Abstract
This report describes a case of West Nile virus (WNV) meningoencephalitis in a child who presented with fever, headache, seizures, and altered mental status, as well as hyponatremia and bronzing of the skin. Findings that led to the diagnosis of WNV included plasma-cell pleocytosis of the cerebrospinal fluid (CSF) and linear chorioretinitis on ophthalmologic exam. The diagnosis was confirmed by a positive serum and CSF WNV IgM. The acute WNV infection triggered an adrenal crisis which uncovered a new diagnosis of underlying Addison's disease. This is the first case report of severe neuroinvasive WNV disease in a pediatric patient with primary adrenal insufficiency. Neuroinvasive WNV disease is uncommon in children, but may have a more severe presentation in those with certain underlying medical conditions.
Keywords: West Nile virus, Encephalitis, Adrenal insufficiency, Addison's disease, Chorioretinitis, Plasma cell pleocytosis
Case presentation
A 9-year-old previously healthy male presented in September 2012 in status epilepticus to a local emergency room. One day prior to presentation he developed subjective fever and headaches. The morning of presentation he was found unresponsive at home with tonic–clonic seizure activity.
He was an active boy who spent much of his summer outdoors. He had multiple animal contacts including dogs, skunks, mice, chickens, frogs, pigs, and turtles. He frequently swam in a nearby river and had a history of multiple mosquito bites.
Initial physical examination was notable for a temperature of 38.1 °C, respiratory rate of 26 per minute, pulse of 136 beats per minute, and blood pressure of 118/57 mmHg. The patient was unresponsive to stimuli but breathing spontaneously. Pupils were equal and reactive. There were no tonic–clonic movements or focal deficits on neurologic exam. The skin exam showed diffuse bronzing, including areas without sun exposure, and hyperpigmentation accentuated in the skin creases. The remainder of the physical examination was normal.
On presentation, the white blood cell (WBC) count was 9.0 × 103/μL (79% neutrophils, 8% bands, 6% lymphocytes, 5% monocytes, 2% eosinophils), hemoglobin was 11.5 g/dL, and platelet count was 219 × 103/μL. Serum sodium was 119 meq./L, potassium was 4.4 meq./L, bicarbonate was 17 meq./L, and serum glucose was 50 mg/dL. Liver function tests were significant for aspartate aminotransferase (AST) of 514 IU/L, alanine aminotransferase (ALT) of 156 IU/L, and total bilirubin of 1.3 mg/dL. The initial C-reactive protein was 3.18 mg/dL (reference range 0–1 mg/dL) and erythrocyte sedimentation rate was 8 mm/h. A lumbar puncture demonstrated cerebrospinal fluid (CSF) with 2 WBCs/μL, 0 red blood cells (RBCs)/μL, protein of 20 mg/dL, glucose of 61 mg/d, and a negative Gram stain. Head CT without contrast and a brain MRI with gadolinium were both normal. An EEG showed a diffuse, slow encephalopathic pattern.
The patient was started on intravenous antibiotics and acyclovir without clinical improvement. His hyponatremia was corrected over the course of several days. Blood, urine, and CSF cultures were negative. The patient's WBC reached a nadir of 2.2 × 103/μL on hospital day (HD) 4 and platelets reached a nadir of 81 × 103/μL on HD 5. The patient developed an S4 gallop and echocardiogram showed a dilated left ventricle with an ejection fraction of 37%. On HD 7, the liver edge was palpable 4 cm below the costal margin and the AST and ALT peaked at 643 IU/L and 250 IU/L, respectively.
The hospital course was significant for ongoing obtundation requiring intubation and mechanical ventilation. He had intermittent seizures for several days which were eventually controlled with antiepileptics. Following cessation of seizure activity, he remained unresponsive to commands without response to painful stimuli.
A repeat lumbar puncture on HD 9 had 41 WBCs/μL with 18% segmented neutrophils, 17% lymphocytes, 48% monocytes, and 15% plasma cells (Fig. 1), 3 RBCs/μL with a protein of 256 mg/dL and glucose of 42 mg/dL. A follow-up MRI with gadolinium of the brain on HD 9 was notable for symmetric T2 hyperintensity in the bilateral caudate heads and putamen, as well as cerebral cortical volume loss. An ophthalmologic exam performed on HD 11 noted multiple small, white, round chorioretinal lesions in linear streaks and clusters bilaterally (Fig. 2).
Fig. 1.
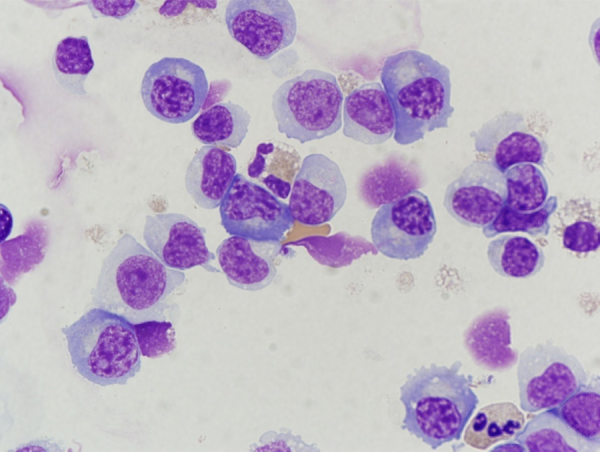
Cerebrospinal fluid cytospin with plasma cell predominant pleocytosis.
