Summary
Common polymorphisms in the first intron of FTO are associated with a increased body weight in adults. Previous studies have suggested that a CUX1 regulatory element within the implicated FTO region controls expression of FTO and the nearby ciliary gene, RPGRIP1L. Given the role of ciliary genes in energy homeostasis, we hypothesized that mice hypomorphic for Rpgrip1l would display increased adiposity. We find that Rpgrip1l+/− mice are hyperphagic, fatter, and display diminished suppression of food intake in response to leptin administration. In the hypothalamus of Rpgrip1l+/− mice, and in human fibroblasts with hypomorphic mutations in RPGRIP1L, the number of AcIII-positive ciliais diminished, accompanied by impaired convening of the leptin receptor to the vicinity of the cilium, and diminished pStat3 in response to leptin. These findings suggest that RPGRIP1L may be partly or exclusively responsible for the obesity susceptibility signal at the FTO locus.
Introduction
Common single nucleotide polymorphisms (SNPs) within a ~47 Kb interval located in the first intron of the Fat Mass and Obesity Associated (FTO) gene are associated with a dose-dependent body weight difference in humans (Frayling et al., 2007;Scuteri et al., 2007;Meyre et al., 2009). FTO encodes a nuclear protein that is orthologous to proteins of the AlkB family of deoxygenases and appears to function as a DNA or RNA demethylase (Han et al., 2010). In mice and rats, manipulations of Fto gene expression have produced changes in body weight, but not in a consistent direction (Stratigopoulos et al., 2008, 2011; Wang et al., 2011; Tung et al., 2010; Church et al., 2010), and humans heterozygous for null alleles of FTO show no consistent effects on body weight (Meyre et al., 2010). RPGRIP1L (Retinitis Pigmentosa GTPase Regulator-Interacting Protein-1 Like) is located >100bp 5′ in the opposite transcriptional orientation of FTO (Fig. 1A), and encodes a protein localized at the transition zone of the primary cilium (Williams et al., 2011; Liu et al., 2011). Functional derangements of the primary cilium in Bardet-Biedl and Alström syndromes are associated with obesity (Baker et al., 2009). We have previously demonstrated that fasting reduces hypothalamic expression of Rpgrip1l in mice and is restored by exogenous leptin administration (Stratigopoulos et al., 2011). The first intron of FTO contains a binding site for the CUX1 p110 isoform - capable of long-range regulation of transcription (Vadnais et al., 2013) – that increases promoter activity and expression of RPGRIP1 Lin vitro, and the obesity-risk allele of intronic SNP rs8050136 (Scuteri et al., 2007) located in the CUX1 binding site lowers the affinity of the CUX1 p110 isoform for DNA (Stratigopoulos et al., 2011). The long isoform of the leptin receptor (Lepr-b) localizes to the vicinity of the primary cilium in cultured neuronal cells, and in vitro knock down of Rpgrip1l reduces Lepr-b localization in the vicinity of the cilium and decreases leptin signaling (Stratigopoulos et al., 2011). Thus, it is possible that some or all of the association with obesity of the intron 1 SNPs of FTO is actually conveyed by reduced expression of RPGRIP1L. In mice, homozygosity for a null allele of Rpgrip1l is embryonically lethal (Vierkotten et al., 2007), but based on the allele-dosage effect of the FTO intronic SNPs on human adiposity, and our linking of aCUX1 regulatory element in FTO intron 1 that controls Rpgrip1l expression, we hypothesized that mice heterozygous for a null allele of Rpgrip1l would be fatter than wild type animals. Here we show that these animals are indeed fatter, and report the details of their metabolic and behavioral phenotypes. We also describe a series of experiments investigating a possible mechanism for an impact of hypomorphism at Rpgrip1l on Lepr-b signaling that could account for these systemic phenotypes.
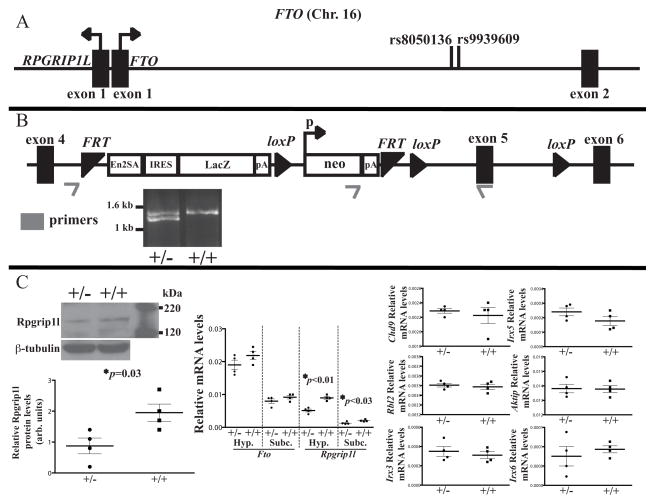
Fig. 1. Targeted disruption of the Rpgrip1l locus.
(A) Genomic organization of the human FTO/RPGRIP1L interval on chromosome 16. SNPs rs8050136 and rs9939609 are associated with increased BMI. Figure not drawn to scale. (B) Targeted disruption of the murine Rpgrip1l allele. A cassette comprised of the promoterless LacZ gene and the promoter-driven Neomycin resistance (neo) gene separated by a loxP site and flanked by FRT sites. The LacZ gene is flanked by the Engrailed-2 exon-2 splice acceptor (En2SA) and an internal ribosome entry site (IRES) designed to integrate the LacZ cDNA with Rpgrip1l exon 4 resulting in premature transcriptional termination of the Rpgrip1l mRNA. Figure not drawn to scale. (C) Fto and Rpgrip1l transcript levels, and Rpgrip1l protein levels assessed by RT-PCR and western blotting respectively in the hypothalamus of Rpgrip1l+/− (+/−) and Rpgrip1l+/+ (+/+) mice. Fto and Rpgrip1l mRNA levels were also assessed in subcutaneous (Subc) fat. Transcript levels of nearby genes Chd9, Rbl2, Aktip, Irx3, Irx5 and Irx6 were unaltered in the the hypothalamus of Rpgrip1l+/− and Rpgrip1l+/+ mice. Error bars represent SEM.
Results
Rpgrip1l+/− mice display increased adiposity due to increased food intake
Deletion of Rpgrip1l in mice, as well as biallelic RPGRIP1L mutations in Joubert patients, is linked with severe brain and craniofacial abnormalities (Vierkotten et al., 2007;Arts et al., 2007; Delous et al., 2007). In addition, Rpgrip1l-deleted embryos display polydactyly (Vierkotten et al., 2007), which is also characteristic of mutations in cilia-related proteins related to human syndromic obesity (e.g. Zaghloul & Katsanis, 2009). Nevertheless, RPGRIP1L mutations have not been directly linked to obesity in mice or humans. Deletion of Rpgrip1l is embryonic lethal in mice; and patients with homozygous or compound heterozygous loss-of-function mutations in RPGRIP1L show severe renal and skeletal developmental defects that may mask a role of RPGRIP1L in energy homeostasis. We obtained mice segregating for a Rpgrip1l allele in which LacZ is knocked into intron 4 (Fig. 1B). LacZ is downstream of the Engrailed-2 exon-2 splice acceptor, resulting in a functionally null Rpgrip1l allele. Interbreeding of heterozygous mice failed to produce viable mice homozygous for the knock-in allele, in agreement with the embryonic lethality previously reported (Vierkotten et al, 2007). Rpgrip1l mRNA and protein levels in the hypothalamus of heterozygous mice were decreased by ~50%, and Rpgrip1l mRNA was also decreased by ~50% in subcutaneous fat (Fig. 1C), demonstrating that these mice are systemically heterozygous for Rpgrip1l (Rpgrip1l+/−). Conversely, Fto mRNA remained unchanged in the hypothalamus and subcutaneous fat of Rpgrip1l+/− mice, and mRNA levels of nearby genes Chd9, Rbl2, Aktip, Irx3–5 in the hypothalami of Rpgrip1l+/− mice were equivalent to control mice. Rpgrip1l+/−mice on a C57BL6/J background generated viable Rpgrip1l+/− mice at expected Mendelian ratios. By 10 weeks of age, male and female Rpgrip1l+/− mice fed regular chow (9% of calories as fat) ad libitum were ~10% heavier than +/+ animals (Fig. 2A, S1A). Thereafter, we concentrated on male Rpgrip1l+/− mice. At 18 weeks of age, male Rpgrip1l+/− mice fed regular chow were ~12% heavier due to a ~40% difference in fat mass (Fig. 2B–D). After 1 week of ad libitum feeding of a High Fat Diet (HFD; 65% of calories as fat), 19-week old male Rpgrip1l+/− mice were ~15% heavier (~4 grams) and had ~80% more body fat (~2.5 grams) and 6% more lean mass (~1.5 grams) than Rpgrip1l+/+ littermates (Fig. 2E–G). Energy intake was significantly increased (~23%) in Rpgrip1l+/−mice consuming the HFD during the 12h light phase compared with Rpgrip1l+/+ littermates (Fig. S1B). No statistically significant differences in energy expenditure or physical activity were detected between Rpgrip1l+/−and Rpgrip1l+/+ mice when ingesting the high-fat diet (Fig. S1C–D). After 18 weeks of ad libitum feeding of regular chow followed by7 days of the high fat diet, male Rpgrip1l+/− mice had ~82% more subcutaneous fat, ~65% more perigonadal and perirenal fat, and ~50% more mesenteric fat than +/+ littermates (Fig. 2H). The ~2-fold increase in serum leptin levels of male Rpgrip1l+/− mice correlated with their increased adiposity (Fig. S1E–F). No notable differences by genotype where observed in blood glucose levels of fed or fasted Rpgrip1l+/− and Rpgrip1l+/+ mice (Fig. S1G).
