Abstract
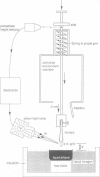
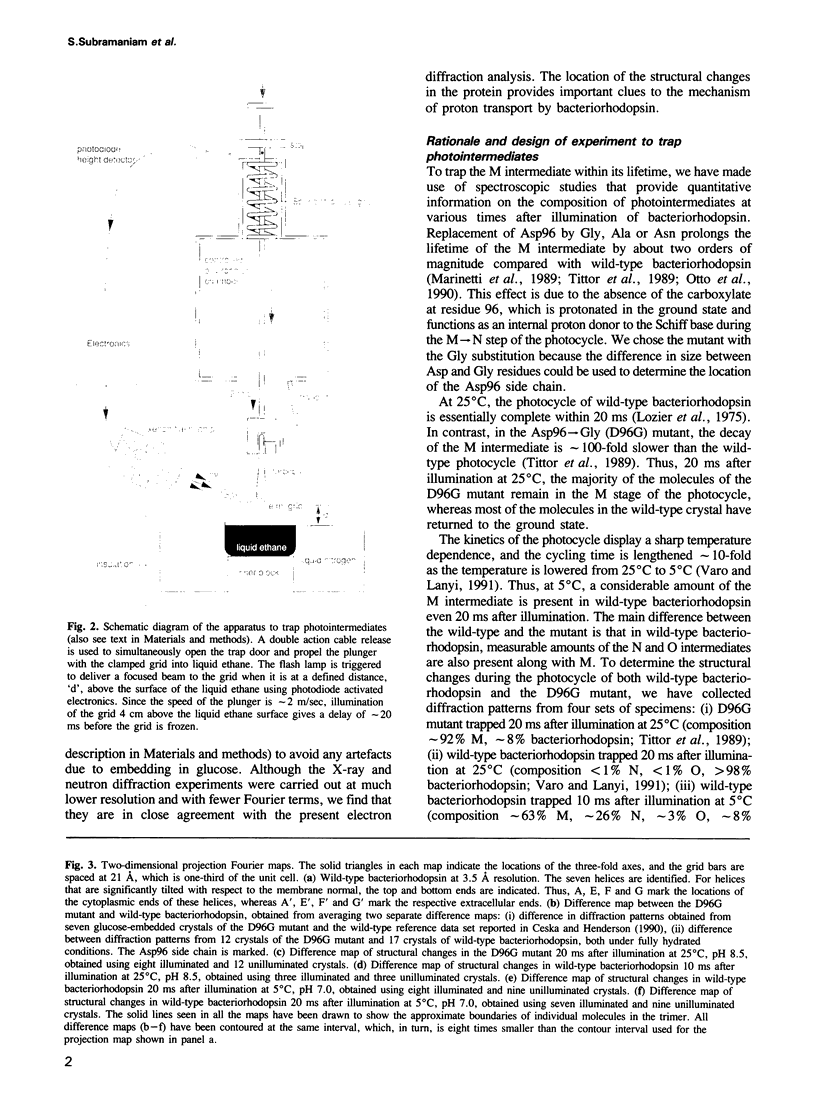
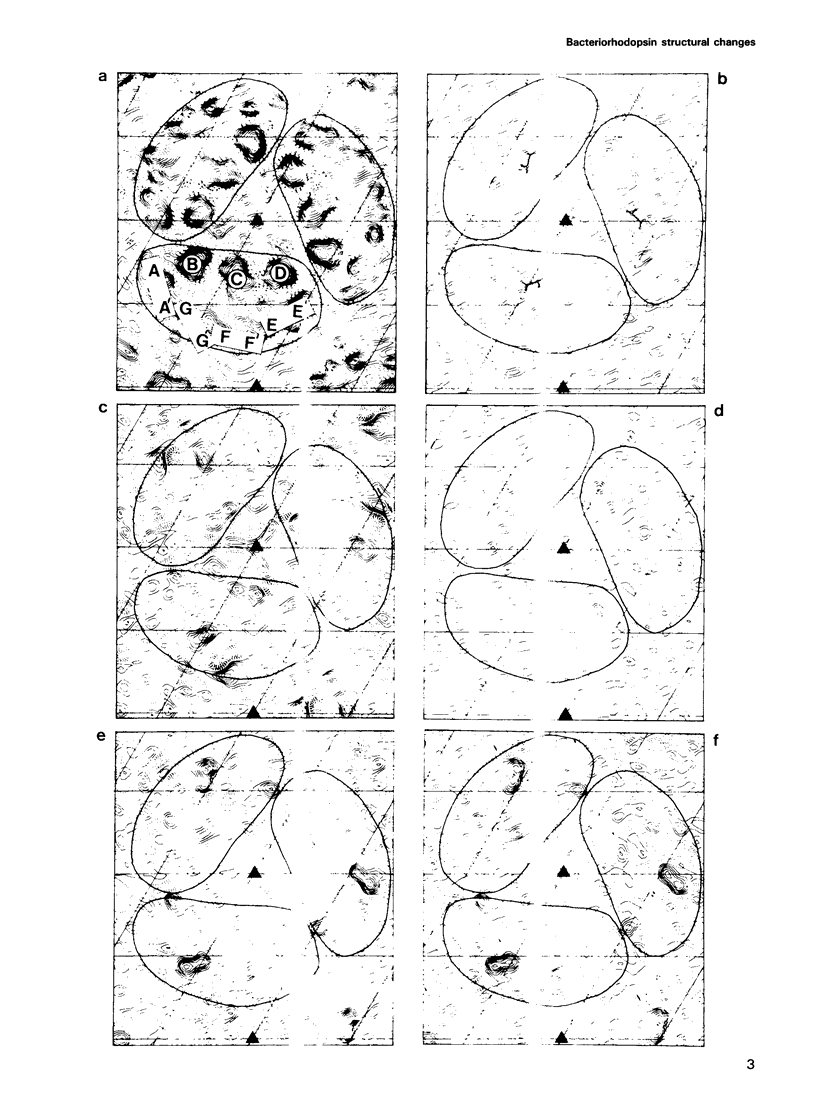
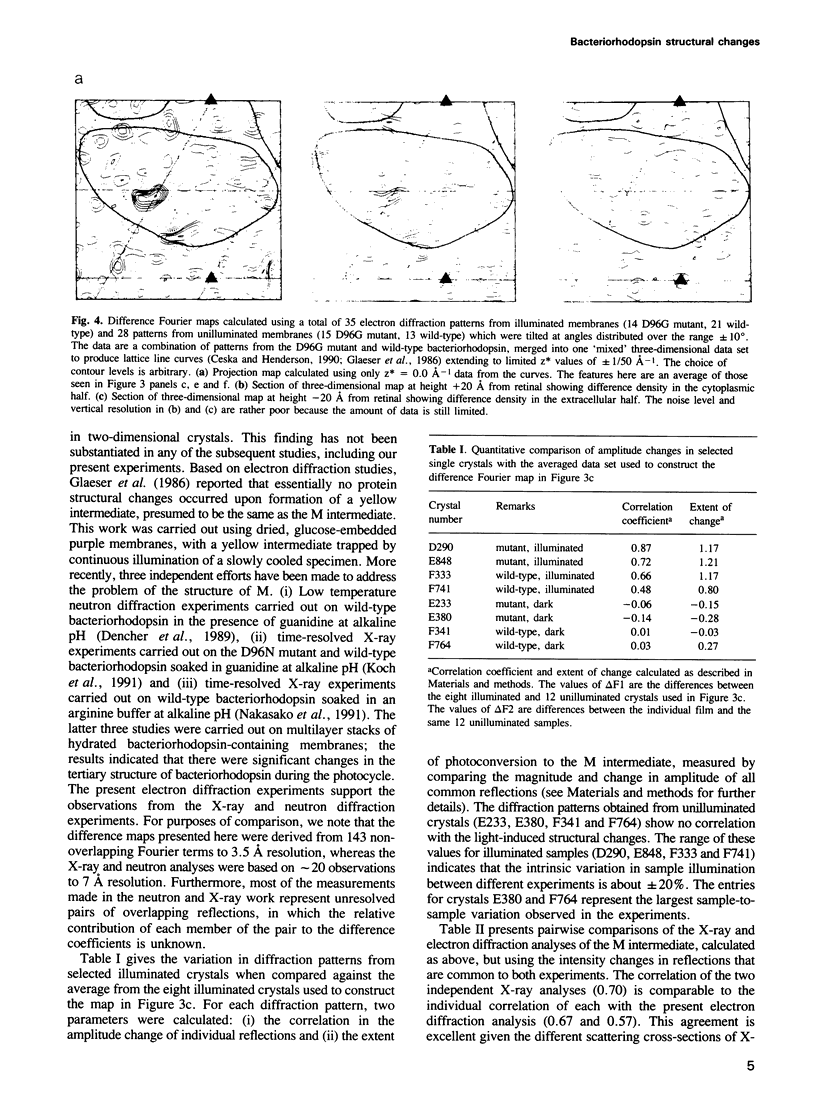
Structural changes are central to the mechanism of light-driven proton transport by bacteriorhodopsin, a seven-helix membrane protein. The main intermediate formed upon light absorption is M, which occurs between the proton release and uptake steps of the photocycle. To investigate the structure of the M intermediate, we have carried out electron diffraction studies with two-dimensional crystals of wild-type bacteriorhodopsin and the Asp96-->Gly mutant. The M intermediate was trapped by rapidly freezing the crystals in liquid ethane following illumination with a xenon flash lamp at 5 and 25 degrees C. Here, we present 3.5 A resolution Fourier projection maps of the differences between the M intermediate and the ground state of bacteriorhodopsin. The most prominent structural changes are observed in the vicinity of helices F and G and are localized to the cytoplasmic half of the membrane.
