Abstract
Glucocorticoids increase osmotic water permeability (Pf) of neonatal rabbit renal brush border membrane vesicles. Am J Physiol Regul Integr Comp Physiol 288: R1417–R1421, 2005. First published January 20, 2005; doi:10.1152/ajpregu.00448.2004.— During postnatal maturation, there is an increase in renal brush border membrane vesicle (BBMV) osmotic water permeability and a parallel increase in aquaporin-1 (AQP1) protein abundance. The mechanisms responsible for these changes remain unknown. Because serum glucocorticoid levels rise postnatally and have previously been linked to other maturational changes in renal function, we examined the effects of glucocorticoids on osmotic (Pf) and diffusional (PDW) water permeability and AQP1 protein abundance of renal BBMV. Neonatal rabbits were treated with dexamethasone (10 μg/100 g) for three days and compared with control neonates and adults. Pf and PDW were measured at 20°C with a stopped-flow apparatus using light-scattering and aminonaphthalene trisulfonic acid (ANTS) fluorescence, respectively. Pf was significantly higher in BBMV from dexamethasone-treated neonates compared with vehicle-treated neonates, but remained lower than in BBMV from adults (P < 0.05). PDW in dexamethasone and vehicle-treated neonatal BBMV was lower than in adult BBMV. Pf/PDW ratio increased from neonate (5.1 ± 0.3) to dexamethasone (7.0 ± 0.1) and adult BBMV (6.3 ± 0.1). AQP1 expression was increased by dexamethasone treatment to adult levels. Membrane fluidity, which is inversely related to generalized polarization (GP) of steady-state laurdan fluorescence, was significantly higher in neonatal BBMV than both dexamethasone and adult BBMV (GP: neonate 0.285 ± 0.002, dexamethasone treatment 0.302 ± 0.006, and adult 0.300 ± 0.005; P < 0.05). These combined results show that dexamethasone-treatment during days 4–7 of life increases BBMV water permeability despite a decrease in membrane fluidity. This occurs by increasing channel-mediated water transport, as reflected in an increase in AQP1 protein abundance and a higher Pf/PDW ratio. This mimics the maturational changes and suggests a physiological role for glucocorticoids in maturation of proximal tubule water transport.
Keywords: stop-flow kinetics, diffusional water permeability, development, aquaporin
The proximal tubule reabsorbs approximately three fourths of the glomerular ultrafiltrate (26). This occurs in a nearly isoosmotic manner due to the high water permeability of this nephron segment provided by the abundance of aquaporin-1 (AQP1), the water channel in the kidney proximal tubule (1, 18, 26). The functional aspects of water transport in the proximal tubule during development have recently been studied and reviewed (21–25). In vitro microperfusion revealed that the osmotic water permeability coefficient (Pf) of the proximal tubule is higher in the neonatal rabbit than that in the adult (21). Studies with isolated membrane vesicles, on the other hand, have indicated that Pf of both the apical (brush border) and basolateral membranes are lower in the neonatal tubule compared with the adult (23, 24). For both membranes, this was attributed primarily to a lower expression of AQP1. The neonatal brush border membrane also had a higher activation energy (Ea) for water permeability and a lower sensitivity to mercury inhibition (24). We have also demonstrated that the intracellular compartment of the tubule cells plays a role in the developmental changes in the water permeability that likely explained the discrepancy (22). Thus the maturational changes in water transport in the proximal tubule involve several factors.
Since the discovery and identification of AQP1, much has been learned about its developmental expression (5, 7, 29, 32, 34). In rats, renal expression of AQP1 mRNA increased greatly in the first week after birth to remain high throughout adult life (5, 29, 34). This increase in mRNA expression is also seen in sheep and humans, but in these species, it is initiated during fetal life (6, 7, 32). The regulation of the developmental changes in proximal tubule water transport remains to be elucidated. Several researchers have provided evidence that glucocorticoids play an important role in maturational changes in the kidney (2–4, 20). Serum glucocorticoid levels rise postnatally in rabbits and rats (9, 10). These have been linked to an increase in proximal tubular acidification (3, 4), Na+/K+-ATPase gene transcription (31) and a decrease in phosphate transport during neonatal life (2, 20). Furthermore, glucocorticoids have been shown to induce AQP1-expression in membranes prepared from whole neonatal rat kidney (11) and in fetal sheep (32). Other factors that affect water permeability, for example, alterations in membrane lipid composition, may also be regulated by glucocorticoids (2, 27, 28).