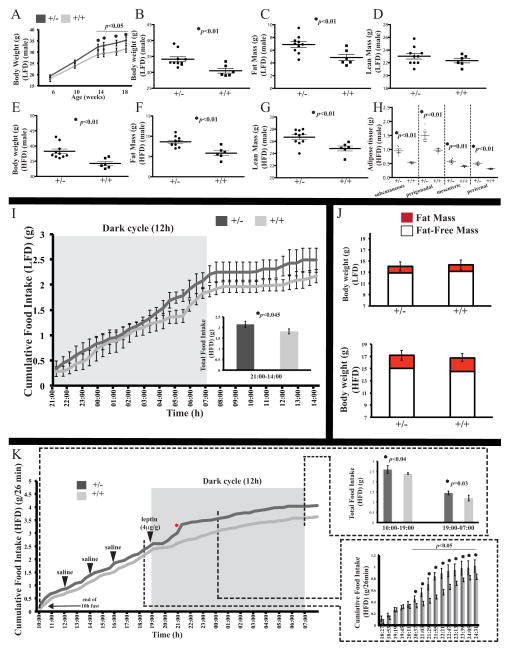
Fig. 2. Positive energy balance in mice heterozygous for a null allele of Rpgrip1l.
(A) Total body weight increased in male Rpgrip1l+/− versus Rpgrip1l+/+ mice fed regular chow (LFD) over an18-week period. Total body weight (B, E), fat mass (C, F), and lean mass (D, G) increased in 18-week old male Rpgrip1l+/− versus Rpgrip1l+/+ mice fed LFD and a high fat diet (HFD) respectively. (H) Increase in weight of various adipose tissue depots of Rpgrip1l+/− compared with Rpgrip1l+/+ mice after being fed regular chow for 18 weeks followed by the HFD for 7 days. Food intake (I, K) and body composition (J) of male Rpgrip1l+/− and Rpgrip1l+/+ mice fed LFD up to 4 weeks of age and subsequently fed HFD for 1 week. (K) While receiving the HFD, male Rpgrip1l+/− and Rpgrip1l+/+ mice were administered leptin intraperitoneally at the start of the dark cycle resulting in a shorter delay in feeding for the Rpgrip1l+/− group compared with control mice, that was followed by greater food intake in the Rpgrip1l+/− mice compared with the control group. After leptin administration, food intake was ~44% and ~46% lower in Rpgrip1l+/− and Rpgrip1l+/+ mice respectively during the dark cycle (19:00-07:00) compared to the light cycle (10:00–19:00). Red asterisk marks the time point when mRNA measurements were taken (Fig. 7B, C). Error bars represent SEM.
Rpgrip1l+/−male mice fed regular chow showed increased food intake as early as 4 weeks old (Fig. 2I). At this age, both groups weighed the same, had the same amount of fat mass, and displayed the same activity levels, whereas energy expenditure was ~25% lower in Rpgrip1l+/− mice (Fig. 2J, S1H–I). By5 weeks of age, after being switched to the high-fat diet for 1 week, Rpgrip1l+/− mice ate more and displayed ~20% lower energy expenditure compared to the control group (Fig. 2K, S1J). At this age, body weight, body composition and physical activity levels were indistinguishable between Rpgrip1l+/− and control mice (Fig. 2J, S1K). During a 5-day period of high fat feeding, a single intra-peritoneal dose of leptin (4 μg/g) administered at the start of the dark cycle led to acute suppression of feeding for ~2 hours in the control group (Fig. 2K). In contrast, Rpgrip1l+/− mice started consuming food again at an earlier time point (~1 hour after leptin administration at 19:00). There was no statistically significant difference in cumulative food intake during the acute suppression of food intake by leptin for the two experimental groups from 19:19 through 20:11. On the other hand, Rpgrip1l+/− mice ate more than littermate controls between 20:37 through 21:29 (slopes: 0.0034 versus 0.0016 grams/min; p=0.03) compared with the feeding behavior of the two groups prior to leptin administration. After leptin administration, Rpgrip1l+/− mice ate ~0.25g (~21%) more HFD than control mice over the ensuing 12h dark cycle period (Fig. 2K). Following leptin administration, Rpgrip1l+/− and Rpgrip1l+/+ mice consumed ~44% and ~46% less food respectively over the 12h dark cycle period (19:00-07:00) compared with food intake during the light cycle (10:00–19:00) (Fig. 2K). Administration of saline 3, 5, and 7h before leptin administration did not affect the feeding behavior of either genotype. At the end of the 5-day high-fat feeding period, there were no statistically significant changes in body weight and composition between Rpgrip1l+/− mice and control group (Fig. 2J).
Diminished acute pStat3 response to leptin and reduced number of ciliated cells in the hypothalamus of Rpgrip1l+/− mice
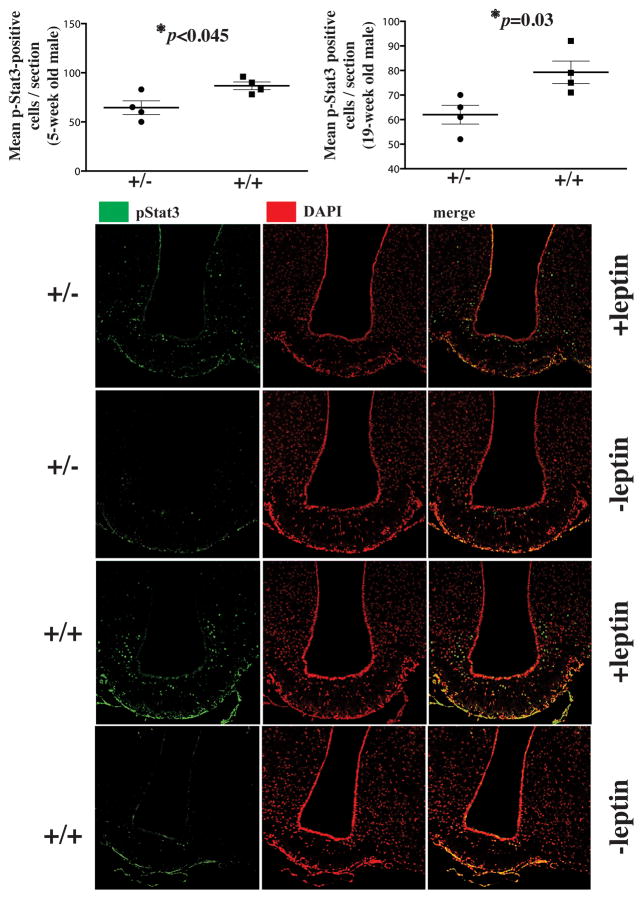
In response to a single dose (2μg/g) of intraperitoneal leptin, the number of pStat3-positive hypothalamic neurons was ~20% and ~35% lower in Rpgrip1l+/− compared with Rpgrip1l+/+ mice fed HFD ad lib at 5 and 19 weeks of age, respectively (Fig. 3). Moreover, in Rpgrip1l+/− 19 week old mice, ~20% fewer Lepr-positive cells were positive for the presence of type III A denylyl Cyclase (ACIII) in the axonemal part of the cilium (Bishop et. al., 2007) (Fig. 4A). By staining of hypothalamic tissue with an anti-LEPR antibody tested for specificity in vitro (Stratigopoulos et al., 2011) and in vivo (Fig. S1L), we observed that only ~55% of Lepr-positive cells in 19-week old Rpgrip1l+/− mice (compared with ~90% in control mice) exhibited Leprlocalization to the vicinity of the cilium (Fig. 4B, Movies S1–2), suggesting impaired subcellular trafficking of Lepr. Moreover, in 5-week old wild-type mice fed HFD ad lib and administered leptin (2μg/g) intraperitoneally, only ~30% of neurons with diffuse Lepr distribution were pStat3-positive, whereas ~70% of cells with localized Lepr distribution were pStat3-positive (Fig. 4C).
