Abstract
Phytochemical investigation of the aerial parts of the monotypic plant, Musella lasiocarpa, led to the isolation of four rare bicyclic diarylheptanoids, musellarins B-E (2–5), two new phenylphenalenones, 2-methoxy-9-(3′,4′-dihydroxyphenyl)-1Hphenalen-1-one (9), 2-methoxy-9-(3′-methoxy-4′-hydroxyphenyl)-1H-phenalen-1-one (10), a new acenaphtylene derivative, trans-(1S,2S)-3-(4′-methoxyphenyl)-acenaphthene-1,2-diol (13), and two new sucrose esters, 1,2′,3′,4′,6′-O-pentaacetyl-3-O-trans-pcoumaroylsucrose (16), 1,2′,3′,4′,6′-O-pentaacetyl-3-O-cis-p-coumaroylsucrose (17), together with nine known compounds. In addition, (4E,6E)-1-(3′,4′-dihydroxyphenyl)-7-(4″-hydroxyphenyl)-hepta-4,6-dien-3-one (15) was isolated for the first time from a natural source. The structures of new compounds were elucidated by analysis of their spectroscopic data. Compounds 2, 6, 8–10, 12, and 14 were cytotoxic toward several of the human tumor cell lines (HL-60, SMMC-7721, A-549, MCF-7, and SW480). Of these, the new compound 9 was the most potent one, with IC50 values of 5.8, 10.3, 6.3, 3.3, and 2.3 µM, respectively. 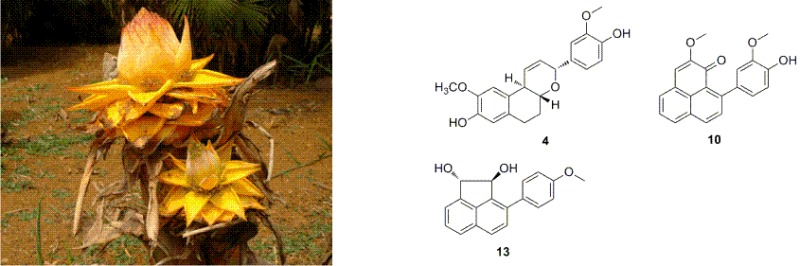
Electronic Supplementary Material
Supplementary material is available for this article at 10.1007/s13659-011-0007-7 and is accessible for authorized users.
Keywords: monotypic, diarylheptanoid, phenylphenalenone, acenaphtylene, sucrose ester, Musella lasiocarpa
Electronic supplementary material
Supplementary material, approximately 1.23 MB.
Footnotes
This article is published with open access at Springerlink.com
References
- [1].Wu D. L., Cai X. T., Li X. W. Flora Reipublicae Popularis Sinicae. Beijing: Science Press; 1981. pp. 3–6. [Google Scholar]
- [2].Liu A. Z., Kress W. J., Long C. L. Econ. Bot. 2003;57:279–281. doi: 10.1663/0013-0001(2003)057[0279:TEOMLM]2.0.CO;2. [DOI] [Google Scholar]
- [3].Yang W. L., Tian J., Bai B., Guan J., Ding L. Chin. Tradit. Herb. Drugs. 2001;32:681–683. [Google Scholar]
- [4].Qin B., Shao Z. Y., Zeng W., Wang H. Q., Zhu D. Y. J. Chin. Chem. Soc. 2006;53:475–478. doi: 10.1002/jccs.200600062. [DOI] [Google Scholar]
- [5].Qin B., Lu R. H., Wang H. Q., Wang M. Nat. Prod. Res. & Dev. 2000;12:41–44. [Google Scholar]
- [6].Dong L. B., He J., Wang Y. Y., Wu X. D., Deng X., Pan Z. H., Xu G., Peng L. Y., Zhao Y., Li Y., Gong X., Zhao Q. S. J. Nat. Prod. 2011;74:234–239. doi: 10.1021/np100694k. [DOI] [PubMed] [Google Scholar]
- [7].Jang D. S., Park E. J., Hawthorne M. E., Vigo J. S., Graham J. G., Cabieses F., Santarsiero B. D., Mesecar A. D., Fong H. H. S., Mehta R. G., Pezzuto J. M., Kinghorn A. D. J. Agric. Food Chem. 2002;50:6330–6334. doi: 10.1021/jf0206670. [DOI] [PubMed] [Google Scholar]
- [8].Kamo T., Kato N., Hirai N., Tsuda M., Fujioka D., Ohigashi H. Biosci. Biotechnol. Biochem. 1998;62:95–101. doi: 10.1271/bbb.62.95. [DOI] [PubMed] [Google Scholar]
- [9].Luis J. G., Lahlou E. H., Andres L. S., Echeverri F., Fletcher W. Q. Tetrahedron. 1997;53:8249–8256. doi: 10.1016/S0040-4020(97)00490-0. [DOI] [Google Scholar]
- [10].Sternhell S., Westerman P. W. J. Org. Chem. 1974;39:3794–3796. doi: 10.1021/jo00939a047. [DOI] [Google Scholar]
- [11].Wang L. X., Wang X. Y., Cui J. N., Ren W. M., Meng N., Wang J. Y., Qian X. H. Tetrahedron: Asymmetry. 2010;21:825–830. doi: 10.1016/j.tetasy.2010.04.048. [DOI] [Google Scholar]
- [12].Imuta M., Kasai M., Ziffer H. Bioorg. Chem. 1985;13:131–134. doi: 10.1016/0045-2068(85)90015-X. [DOI] [Google Scholar]
- [13].Shimazaki N., Mimaki Y., Sashida Y. Phytochemistry. 1991;30:1475–1480. doi: 10.1016/0031-9422(91)84190-4. [DOI] [Google Scholar]
- [14].Sasaya T. Enshurin Kenkyu Hokoku (Hokkaido Daigaku Nogakubu) 1985;42:191–205. [Google Scholar]
- [15].Cooke R. G., Thomas R. L. Aust. J. Chem. 1975;28:1053–1057. doi: 10.1071/CH9751053. [DOI] [Google Scholar]
- [16].Kamo T., Hirai N., Iwami K., Fujioka D., Ohigashi H. Tetrahedron. 2001;57:7649–7656. doi: 10.1016/S0040-4020(01)00749-9. [DOI] [Google Scholar]
- [17].Luis J. G., Fletcher W. Q., Echeverri F., Grillo T. A. Tetrahedron. 1994;50:10963–10970. doi: 10.1016/S0040-4020(01)85707-0. [DOI] [Google Scholar]
- [18].Ali M. S., Tezuka Y., Awale S., Banskota A. H., Kadota S. J. Nat. Prod. 2001;64:289–293. doi: 10.1021/np000496y. [DOI] [PubMed] [Google Scholar]
- [19].Baranovsky A., Schmitt B., Fowler D. J., Schneider B. Synth. Commun. 2003;33:1019–1045. doi: 10.1081/SCC-120016367. [DOI] [Google Scholar]
- [20].Hirai N., Ishida H., Koshimizu K. Phytochemistry. 1994;37:383–385. doi: 10.1016/0031-9422(94)85064-X. [DOI] [Google Scholar]
- [21].Dias D. A., Goble D. J., Silva C. A., Urban S. J. Nat. Prod. 2009;72:1075–1080. doi: 10.1021/np900016h. [DOI] [PubMed] [Google Scholar]
- [22].Mosmann T. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- [23].Reed L. J., Muench H. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material, approximately 1.23 MB.


