10.1007/s00534-007-1265-y. [DOI] [PubMed] [Google Scholar]
- 25.Ida S, Okajima H, Hayashida S, et al. Undifferentiated sarcoma of the liver. Am J Surg. 2009;198(1):e7–9. doi: 10.1016/j.amjsurg.2008.08.019. doi:S0002-9610(08)00831-3 [pii] 10.1016/j.amjsurg.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Uchiyama M, Iwafuchi M, Yagi M, et al. Treatment of ruptured undifferentiated sarcoma of the liver in children: A report of two cases and review of the literature. J Hepatobiliary Pancreat Surg. 2001;8(1):87–91. doi: 10.1007/s005340170055. [DOI] [PubMed] [Google Scholar]