Fig. 3. Reduced leptin signaling in Rpgrip1l+/−mice.
pStat3-positive cells in the hypothalamus of Rpgrip1l+/− and Rpgrip1l+/+ 5-week and 19-week old male mice fed regular chow to whom leptin (2μg/g of body weight) was administered 30 min prior to sacrifice. pStat3-positive cells were counted in 10μm-thick cryosections encompassing the whole arcuate hypothalamus. Nuclear stain is shown in red to improve contrast with pStat3-specific green stain. Error bars represent SEM.
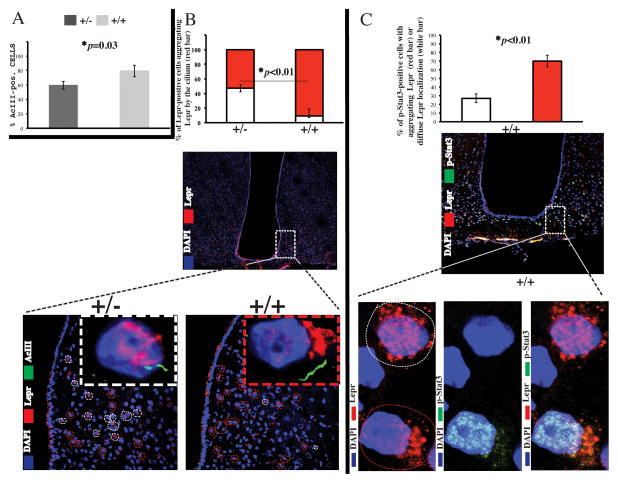
Fig. 4. Reduced AcIII-positive cilia and diminished aggregation of Leprin the vicinity of cilia in hypothalamic cells of Rpgrip1l+/− mice.
(A) Percentage of AcIII-positive positive cells in Z-stacks of 10μm cryosections spanning the arcuate hypothalamus of 19-week old male Rpgrip1l+/− and Rpgrip1l+/+ mice fed regular chow. (B) Comparison of frequency of Leprin the vicinity of cilia in arcuate hypothalamic cells of Rpgrip1l +/+ (red dashed-line circles) versus Rpgrip1l+/− (white dashed-line circles). See also Movies S1–2. (C) Higher percentage of pSTAT3-positive arcuate neurons with polarized (red dashed-line circle) versus scattered (white dashed-line circle) localization of Lepr in wild type mice administered leptin intraperitoneally. Each bar represents N of 4. Error bars represent SEM.
Rpgrip1l interacts with Lepr-b and coordinates leptin signaling
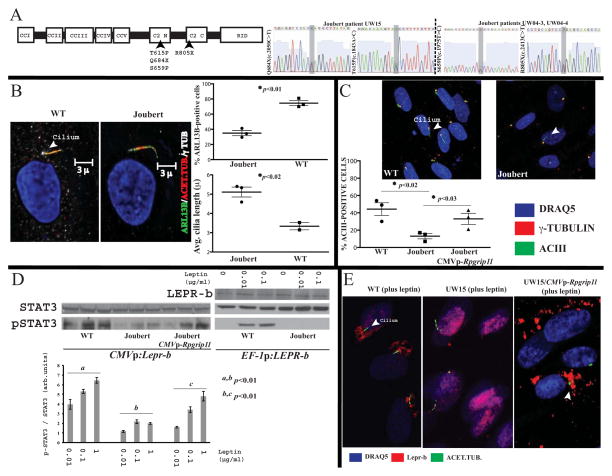
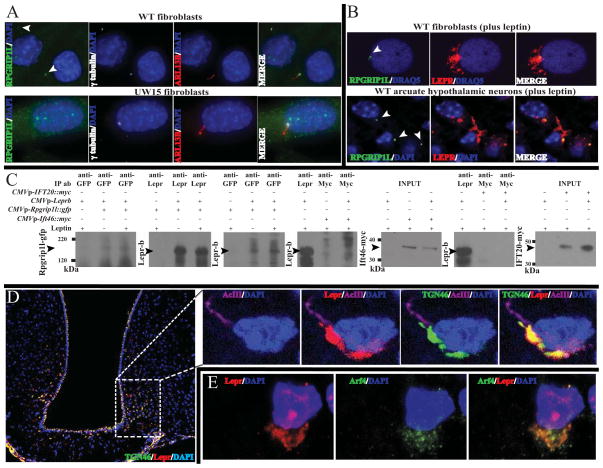
RPGRIP1L encodes a 1,253-amino-acid-long protein that contains several protein-protein interaction domains (Fig. 5A). These comprise five coiled-coil domains (CC1, CC2, CC3, CC4, CC5), which are possibly involved in homopolymer formation (Zhao et al., 2003); a C2 domain (C2 N′terminal-C2C′terminal), which is a Protein Kinase C-like domain that interacts with Nephrocysteine 4 and facilitates recruitment of other proteins to the base of the cilium by RPGRIP1L (Arts et al., 2007, Williams et al., 2011); and a domain in the C′ terminus homologous to the RPGR (Retinitis Pigmentosa GTPase Regulator)-interacting RID domain of RPGRIP1. RPGR is a component of the ciliary base and physically interacts with RPGRIP1L (Khanna et al., 2009). Because Lepr-b localizes to the vicinity of the cilium upon leptin stimulation, and Rpgrip1l knock-down diminishes leptin signaling in vitro (Stratigopoulos et al., 2011), we hypothesized that RPGRIP1L physically interacts with LEPR-b and there by co-ordinates leptin signaling. We examined three skin-derived primary fibroblast cell lines (UW15, UW04-3 and UW04-4) with compound heterozygous mutations in the RPGRIP1L C2 domain derived from two patients with Joubert Syndrome (Fig. 5A). These mutations severely disrupt – or are predicted to disrupt – nephrocystin-4 binding (Arts et al., 2007; Doherty et al., 2010). Control primary skin fibroblasts from healthy individuals transiently expressing murine Lepr-b or stably expressing human LEPR-b-display single primary cilia[assessed by staining for acetylated tubulin, or ADP-Ribosylation factor-Like protein 13B (ARL13B)] and dose-dependently phosphorylate STAT3 in response to leptin (Fig. 5B, D). RPGRIP1L mutant fibroblasts had ~50% and ~70% fewer ARL13B-positive and ACIII-positive cilia respectively, which were, on average, ~50% longer (Fig. 5B, C). Joubert fibroblasts transiently expressing Lepr-b or stably expressing LEPR-b failed to phosphorylate STAT3 in a dose-dependent mannerin response to leptin (Fig. 5D). Lepr-b localized to the vicinity of the cilium upon leptin stimulation of primary skin fibroblasts from unaffected individuals (Fig. 5E). In contrast, in RPGRIP1L mutant fibroblasts, Lepr-b did not do so; in these cells, Lepr-b localization appeared to be perinuclear. The expression of intact RPGRIP1L in mutant fibroblasts partially restored the number of ACIII-positive cilia, localization of Lepr-b around the base of the cilium, and pSTAT3 levels in response to leptin (Fig. 5C–E). By immunofluorescence, primary fibroblasts from unaffected individuals localized RPGRIP1L in the transition zone of the cilium (Fig. 6A). In contrast, all UW15 Joubert fibroblasts did not incorporate RPGRIP1L in the transition zone. In primary fibroblasts from unaffected individuals, and in hypothalamic neurons of 19-week-old male B6 mice following IP administration of leptin, transiently expressed Lepr-b and endogenous Lepr, respectively, partly co-localized with endogenous RPGRIP1L in the vicinity of the cilium, upon leptin treatment (Fig. 6B, Movie S3). In agreement with a possible physical interaction between RPGRIP1L and LEPR-b implied by immunofluorescence, Lepr-b co-immunoprecipitates with RPGRIP1L at a higher concentration in primary fibroblasts treated with leptin compared with untreated cells (Fig. 6C). In contrast, Lepr-b does not co-immunoprecipitate with intraflagellar transport proteins, Ift46 or IFT20 (Lucker et al., 2010; Follit et al., 2006) in the presence of leptin (Fig. 6C), suggesting that Lepr-b interacts selectively with RPGRIP1L in the transition zone of the cilium. It has been previously reported that TGN46, a marker for the post and Trans-Golgi Network (TGN), co-localizes with LEPR-bin ARPE-19 cells (Seo et al., 2009). Levels of Tgn46 mRNA are high in mouse hypothalamus (Allen Brain Atlas; http://mouse.brain-map.org/gene/show/21892). In the arcuate hypothalamus of 19-week old male wild type mice administered leptin via a single IP injection, Tgn46 protein co-localized with Leprin the vicinity of the cilium (Fig. 6D). Co-localization of Lepr and Tgn46 persisted in cells with more diffuse Lepr distribution (Fig. S1M), suggesting that assembly of the TGN near the base of cilium is required for the aggregation of Lepr in the vicinity of the cilium. In the same mice, Lepr subcellular localization at the TGN in arcuate hypothalamic cells was also evidenced by staining with another TGN marker, ARF4 (ADP-Ribosylation Factor 4) (Fig. 6E), a small GTPase that localizes to the TGN and regulates aspects of trafficking to the cilium (Lim et al., 2011).