Full text
PDF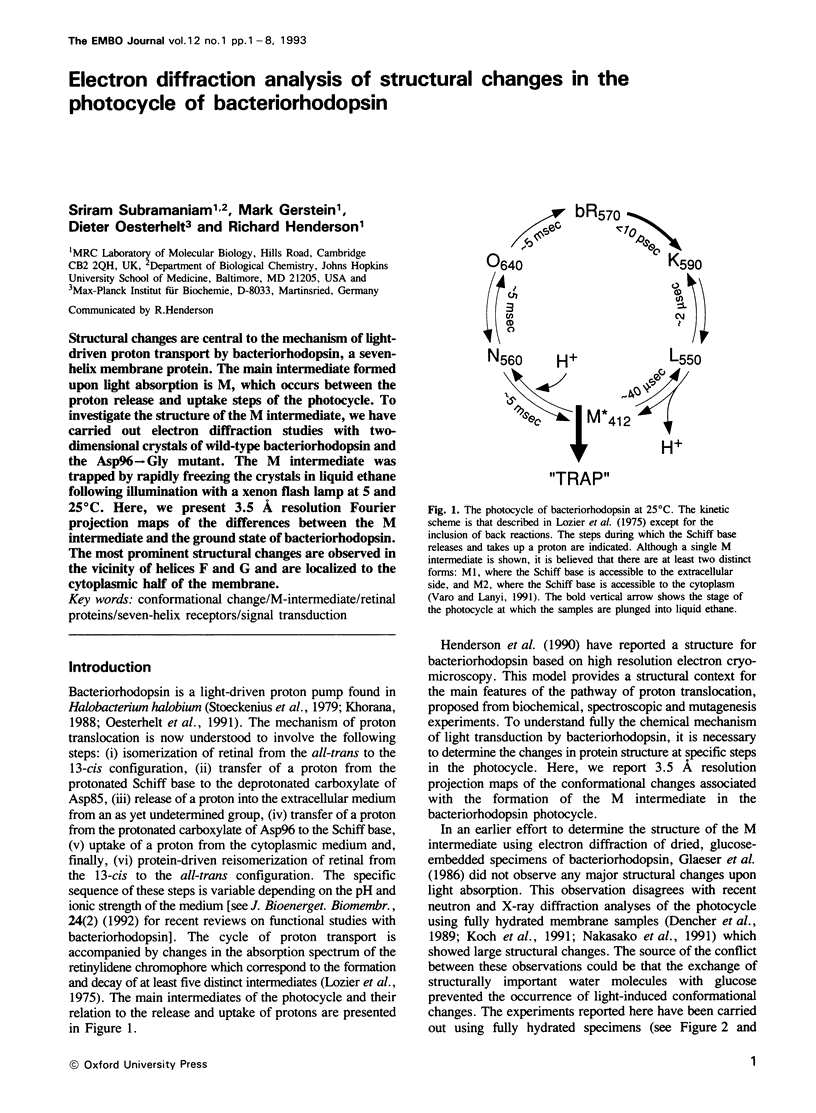




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellare J. R., Davis H. T., Scriven L. E., Talmon Y. Controlled environment vitrification system: an improved sample preparation technique. J Electron Microsc Tech. 1988 Sep;10(1):87–111. doi: 10.1002/jemt.1060100111. [DOI] [PubMed] [Google Scholar]
- Ceska T. A., Henderson R. Analysis of high-resolution electron diffraction patterns from purple membrane labelled with heavy-atoms. J Mol Biol. 1990 Jun 5;213(3):539–560. doi: 10.1016/S0022-2836(05)80214-1. [DOI] [PubMed] [Google Scholar]
- Dencher N. A., Dresselhaus D., Zaccai G., Büldt G. Structural changes in bacteriorhodopsin during proton translocation revealed by neutron diffraction. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7876–7879. doi: 10.1073/pnas.86.20.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Frankel R. D., Forsyth J. M. Time-resolved x-ray diffraction study of photostimulated purple membrane. Biophys J. 1985 Mar;47(3):387–393. doi: 10.1016/S0006-3495(85)83930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Baldwin J., Ceska T. A., Henderson R. Electron diffraction analysis of the M412 intermediate of bacteriorhodopsin. Biophys J. 1986 Nov;50(5):913–920. doi: 10.1016/S0006-3495(86)83532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Khorana H. G. Bacteriorhodopsin, a membrane protein that uses light to translocate protons. J Biol Chem. 1988 Jun 5;263(16):7439–7442. [PubMed] [Google Scholar]
- Koch M. H., Dencher N. A., Oesterhelt D., Plöhn H. J., Rapp G., Büldt G. Time-resolved X-ray diffraction study of structural changes associated with the photocycle of bacteriorhodopsin. EMBO J. 1991 Mar;10(3):521–526. doi: 10.1002/j.1460-2075.1991.tb07978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti T., Subramaniam S., Mogi T., Marti T., Khorana H. G. Replacement of aspartic residues 85, 96, 115, or 212 affects the quantum yield and kinetics of proton release and uptake by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Jan;86(2):529–533. doi: 10.1073/pnas.86.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhalen C. A., Vincent M. G., Picot D., Jansonius J. N., Lesk A. M., Chothia C. Domain closure in mitochondrial aspartate aminotransferase. J Mol Biol. 1992 Sep 5;227(1):197–213. doi: 10.1016/0022-2836(92)90691-c. [DOI] [PubMed] [Google Scholar]
- Nakasako M., Kataoka M., Amemiya Y., Tokunaga F. Crystallographic characterization by X-ray diffraction of the M-intermediate from the photo-cycle of bacteriorhodopsin at room temperature. FEBS Lett. 1991 Nov 4;292(1-2):73–75. doi: 10.1016/0014-5793(91)80837-s. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Bräuchle C., Hampp N. Bacteriorhodopsin: a biological material for information processing. Q Rev Biophys. 1991 Nov;24(4):425–478. doi: 10.1017/s0033583500003863. [DOI] [PubMed] [Google Scholar]