Fig. 2.
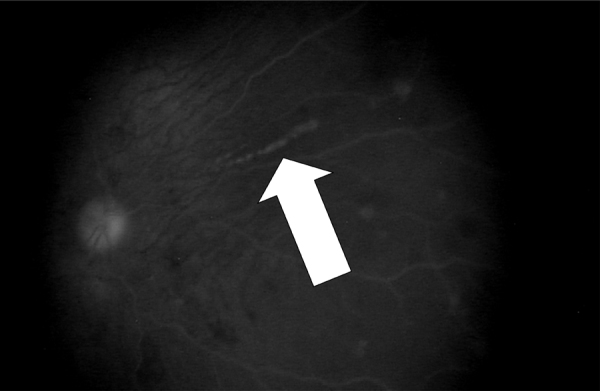
Linear and scattered round chorioretinal lesions on ophthalmologic exam.
The following tests were conducted to identify an etiology of his meningoencephalitis, which were negative: CSF PCRs for enterovirus, herpes simplex virus, varicella zoster virus, cytomegalovirus, and Epstein Barr virus; nasopharyngeal respiratory viral PCR panel (including influenza viruses, adenoviruses, and enteroviruses); serologic testing for Mycoplasma pneumoniae, Rickettsiae, Bartonella henselae, Leptospira, and Borrelia borgdorferi; anti-NMDA receptor autoantibodies; and tuberculin skin test. On HD 3, an acute arboviral serology panel, including West Nile virus (WNV) IgM and IgG, was negative. On HD 9, serum WNV IgM and CSF WNV IgM were positive by microsphere immunoassay and confirmed by serum dilution-plaque reduction neutralization testing.
In addition, a random cortisol level was found to be low at 0.7 mcg/dL (morning reference range: 4.5–22.7 mcg/dL) and ACTH elevated at 652 pg/mL (reference range 0–46 pg/mL) suggesting glucocorticoid deficiency due to primary adrenal insufficiency. An aldosterone level was undetectable and plasma renin activity was greater than 1000 ng/dL/h suggesting mineralocorticoid deficiency. Stress-dose glucocorticoids and mineralocorticoids were administered for hormone replacement. CT of the abdomen demonstrated normal appearing adrenal glands. Very long chain fatty acid testing was negative. Further testing revealed the presence of 21-hydroxylase antibodies which persisted at three months after discharge.
The patient remained intubated for 16 days in the pediatric intensive care unit and was hospitalized for a total of 43 days, including 13 days on the inpatient rehabilitation service. At discharge, he required assistance with ambulation and activities of daily living. Cognitive deficits were noted in the realms of attention, problem solving, reading comprehension, short-term memory, and spontaneous use of language. Neuropsychiatric testing demonstrated verbal and non-verbal skills at <5th percentile for age. Three months after discharge, the patient returned to school in special education classes, but continues to suffer from persistent tremors, emotional lability, and attention issues. He remains on daily maintenance steroid replacement, with stress dosing in times of illness.
Discussion
Since 1999, over 30,000 U.S. cases of WNV disease have been reported to the CDC and it is estimated that over 3 million people have been infected [1]. Approximately 80% of those infected are asymptomatic, 20% have an acute self-limited influenza-like illness known as West Nile Fever, and less than 1% have neuroinvasive disease, defined as either meningitis, encephalitis or acute flaccid paralysis [2]. Children account for only 4% of all WNV neuroinvasive cases [3]. In 2012, the United States experienced its largest WNV epidemic to date with 5674 cases reported to the CDC, including 2873 neuroinvasive infections and 286 deaths [4]. This report documents a severe case of pediatric neuroinvasive WNV disease during the 2012 epidemic.
WNV should be considered as a possible cause of encephalitis in children without an identified etiology, especially during periods of increased activity. However, due to the relatively nonspecific presentation, neuroinvasive WNV disease is likely under-recognized and under-diagnosed in children. One finding that triggered repeat WNV testing in this case was the presence of CSF plasma cell pleocytosis (Fig. 1), which has been described in an adult case series of WNV encephalitis [5]. Plasma cell CSF pleocytosis should prompt consideration of WNV as a possible etiology, however this is not a sensitive or specific finding [6]. The differential diagnosis for CSF plasmacytosis includes infectious conditions (Lyme disease, syphilis, cysticercosis, tuberculosis, other viral causes of encephalitis), autoimmune conditions (multiple sclerosis, Sjögren syndrome), and malignancy (leukemia, lymphoma) [5].