Fig. 5. Reduced ciliogenesis, perturbed Lepr-b localization and diminished leptin signaling in human fibroblasts with hypomorphic mutations of RPGRIP1L.
(A) RPGRIP1L domain organization. Biallelic mutations, Q684X/T615P, and S659P/R805X, in the C2 domain of RPGRIP1L disrupt - or predicted to disrupt - nephrocystin-4 binding in Joubert patients UW15 and UW04-3/UW04-4 respectively (Arts et al., 2007; Doherty et al., 2010). (B) Number of cilia positive for ARL13B [membrane-associated protein of the ARL family of small GTPases that regulates ciliary transport (Lim et al., 2011)], acetylated tubulin and γ tubulin is reduced, where asaxoneme length is increased, in cultured primary skin fibroblasts from Joubert patients compared with primary fibroblasts from healthy individuals. Each data point represents a unique Joubert cell line. (C) Number of AcIII-positive ciliated cells is reduced in cultured primary skin fibroblasts from Joubert patients, and number is partially restored in Joubert fibroblasts transiently expressing Rpgrip1l. Each data point represents a Joubert cell line. (D) Western blot showing reduced pSTAT3 levels in whole protein extracts from unaffected compared with UW15 fibroblasts transiently expressing murine Lepr-b (CMVp:Lepr-b) or stably expressing human LEPR-b (EF-1p:LEPR-b), and partially restored pSTAT3 levels in UW15 primary fibroblasts transiently co-expressing Lepr-b and Rpgrip1l. Results were replicated in Joubert fibroblasts from patients UW04-3 and UW04-4 (data not shown). (E) Immunofluorescence of primary skin fibroblasts from unaffected individuals or UW15 fibroblasts transiently expressing Lepr-b or Lepr-b and Rpgrip1l. Lepr-b fails to localize in the vicinity of the cilium in the presence of leptinin UW15 fibroblasts transiently expressing Lepr-b. Lepr-b localization in the presence of leptin is partly restored in UW15 fibroblasts transiently expressing Lepr-b and Rpgrip1l. Results were replicated in Joubert fibroblasts from patients UW04-3 and UW04-4 (data not shown). Error bars represent SEM.
Fig. 6. Co-localization of RPGRIP1L, Lepr, Tgn46 and Arf4.
(A) Localization of endogenous RPGRIP1L in ciliated wild type primary fibroblasts. Increased gain of the green channel confirmed the absence of RPGRIP1L at the transition zone of fibroblasts from patient UW15 segregating for biallelic mutations in RPGRIP1L. Arrows point at the transition zone.(B) RPGRIP1L co-localizes with Lepr-b transiently expressed in primary fibroblasts from an unaffected subject, as well as endogenous Leprin arcuate neurons. See also Movie S3. (C) Increased levels of co-immunoprecipitated Lepr-b with GFP-tagged Rpgrip1l in the presence of leptin compared with proteins extracts from untreated primary fibroblasts from unaffected human subjects. Lepr-b did not co-immunoprecipitate with myc-tagged ciliary protein Ift46 or IFT20 in the presence or absence of leptin. (D) Subcellular co-localization by immunohistochemistry of Lepr and Tgn46 (See also Fig. S1M) or (E) Lepr and Arf4in the arcuate hypothalamus of mice stimulated with leptin.
Expression of genes conveying effects on energy homeostasis in hypothalami of Rpgrip1l+/− mice
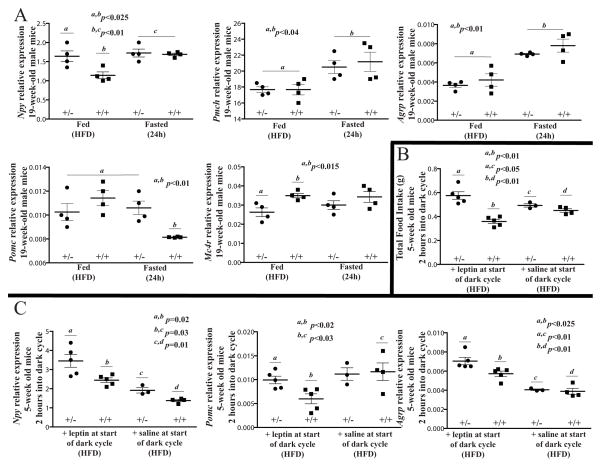
We assessed by RT-PCR the expression levels of selected genes with known actions on ingestive behavior in the hypothalami of Rpgrip1l+/− and wild type mice that had been fed chow for 18 weeks followed either by high-fat diet for 7 days, or fed high-fat diet for 6 days and subsequently fasted for 24h. As expected, expression of orexigenic genes Neuropeptide Y (Npy), Agouti-Related Protein (AgRP) and Pro-Melanin-Concentrating Hormone (Pmch) were increased in hypothalami of fasted wild type mice by ~40%, ~50% and ~30%, respectively (Fig. 7A). Importantly, Npy expression was ~40% higher in HFD-fed Rpgrip1l+/− mice compared to Rpgrip1l+/+ control mice, consistent with the increased food intake displayed by Rpgrip1l+/− mice fed high-fat diet and the proposed impact of RPGRIP1L on leptin signaling. As expected, mRNA levels of anorexigenic pro-opiomelanocortin (Pomc) decreased by ~30% in fasted wild type compared with fed wild type mice. In contrast, expression of Pomc was not decreased in fasted Rpgrip1l+/− mice and expression of the a-Msh receptor (Mc4r) was ~27% lower in fed Rpgrip1l+/− compared with fed Rpgrip1l+/+ mice, suggesting dysregulation of leptin signaling.
Fig. 7. Alterations in Npy, AgRP, Pomc and Mc4r expression in Rpgrip1l+/− mice.
(A) mRNA levels assessed by RT-PCR using as template total hypothalamic RNA extracted from 4 Rpgrip1l+/− and 4Rpgrip1l+/+ mice fed regular chow ad lib for 18 weeks followed by the high fat diet (HFD) for 6 days before a 24-hour fast; or 4Rpgrip1l+/− and 4Rpgrip1l+/+ littermatesfed regular chow ad lib for 18 weeks followed by HFD for a week. Food intake (B) and transcript levels (C) in 8 Rpgrip1l+/− and 9 Rpgrip1l+/+ mice fed HFD at 4-weeks of age ad lib for one week. 5 Rpgrip1l+/− and 5 Rpgrip1l+/+ mice were administered leptin intraperitoneally at the start of the dark cycle, whereas 3 Rpgrip1l+/− and 4 Rpgrip1l+/+ mice were administered saline at the same time point. All mice were sacrificed 2 hours after being injected with leptin or saline (red asterisk; Fig. 2K). Npy, AgRP and Pomc mRNA levels were higher in leptin-treated Rpgrip1l+/− compared with leptin-treated Rpgrip1l+/+ mice. Each data set is annotated with a letter to facilitate comparison. Error bars represent SEM.