- Otto H., Heyn M. P. Between the ground- and M-state of bacteriorhodopsin the retinal transition dipole moment tilts out of the plane of the membrane by only 3 degrees. FEBS Lett. 1991 Nov 18;293(1-2):111–114. doi: 10.1016/0014-5793(91)81163-3. [DOI] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Stern L. J., Engel F., Khorana H. G., Heyn M. P. Substitution of amino acids Asp-85, Asp-212, and Arg-82 in bacteriorhodopsin affects the proton release phase of the pump and the pK of the Schiff base. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1018–1022. doi: 10.1073/pnas.87.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertler G. F., Lozier R., Michel H., Oesterhelt D. Chromophore motion during the bacteriorhodopsin photocycle: polarized absorption spectroscopy of bacteriorhodopsin and its M-state in bacteriorhodopsin crystals. EMBO J. 1991 Sep;10(9):2353–2361. doi: 10.1002/j.1460-2075.1991.tb07774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppa J., Oesterhelt D. Bacteriorhodopsin mutants of Halobacterium sp. GRB. I. The 5-bromo-2'-deoxyuridine selection as a method to isolate point mutants in halobacteria. J Biol Chem. 1989 Aug 5;264(22):13043–13048. [PubMed] [Google Scholar]
- Stern L. J., Khorana H. G. Structure-function studies on bacteriorhodopsin. X. Individual substitutions of arginine residues by glutamine affect chromophore formation, photocycle, and proton translocation. J Biol Chem. 1989 Aug 25;264(24):14202–14208. [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Greenhalgh D. A., Rath P., Rothschild K. J., Khorana H. G. Replacement of leucine-93 by alanine or threonine slows down the decay of the N and O intermediates in the photocycle of bacteriorhodopsin: implications for proton uptake and 13-cis-retinal----all-trans-retinal reisomerization. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6873–6877. doi: 10.1073/pnas.88.15.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittor J., Soell C., Oesterhelt D., Butt H. J., Bamberg E. A defective proton pump, point-mutated bacteriorhodopsin Asp96----Asn is fully reactivated by azide. EMBO J. 1989 Nov;8(11):3477–3482. doi: 10.1002/j.1460-2075.1989.tb08512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Distortions in the photocycle of bacteriorhodopsin at moderate dehydration. Biophys J. 1991 Feb;59(2):313–322. doi: 10.1016/S0006-3495(91)82225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5016–5022. doi: 10.1021/bi00234a025. [DOI] [PubMed] [Google Scholar]