In addition, the pattern of chorioretinitis seen on ophthalmologic exam raised suspicion for WNV infection (Fig. 2). Chorioretinitis is the most common ocular manifestation of WNV. The characteristic pattern of lesions distributed in scattered or linear arrays distinguishes the chorioretinitis of WNV from that of other infectious etiologies of encephalitis, such as syphilis, histoplasmosis, tuberculosis, and sarcoidosis [7]. Ophthalmalogic evaluation should be considered in patients with encephalitis during WNV season as it can aid in diagnosis.
Serologic tests for WNV can be negative early in the disease course. Only half of patients will have a positive IgM by day 4 of illness, but 95% will be positive by day 10 [8]. It is important to repeat testing 8–10 days into the illness if early testing is negative for WNV. Serum WNV IgM persists for an average of 5–6 months and could represent an unrelated prior exposure [8]; however, CSF WNV IgM is confirmatory of an acute neuroinvasive WNV infection.
Severe WNV infection is commonly associated with secondary adrenal insufficiency [9], however WNV disease has not been described in the setting of primary adrenal insufficiency. The differential diagnosis for primary adrenal insufficiency includes congenital adrenal hypoplasia or hyperplasia, adrenoleukodystrophy, adrenal hemorrhage, infectious destruction, and Addison's disease. It is unlikely that this patient had congenital adrenal hypoplasia or hyperplasia due to his age at presentation and lack of virilization. Very long chain fatty acid testing rules out adrenoleukodystrophy. Adrenal imaging did not show signs of hemorrhage or infectious destruction in this case, which is consistent with the lack of histopathologic evidence of adrenal invasion found in a case series of six fatal systemic WNV infections [10]. The patient's skin bronzing was likely due to high levels of melanocyte stimulating hormone produced along with ACTH, suggestive of long-standing Addison's disease predating this illness. The presence and persistence of 21-hydroxylase auto-antibodies following the resolution of his illness confirms the diagnosis of Addison's disease and suggests these are not merely infectious mediated cross-reactive antibodies.
This is the first published report of severe WNV disease in a pediatric patient with underlying primary adrenal insufficiency. In this case, WNV infection likely triggered the adrenal crisis which contributed to his particularly severe presentation. It is likely that children with certain underlying medical conditions are more susceptible to severe neuroinvasive WNV disease, similar to what has been found in adults [11]. Characteristic chorioretinitis and plasma cell pleocytosis may be supportive of the diagnosis of WNV infection prior to laboratory confirmation.
Conflict of interest and source of funding
Kevin Messacar is currently receiving a grant from the NIH/NCATS Colorado CTSI Grant Number UL1 TR000154. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
References
- 1.Petersen L.R., Carson P.J., Biggerstaff B.J., Custer B., Borchardt S.M., Busch M.P. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiol Infect. 2012:1–5. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis L.E., DeBiasi R., Goade D.E., Haaland K.Y., Harrington J.A., Harner J.B. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 3.Lindsey N.P., Hayes E.B., Staples J.E., Fischer M. West Nile virus disease in children, United States, 1999–2007. Pediatrics. 2009;123:e1084–e1089. doi: 10.1542/peds.2008-3278. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention: National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Vector-Borne Diseases (DVBD) 2012. 2012 West Nile virus human infections in the United States. Available from: http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount12_detailed.htm [accessed 22.5.13] [Google Scholar]
- 5.Carson P.J., Steidler T., Patron R., Tate J.M., Tight R., Smego R.A., Jr. Plasma cell pleocytosis in cerebrospinal fluid in patients with West Nile virus encephalitis. Clin Infect Dis. 2003;37:e12–e15. doi: 10.1086/375692. [DOI] [PubMed] [Google Scholar]
- 6.Jordan M., Nagpal A., Newman W., Thompson P.A., Carson P.J. Plasma cell cerebrospinal fluid pleocytosis does not predict West Nile virus infection. J Biomed Biotechnol. 2012:697418. doi: 10.1155/2012/697418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg S., Jampol L.M. Systemic and intraocular manifestations of West Nile virus infection. Surv Ophthalmol. 2005;50:3–13. doi: 10.1016/j.survophthal.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Busch M.P., Kleinman S.H., Tobler L.H., Kamel H.T., Norris P.J., Walsh I. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis. 2008;198:984–993. doi: 10.1086/591467. [DOI] [PubMed] [Google Scholar]
- 9.Abroug F., Ouanes-Besbes L., Ouanes I., Nciri N., Dachraoui F., Najjar F. Adrenal insufficiency in severe West Nile Virus infection. Intensive Care Med. 2006;32:1636–1639. doi: 10.1007/s00134-006-0298-z. [DOI] [PubMed] [Google Scholar]
- 10.Armah H.B., Wang G., Omalu B.I., Tesh R.V., Gyure K.A., Chute D.J. Systemic distribution of West Nile virus infection: postmortem immunohistochemical study of six cases. Brain Pathol. 2007;17:354–362. doi: 10.1111/j.1750-3639.2007.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsey N.P., Staples J.E., Lehman J.A., Fischer M. Medical risk factors for severe West Nile Virus disease, United States, 2008–2010. Am J Trop Med Hyg. 2012;87:179–184. doi: 10.4269/ajtmh.2012.12-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]