We assessed the expression of feeding-related peptides in the hypothalami of5-week old Rpgrip1l+/− and Rpgrip1l+/+ high fat-fed male mice in response to a single intraperitoneal dose of leptin (4 μg/g) at the start of the dark cycle (Fig. 7B, C). Rpgrip1l+/− mice ate more than wild type animals ~2 hours after the administration of leptin (Fig. 7B), and Npy and AgRP expression levels were~45% and ~35% higher, respectively, in Rpgrip1l+/− compared with Rpgrip1l+/+ mice (Fig. 7C). Pomc expression was also ~70% higher in Rpgrip1l+/−compared with Rpgrip1l+/+ mice 2 hours after leptin administration. mRNA measurements were taken shortly before Rpgrip1l+/− mice, unlike Rpgrip1l+/+ mice, exhibited reduced food intake (red asterisk, Fig. 2K). Therefore, the increase in Pomc expression in Rpgrip1l+/− mice may have caused this reduction in food intake.
Discussion
Primary cilia are ubiquitous in the brain, including the hypothalamus, hippocampus and olfactory bulb (Bishop et al., 2007). The melanin-concentrating hormone receptor 1 (Mchr1) localizes to neuronal primary cilia. Mchr1 fails to do so in mice lacking the ciliary proteins, BBS2 or BBS4, implicated in Bardet-Biedl Syndrome, a disorder associated with obesity and anosmia (Berbari et al., 2008). Adult mice with impaired ciliary function due to absence of functional Kif3ain Pomc-expressing neurons, display increased food intake leading to obesity (Davenport et al., 2007). Knocking down ciliary proteins BBS1 or BBS2 in human retinal pigment epithelial cells disrupts LEPR-b trafficking to the TGN, resulting in retention of LEPR-b molecules in large cytoplasmic vesicles (Seo et al., 2009). In the present study, Lepr-b partially co-localized and co-immunoprecipitated with RPGRIP1L in vitro, and Leprco-localized with TGN-specific markers in arcuate hypothalamic cells. Additional evidence that LEPR-b may be part of a cilium-related protein complex includes the observations that LEPR-b co-localizes at the ciliary basal body (Han et al., 2014), co-immunoprecipitates with ciliary basal-body-associated BBS1 in vitro (Seo et al., 2009), and our finding that Leprco-localizes with ARF4, which participates in mediating trafficking of molecules to the cilium (Lim et al., 2011). A model that includes LEPR-b clustering for optimal JAK/STAT signaling (Zabeau et al., 2004; Peelman et al., 2006) may provide a mechanism for the apparent functional role of cilia in leptin signaling by virtue of the convening of LEPR molecules in close proximity to each other. Our findings in the arcuate nucleus of Rpgrip1l+/− mice and fibroblasts from Joubert patients with regard to physical size of cilia, clustering of Lepr-b, and pStat3 generation in response to exogenous leptin, are consistent with this formulation. Nevertheless, the data presented here suggest that convening of LEPR molecules does not occur in the axonemal part of primary cilia. The mechanism underlying enhanced leptin signaling that involves Lepr-blocalization in the vicinity of, but not within, the cilium is unclear. The possibility that TGN assembly near the base of the cilium is important for efficient trafficking and clustering of Lepr-b to the cell membrane vicinal to the cilium warrants further investigation.
AcIII hypothalamic mRNA levels in Rpgrip1l+/− and Rpgrip1l+/+ mice are indistinguishable (Fig. S1N). Therefore, the reduction of AcIII-positive cilia in the hypothalamus of mice hypomorphic for Rpgrip1l, as well as in Joubert fibroblasts that fail to segregate RPGRIP1L at the transition zone, suggests that RPGRIP1L facilitates the transport of ACIII to the cilium. In agreement with this inference, mouse fibroblasts derived from embryos lacking proteins associated with the transition zone - Tectonic 1&2, Cc2d2a or Tmem67 - fail to localize AcIII in their cilia (Garcia-Gonzalo et al., 2011). Adenylyl cyclases catalyze the synthesis of cyclic 3353-AMP (cAMP) from ATP, and elevated cAMP levels in the hypothalamus have been reported to reduce the anorexigenic effect of leptin (Zhao et al., 2002; Fukuda et al., 2011). Moreover, congenital systemic deletion of AcIII is associated with increased bodyweight (Wang et. al., 2009), suggesting a potential role of AcIII in the increased food intake of Rpgrip1l+/− mice that may be partially dependent on reduced leptin signaling. However, it is unclear whether some or all of the dose-dependent effect of Rpgrip1l on energy balance is mediated by AcIII; the phenotypes of mice heterozygous for a null allele of AcIII have not been reported. Given the plethora of receptors and signaling molecules that convene in or around the cilium (Mukhopadhyay & Jackson, 2013), it is likely that multiple signaling pathways are affected by Rpgrip1l hypomorphism. The implication of AcIII in energy homeostasis in this context is certainly consistent with this formulation.
LEPR-b signaling, which is diminished in Rpgrip1l+/− mice, is important for development of energy homeostasis-related neuronal circuitry in the hypothalamus (Bouret et al., 2004, 2012). It is possible that physiological sensitivity to leptin-mediated by the cilium and RPGRIP1L is important for the development of the feeding neuro circuitry. In support of this hypothesis, induced ablation of ciliary formation in adult mice (week 8) has no apparent effect on responses in feeding behavior to exogenous leptin (Berbari et al., 2013). Nevertheless, transient perturbation of ciliary formation in the mediobasal hypothalamus of adult mice by stereotaxic injection of siRNA specific to ciliary structural components resulted in diminished response to exogenous leptin leading to increased food intake, suggesting that hypothalamic primary cilia modulate leptin sensitivity independent of their role (s) in neurodevelopment (Han et al., 2014).
Adult male mice heterozygous for null mutations of Lepr-b (Lepdb) or leptin (Lepob) have ~50% and ~30% increased body fat, respectively, compared to wild type mice (Chung et al., 1998). Likewise, individuals heterozygous for hypomorphicalleles of LEP have increased body fat (Farooqi et al., 2001). Reduced LEPR-b (or other weight-homeostatic) signaling as a consequence of alterations in the function of the primary cilium, might account for some or all of the increase in the fat mass of Rpgrip1l+/− mice compared with their control littermates.
We have previously shown that the protective C allele of rs8050136, one of the FTO intronic SNPs associated with increased BMI (Scuteri et al., 2007), favors binding of the short isoform of Cut-like homeobox 1 (CUX1 P110) that acts as a transcriptional activator of RPGRIP1L and FTO (Stratigopoulos et al., 2011). As in the case of mouse embryos lacking functional Rpgrip1l, loss of Ftoin zebra fish results in multiple developmental defects that are due in part to ciliary structural defects accompanied by disregulated β-catenin-dependent Wnt signaling (Vierkotten et al., 2007; Huang et al., 2011; Osborn et al., 2014). Similarly, CUX1 p110 controls the expression of genes that participate in the β-catenin-dependent Wnt pathway (Cadieux et al., 2009). Moreover, CUX1 P110 is a positive regulator of leptin signaling, whereas FTO knock-down results in diminished leptin signaling in vitro (Stratigopoulos et al., 2011). Therefore, it is conceivable that individuals segregating for the obesity-risk A allele at rs8050136 have lower hypothalamic RPGRIP1L and FTO expression levels due to lower DNA binding affinity of P110 at that cognate CUX1-binding site. Consequently, due to pre- and/or postnatal events, these individuals would have diminished leptin signaling resulting in increased food intake and adiposity, thus providing a mechanism that links the allelic variation at FTO intron 1 with increased food intake and adiposity.
The possibility that SNPs associated with adiposity are embedded in regulatory elements of nearby genes that extend within the first intron of FTO is supported by the finding that the promoter of the homeobox gene IRX3 interacts with the obesity-associated FTO interval through long-range looping (Smemo et al., 2014). This recent report has suggested that the intronic region of FTO implicated in obesity interacts at a distance (of ~0.5 Mbp) with IRX3 to increase adiposity primarily by effects on energy expenditure. The susceptibility allele of FTO has been proposed to increase IRX3 expression resulting in lower energy expenditure due to diminished activation of brown adipose tissue and limited “browning” of white adipose tissue (Smemo et al., 2014). In humans, the implicated FTO allele conveys its impact on adiposity, proportional to allele dose, primarily by effects on energy intake (Cecil et al., 2008; Haupt et al., 2009; Speakman et al., 2008; Wardle et al., 2009; Den et al., 2009; Wardle et al., 2008; Rutters et al., 2010; Tanofsky-Kraff et al., 2009). Irx3 expression was not affected in proportion to dosage of the FTO obesity-risk-allele in human brain, and an intermediate adiposity phenotype in mice segregating for a single Irx3 null allele was not reported (Smemo et al., 2014). It is certainly possible, of course, that intronic FTO has effects on the expression of several genes (Jowett et al., 2010). Our data support a quantitatively important role for RPGRIP1L in conveying the orexigenic effects of intronic sequence variants in FTO.
Experimental Procedures
Mouse strains
Mice heterozygous for the Rpgrip1l floxed-LacZ allele (Rpgrip1l™1a(EUCOMM) Wtsi; referred to here as Rpgrip1l+/−) were derived from targeted embryonic stem cells on a C57BL/6NTac background, purchased from the International Knockout Mouse Consortium (http://www.knockoutmouse.org), and maintained on a C57BL/6J background. Targeting was confirmed by the International Knockout Mouse Consortium. Compound heterozygous Lepr-bcre/+, Gt (ROSA) 26 Sor™2Sho/+ mice were a gift by Dr. Martin Myers (University of Michigan) (Leshan et al., 2006).
Diet and dietary treatment
Female Rpgrip1l+/− (n=6) and control (Rpgrip1l+/+) littermates (n=5) were fed regular chow (9% Kcal from fat; Picolab 5058; Purina Mills, USA) until 12 weeks of age. Male Rpgrip1l+/− (n=10) and control (Rpgrip1l+/+) littermates (n=6) were fed regular chow ad libitum until 18 weeks of age and switched to a high fat (65% of calories as fat; Cat. No D12492; Open Source Diets™, USA) for one week. An additional 5 Rpgrip1l+/− and 7 control (Rpgrip1l+/+) male mice were fed regular chow ad libitum until 13 weeks of age, and their body weights measured at 8, 12 and 13 weeks of age. A second experimental group of male Rpgrip1l+/− (n=6) and control (Rpgrip1l+/+) mice (n=6) were fed regular chow ad libitum until 4 weeks of age and then switched to the high fat diet for one week. After 2 days of ad libitum ingestion of the high fat diet, mice were fasted overnight for 10 hours in order to assure that both groups were identical with regard to metabolic and feeding status. Food was re-introduced and serial intraperitoneal saline injections were performed in all mice to accustom them to handling. 9 hours after re feeding, mice were administered leptin (4 μg/g). This higher than customary (2 μg/g) dose was used in order to accentuate distinctions in behavioral responses between +/+ and +/− animals. Room temperature was constant at 23°C on a 12h light/12h dark cycle (lights were turned off at 7 PM), and mice had ad libitum access to food and water.
Indirect calorimetry
Mice were weighed and their body composition determined before individually caged in a LabMaster-CaloSys-Calorimetry System (TSE Systems, USA). Calorimetry was performed on the 18-week old Rpgrip1l+/− (n=10) and control (Rpgrip1l+/+; n=6) experimental groups for ~72h while fed regular chow ad libitum, and then for another 3 days after the groups were switched to the high fat diet ad libitum. Calorimetry was also performed on the 3-week old Rpgrip1l+/− (n=6) and control (Rpgrip1l+/+; n=6) groups for one week while being fed regular chow ad libitum, and then for another week after the groups were switched to the high fat diet ad libitum. 40h after they were switched to the high fat diet, mice were fasted overnight for 10 hours, administered saline intraperitoneally at 2, 4 and 6 hours after a 10h overnight fast, followed by intraperitoneal leptin administration (4μg/g) at the start of the dark cycle. During this period, food intake and energy expenditure were automatically recorded every 26 minutes by the calorimeter. Mice were allowed to acclimate during the first ~24h. Energy intake was calculated by multiplying cumulative food intake for a 24-hour period by the metabolizable energy present in the high fat (5.24 Kcal/g) diet and regular chow (3.56 Kcal/g).
Body mass and composition measurements
Mice were weighed on an electronic scale. Body composition was determined by TD-NMR using a Minispec Analyst AD lean fat analyzer (Bruker Optics, Silberstreifen Germany). The TD-NMR was calibrated using mouse carcasses that ranged from 5 to 70 grams in mass (Halldorsdottir et al., 2009).
Tissue culture
Human primary skin fibroblasts from healthy individuals [Protocol: IRB-AAAK6905(Y1Moo)] were derived and maintained in AmnioMAX™ - II Complete Medium (Invitrogen, Carlsbad, CA) in a humidified atmosphere at 37°C and 5% CO2. Primary skin fibroblasts from Joubert patients UW15, UW04-3 and UW04-4 were maintained in AmnioMAX™ - II Complete Medium. For the phosphorylated Stat3 assay or immunofluorescence studies, confluent primary skin fibroblasts cells were made quiescent 48h after transfection by incubation in DMEM with 0.5% BSA overnight. Cells were then treated with leptin for 30 minutes or 3 hours prior to protein extraction. Expression vectors were introduced in cultured cells via transfection (using Lipofectamine®, Invitrogen) or transduction (via Lentiviral packaging, Invitrogen).
Immunohistochemistry and cellular Immunofluorescence
Cultured-cell slides were fixed with ice-cold methanol:acetone (1:1). Primary antibodies against LEPR (1–4 μg/ml for immunohistochemistry, 0.5–1 μg/ml for immunofluorescence; Mouse, Human; Cat # AF497, R&D SYSTEMS, Minneapolis, MN), Acetylated tubulin (1:500; Mouse; Cat # T6793-.2ML, Sigma, St. Louis, MO), and RPGRIP1L (1:400; Human and mouse; provided by Daniel Doherty, University of Washington) were used in conjunction with Alexa Fluor® 488, 555 and 647 secondary antibodies (Invitrogen, Carlsbad, CA). ProLong® Gold antifade reagent with either DAPI (Invitrogen) or DRAQ5 (Axxora, San Diego, CA) were used for mounting and nuclear staining. Brain perfusion, cryo sectioning and immunohistochemical analysis of the murine hypothalamus were performed as described elsewhere (Stratigopoulos et al., 2011). Mice were treated with an intraperitoneal injection of leptin (2μg/g of body weight) 30 minutes prior to sacrifice and brain perfusion. For pSTAT3 staining, brain sections were treated with 1% NaOH/1% H2O2 solution, 0.3% Glycine and 0.03% SDS prior to antibody treatment. Anti-pSTAT3 (Tyr-705), anti-Arf4 and anti-TGN46 antibodies antibody was purchased from Cell Signaling Technology (1: 200; Cat no. 9131, Danvers, MA), Protein Tech (1:100; Cat no 11673-1-AP, Chicago, IL) and Lifespan Biosciences (1:500; Cat no LS-C124646, Seattle, WA) respectively. Tomato-positive arcuate cryosections from Lepr-bcre/+ Gt (ROSA)26Sor™2Sho/+ compound heterozygous mice were visualized using a confocal microscope, treated with 50% MeOH for 10 min followed by overnight incubation with PBS at 64°C, and subsequent overnight incubation with anti-LEPR antibody.
Anti-ARL13B (1:500; Cat no 17711-1-AP, Proteintech, Chicago, IL), anti-Acetylated Tubulin (1:1,000; Cat no T7451, Sigma), and anti-γ Tubulin (1:200; Cat no sc-7396, Santa Cruz) were utilized for the measurement of cilia number and length determination. Fibroblasts were grown for 2 days to ~85–95% confluency, serum-starved for ~48 hours in DMEM, and subsequently fixed in 4% PFA for 15 min followed by 100% methanol for 10 minutes at −20°C.
Isolation of total RNA and nuclear protein extracts
Total RNA was extracted and DNase-treated using the RN easy Lipid Tissue Mini Kit (Qiagen, Gaithersburg, MD) according to the manufacturer’s instructions. Nuclear protein extracts were isolated in the presence of Halt Protease and Phosphatase Inhibitor cocktail (Pierce, Rockford, IL) using the NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Pierce) according to the manufacturer’s instructions. Total protein levels were measured using a Bradford Assay (Pierce).
cDNA synthesis
cDNA synthesis from 13g of total RNA was performed utilizing the Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN) with Oligo (dT) 20 and Random Hexamer primer mix according to the manufacturer’s instructions.
qPCR- For expression analysis, a set of male mice were kept singly caged, fed regular chow till 18 weeks of age, and, thereafter, fed either the high fat diet for 7 days (4 Rpgrip1l+/−and 4 Rpgrip1l+/+ males) or fed the high fat diet for 6 days and fasted for 24h (4 Rpgrip1l+/−and 4 Rpgrip1l+/+ males). Murine whole brains were placed on a dissection block and the hypothalamus was dissected from the rostral border of the optic chiasm to the rostral border of the mammillary body. Fto and Rpgrip1l mRNA levels were measured by quantitative PCR as described elsewhere (Stratigopoulos et al., 2011). Similarly, quantitative PCR was performed to validate mRNA levels of Npy(F-GACAATCCGGGCGAGGACGC, R-CACCACATGGAAGGGTCTTCAAG), Pmch(F-GATCCGTAGCCTTCCCAGCTGAG, R-CCAACATGGTCGGTAGACTCTTCCC), Pomc(F-GCCTGGAGAGCAGCCAGTGCCAG, R-GACGTACTTCCGGGGGTTTTCAGTC), AgRP (F-GGGCGTGGCTCCACTGAAGGGCAT, R-CTGCAGTTGTCTTCTTGAGGCCAT), Mc4r (F-CACCATGGCATGTATACTTCCCTCC, R-GATCACTAGAATGTTCTCCAACA), Lepr-b (F-AACCCCAAGAATTGTTCCTGG, R-GGAGACCATAGCTGCTGGGACC), Cdh9 (F-CATGGCTCAAACTCAGCTGCAA, R-GCAGCTTAGTGTATGTCCCAG), Rbl2 (F-GGCCTGGAGCAGCTACCGCAG, R-GATACATAGTTTCCTTCAGCGGT), Aktip (F-GCAGCGCAGTCAACAAACGGC, R-CAGAGCGGTAAGATGGCTGTAC), Irx3 (F-CAATGTGCTTTCATCAGTGTACG, R-GGATGCTGGACGCCAGGGCTGT), Irx5 (F-GCACGGATGAGCTCGGCCGCTC, R-GGGTGATATCCCAAGGAACCTG), Irx6 (F-CTCAGTATGAGTTCAAGGATGCTG, R-CTCCCTTGTGGCATTCTTCCTG) and Adcy3 (F-CCAAGCACGTGGCTGACGAG, R-CAGAGGACAGCTGGGTAAAGCC) in the hypothalamus of Rpgrip1l+/−and Rpgrip1l+/+ littermates. mRNA levels were normalized as described elsewhere (Stratigopoulos et al., 2011).
Antibodies
Anti-RPGRIP1L (1:200; Human &Mouse; provided by Daniel Doherty) was used for determining Rpgrip1l protein levels in Rpgrip1l+/− mice by Western blotting. Phospho-Stat3 (Tyr705) antibody purchased from Cell Signaling Technology (1:1000; Cat# 9131, Danvers, MA) was also utilized for western blotting. 3-TUBULIN (1:2000; Cat# 05-661; Millipore, Bedford MA) and STAT3 (1:1000; Cat# 9132) were used as internal controls. In murine, tissue, Lepr and AcIII were stained with antibodies as described elsewhere (Stratigopoulos et al., 2011).
Plasmids
The full-length murine Rpgrip1lc DNA (fused to GFP) under the CMV promoter was purchased from Origene (Cat# MG218118; Rockville, MD). The overexpression Myc-tagged Ift46 and IFT20c DNA were also purchased from Origene (Cat # MR204208, RC201337). The mouse leptin receptor isoform b (Lepr-b) cDNA (Stratigopoulos et al., 2011) with 3 stop codons at the 3′end (introduced by PCR) was cloned downstream of the CMV promoter using the pcDNA3.1 Directional TOPO Expression Kit (Invitrogen). The human LEPR-b cDNA was amplified from a plasmid purchased by Sino Biological Inc. (NM_002303.3; Beijing, China) using primers5′-GCGCTAGCATGATTTGTCAAAAATTCTGTGTGG, 5′ATGGATCCCACAGTTAGGTCACACATCT, and cloned in CD520A-RFP at the NheI-BamHI restriction sites after digestion of the insert with NheI, BamHI and Mung Bean nucleases (NEB). CD520A-RFP was constructed by cloning RFP - amplified from pDsRed-Monomer-N1 (Clontech)using primers 5′-AATCGGATCCACCGGTCGCCACCAT and 5′-AATTCAGCGGCCGCTCTCTGGGAGCCGGAGTG - at the BamHI-NotI restriction sites of CD520A (System Biosciences, Mountain View, CAL).
Co-Immunoprecipitation Assay
Primary skin fibroblasts - isolated from a healthy individual - at 80% confluency in a T75 flask were transfected with 23g of specified overexpression construct using Lipofectamine 2000™ reagent (Invitrogen). After 48 h, cells were starved in DMEM with 0.5% BSA for 6h before treatment with leptin (1μg/ml). After 30 minutes or 1 hour of incubation with leptin, cells were lysed (1M Tris-HCl pH=8.0, 5M NaCl, 0.5M EDTA, 10% NP40) in the presence of protease/phosphatase inhibitor (Cat # 1861280; Pierce) and protein was immunoprecipitated by incubation with 5% w/v Protein G Sepharose beads (Cat # 17-0618-01, GE Healthcare Life Sciences, Piscataway, NJ) and anti-GFP (1/200; Mouse; Cat# 11814460001, Roche), anti-Lepr (1/200; Mouse; Cat # AF497, R&D SYSTEMS) or anti-myc (1/200; Mouse; Cat # R951-25, Invitrogen) antibodies. Bound material was detected by immunoblotting with anti-LEPR or anti-GFP antibodies (Sigma).
Statistical analysis
Data are expressed as means +/− standard error. Statistical analysis was performed using Student’s T-test (StatView 5.0, SAS Institute Inc.). Levels of statistical significance were set at 2-tailed palpha<0.05. All error bars represent Standard Error of the Mean (SEM).
Supplementary Material
Highlights.
Mice heterozygous for a null Rpgrip1l allele are fatter than wild type controls.
Leptin signaling is diminished in the hypothalami of Rpgrip1l+/− mice.
The localization of the leptin receptor is perturbed in Rpgrip1l+/− mice.
RPGRIP1L may account for part or all of the association of the FTO locus with BMI.
Acknowledgments
We would like to thank Richard Rausch and Alicja Skowronski for technical support. We also thank Alain Juan de Solis for technical help and constructive discussions. Skin cells were kindly provided by participants AL, and UW15, UW04-3, UW04-04 and family. This work was supported by RO1 DK52431-15, P30 DK26687-30, RO1 NS064077 and RO1 DK097399-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Märker T, Voesenek K, Kartono A, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–8. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151:281–95. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Lewis JS, Bishop GA, Ask with CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. ProcNatlAcadSci U S A. 2008;105:4242–6. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Pasek RC, Malarkey EB, Yazdi SM, McNair AD, Lewis WR, Nagy TR, Kesterson RA, Yoder BK. Leptin resistance is a secondary consequence of the obesity in ciliopathy mutant mice. ProcNatlAcadSci U S A. 2013;110:7796–801. doi: 10.1073/pnas.1210192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–71. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Bates SH, Chen S, Myers MG, Jr, Simerly RB. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J Neurosci. 2012;32:1244–52. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Cadieux C, Kedinger V, Yao L, Vadnais C, Drossos M, Paquet M, Nepveu A. Mouse mammary tumor virus p75 and p110 CUX1 transgenic mice develop mammary tumors of various histologic types. Cancer Res. 2009;69:7188–97. doi: 10.1158/0008-5472.CAN-08-4899. [DOI] [PubMed] [Google Scholar]
- Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–2566. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- Chung WK, Belfi K, Chua M, Wiley J, Mackintosh R, Nicolson M, Boozer CN, Leibel RL. Heterozygosity for Lep (ob) or Lep (rdb) affects body composition and leptin homeostasis in adult mice. Am J Physiol. 1998;274:R985–90. doi: 10.1152/ajpregu.1998.274.4.R985. [DOI] [PubMed] [Google Scholar]
- Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L, Wells S, Brüning JC, Nolan PM, Ashcroft FM, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42:1086–92. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–94. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–81. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- Den HM, Westerterp-Plantenga MS, Bouwman FG, Mariman EC, Westerterp KR. Postprandial responses in hunger and satiety are associated with the rs9939609 single nucleotide polymorphism in FTO. Am J Clin Nutr. 2009;90:1426–1432. doi: 10.3945/ajcn.2009.28053. [DOI] [PubMed] [Google Scholar]
- Doherty D, Parisi MA, Finn LS, Gunay-Aygun M, Al-Mateen M, Bates D, Clericuzio C, Demir H, Dorschner M, van Essen AJ, et al. Mutations in 3 genes (MKS3, CC2D2A and RPGRIP1L) cause COACH syndrome (Joubert syndrome with congenital hepatic fibrosis) J Med Genet. 2010;47:8–21. doi: 10.1136/jmg.2009.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Keogh JM, Kamath S, Jones S, Gibson WT, Trussell R, Jebb SA, Lip GY, O’Rahilly S. Metabolism: Partial leptin deficiency and human adiposity. Nature. 2001;414:34–35. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. MolBiol Cell. 2006;17(9):3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Williams KW, Gautron L, Elmquist JK. Induction of leptin resistance by activation of cAMP-Epac signaling. Cell Metab. 2011;13:331–9. doi: 10.1016/j.cmet.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. 2011;43:776–84. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldorsdottir S, Carmody J, Boozer CN, Leduc CA, Leibel RL. Reproducibility and accuracy of body composition assessments in mice by dual energy x-ray absorptiometry and time domain nuclear magnetic resonance. Int J Body Compos Res. 2009;7:147–154. [PMC free article] [PubMed] [Google Scholar]
- Han YM, Kang GM, Byun K, Ko HW, Kim J, Shin MS, Kim HK, Gil SY, Yu JH, Lee B, et al. Leptin-promoted cilia assembly is critical for normal energy balance. J Clin Invest. 2014 doi: 10.1172/JCI69395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Huang N, Niu T, Chai J. A loop matters for FTO substrate selection. Protein & Cell. 2010;1:616–620. doi: 10.1007/s13238-010-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt A, Thamer C, Staiger H, Tschritter O, Kirchhoff K, Machicao F, Häring HU, Stefan N, Fritsche A. Variation in the FTO gene influences food intake but not energy expenditure. Exp Clin Endocrinol Diabetes. 2009;117:194–197. doi: 10.1055/s-0028-1087176. [DOI] [PubMed] [Google Scholar]
- Huang L, Szymanska K, Jensen VL, Janecke AR, Innes AM, Davis EE, Frosk P, Li C, Willer JR, Chodirker BN, et al. TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am J Hum Genet. 2011;89:713–30. doi: 10.1016/j.ajhg.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowett JB, Curran JE, Johnson MP, Carless MA, Göring HH, Dyer TD, Cole SA, Comuzzie AG, MacCluer JW, Moses EK, Blangero J. Genetic variation at the FTO locus influences RBL2 gene expression. Diabetes. 2010;59:726–32. doi: 10.2337/db09-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41:739–745. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan RL, Björnholm M, Münzberg H, Myers MG., Jr Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 2006;14(Suppl 5):208S–212S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- Lim YS, Chua CE, Tang BL. Rabs and other small GTPases in ciliary transport. Biol Cell. 2011;103:209–21. doi: 10.1042/BC20100150. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang M, Xia Z, Xu P, Chen L, Xu T. Caenorhabditiselegans ciliary protein NPHP-8, the homologue of human RPGRIP1L, is required for ciliogenesis and chemosensation. Biochem Biophys Res Commun. 2011;410:626–31. doi: 10.1016/j.bbrc.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Lucker BF, Miller MS, Dziedzic SA, Blackmarr PT, Cole DG. Direct interactions of intraflagellar transport complex B proteins IFT88, IFT52, and IFT46. J Biol Chem. 2010;285:21508–18. doi: 10.1074/jbc.M110.106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, Durand E, Vatin V, Degraeve F, Proenca C, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41:157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- Meyre D, Proulx K, Kawagoe-Takaki H, Vatin V, Gutiérrez-Aguilar R, Lyon D, Ma M, Choquet H, Horber F, Van Hul W, et al. Prevalence of loss-of-function FTO mutations in lean and obese individuals. Diabetes. 2010;59:311–318. doi: 10.2337/db09-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Jackson PK. Cilia, tubby mice, and obesity. Cilia. 2013;2:1. doi: 10.1186/2046-2530-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn DP, Roccasecca RM, McMurray F, Hernandez-Hernandez V, Mukherjee S, Barroso I, Stemple D, Cox R, Beales PL, Christou-Savina S. Loss of FTO Antagonises Wnt Signaling and Leads to Developmental Defects Associated with Ciliopathies. PLoS One. 2014;9:e87662. doi: 10.1371/journal.pone.0087662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelman F, Iserentant H, De Smet AS, Vandekerckhove J, Zabeau L, Tavernier J. Mapping of binding site III in the leptin receptor and modeling of a hexamericleptin. leptin receptor complex. J BiolChem. 2006;281:15496–15504. doi: 10.1074/jbc.M512622200. [DOI] [PubMed] [Google Scholar]
- Rutters F, Lemmens SG, Born JM, Bouwman F, Nieuwenhuizen AG, Mariman E, Westerterp-Plantenga MS. Genetic associations with acute stress-related changes in eating in the absence of hunger. Patient Educ Couns. 2010;79:367–371. doi: 10.1016/j.pec.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orru M, Usala G, et al. Genome-Wide Association Scan Shows Genetic Variants in the FTO Gene Are Associated with Obesity-Related Traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18:1323–31. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gómez-Marín C, Aneas I, Credidio FL, Sobreira DR, Wasserman NF, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507:371–5. doi: 10.1038/nature13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Rance KA, Johnstone AM. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity (Silver Spring) 2008;16:1961–1965. doi: 10.1038/oby.2008.318. [DOI] [PubMed] [Google Scholar]
- Stratigopoulos G, LeDuc CA, Cremona ML, Chung WK, Leibel RL. Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. J Biol Chem. 2011;286:2155–70. doi: 10.1074/jbc.M110.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratigopoulos G, Padilla SL, LeDuc CA, Watson E, Hattersley AT, McCarthy MI, Zeltser LM, Chung WK, Leibel RL. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1185–1196. doi: 10.1152/ajpregu.00839.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Han JC, Anandalingam K, Shomaker LB, Columbo KM, Wolkoff LE, Kozlosky M, Elliott C, Ranzenhofer LM, Roza CA, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am J Clin Nutr. 2009;90:1483–1488. doi: 10.3945/ajcn.2009.28439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung YC, Ayuso E, Shan X, Bosch F, O’Rahilly S, Coll AP, Yeo GS. Hypothalamic-specific manipulation of Fto, the ortholog of the human obesity gene FTO, affects food intake in rats. PLoS One. 2010;5:e8771. doi: 10.1371/journal.pone.0008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadnais C, Awan AA, Harada R, Clermont PL, Leduy L, Bérubé G, Nepveu A. Long-range transcriptional regulation by the p110 CUX1 homeodomain protein on the ENCODE array. BMC Genomics. 2013;14:258. doi: 10.1186/1471-2164-14-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierkotten J, Dildrop R, Peters T, Wang B, Ruther U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–77. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- Wang P, Yang FJ, Du H, Guan YF, Xu TY, Xu XW, Su DF, Miao CY. Involvement of Leptin Receptor (LepRb)-STAT3 Signaling Pathway in Brain FTO Downregulation during Energy Restriction. Mol Med. 2011;17:523–32. doi: 10.2119/molmed.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li V, Chan GC, Phan T, Nudelman AS, Xia Z, Storm DR. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One. 2009;4:e6979. doi: 10.1371/journal.pone.0006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J, Carnell S, Haworth CM, Farooqi IS, O’Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab. 2008;93:3640–3. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obes (Lond) 2009;33:42–45. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
- Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–41. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabeau L, Defeau D, Van der Heyden J, Iserentant H, Vandekerckhove J, Tavernier J. Functional analysis of leptin receptor activation using a Janus kinase/signal transducer and activator of transcription complementation assay. Mol Endocrinol. 2004;18:150–161. doi: 10.1210/me.2003-0078. [DOI] [PubMed] [Google Scholar]
- Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest. 2009;119:428–37. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–8. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hong DH, Pawlyk B, Yue G, Adamian M, Grynberg M, Godzik A, Li T. The retinitis pigmentosa GTPase regulator (RPGR)- interacting protein: subserving RPGR function and participating in disk morphogenesis. ProcNatlAcadSci U S A. 2003;100:3965–70. doi: 10.1073/pnas.0637349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.