The purpose of this study was to examine the role of glucocorticoids in the maturational changes in water permeability of the brush border membrane of the proximal tubule. We determined Pf and the diffusional water permeability (PDW) using stop-flow kinetics in brush border membrane vesicles (BBMV) from control and dexamethasone-treated neonates and adult rabbits. The physiological significance of measuring Pf and PDW in the same preparation in this study is that it enables us to calculate the Pf/PDW ratio. In membranes that lack water channels, this ratio is ~1, but in channel-containing membranes it is greater than 1 (8). It is, therefore, an informative characteristic regarding the predominant pathway for water to move across membranes. In addition, we measured the protein expression of AQP1 and membrane fluidity using the generalized polarization (GP) of laurdan.
METHODS
Animals
New Zealand white rabbits were housed at the University of Texas Southwestern Medical Center at Dallas. Pregnant does were received at least 9 days before delivery to care for their neonates. They were fed a standard laboratory chow. Neonates received daily, subcutaneous injections with dexamethasone (10 μg/100 g body weight) or vehicle (in mM): 150 NaCl, 2 K2HPO4, pH 7.5 for 3 days, including 2 h before death. Neonates were studied at 7 days of age. Adult rabbits (“adult” hereafter) were >9 wk of age. The protocols for this study were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Materials
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) and Fluka BioChemika (Buchs, Germany). 8-Aminonaphthalene- 1,3,6-trisulfonic acid (ANTS) and 6-dodecanoyl-2-dimethylaminonaphthalene (laurdan) were obtained from Molecular Probes (Eugene, OR). Solution osmolalities were measured by freezing point depression (Advanced Instruments, Norwood, MA; osmometer model 3D3). Osmolalities of solutions prepared with D2O could not be determined in this manner, but small osmotic gradients were previously shown not to affect measurements of PDW (35).
Brush border membrane vesicle preparation
BBMV were prepared as described previously (24). Animals were killed by decapitation after sedation. Kidneys from 3–7 neonates were pooled, while adults were studied individually. Kidneys were removed and immediately placed in ice-cold PBS in mM: 137 NaCl, 2.7 KCl, 10.1 Na2HPO4, 1.7 KH2PO4, with pH 7.4). The capsule was removed, and the cortex was dissected. After mincing, the cortex was put in 15 ml of isolation buffer (in mM) 300 D-mannitol, 16 HEPES, 5 EGTA, adjusted to pH 7.5 with Tris-HCl; containing the protease inhibitors aprotinin (2 μg/ml), leupeptin (2 μg/ml), and phenylmethylsulfonyl fluoride (175 μg/ml). Tissue was homogenized by 15 strokes with a Potter homogenizer, after which precipitation was induced using 230 μl of 1.0 M MgCl2. The homogenate was shaken vigorously for 10 s every 5 min for 20 min. Subsequently, the homogenate was centrifuged at 2,500 g for 15 min at 4°C. 230 μl 1.0 M MgCl2 was added to the supernatant and shaken vigorously for 10 s every 5 min for 20 min. After this, it was centrifuged for 15 min at 2,500 g at 4°C. The supernatant was then centrifuged at 48,400 g for 30 min at 4°C. The pellets were resuspended in ice-cold 1.5 mL resuspension buffer (5.0 mM HEPES, pH 7.4; osmolality adjusted to 80 mosmol/kgH2O with D-mannitol) using 22- and 25-gauge needles. The protein content was determined in the crude homogenate and BBMV using bicinchoninic acid protein assay (Pierce, Rockford, IL). Alkaline phosphatase activity was determined to assess enrichment of brush border membrane (BBM), as was previously described (2, 24). This was not different between control neonates, dexamethasone-treated neonates, and adults (fold increase: 7.6 ± 0.5, 8.4 ± 0.5, and 6.6 ± 0.7, respectively; P = NS).
Pf measurements
Fresh BBMV were brought to a concentration of 0.6 mg protein/ml. BBMV (100 μl) were rapidly mixed with 240 mosmol/kgH2O resuspension buffer (100 μl) using a stopped-flow apparatus (SFM-3, Biologic, France). The cuvet was illuminated using a 75-W xenon arc lamp and a monochrometer, which was set at 400 nm. Light scattering was measured using a photomultiplier tube (Biologic, France) oriented at a 90° angle with regard to the illumination axis. Data were collected at 10-ms intervals for 2–3 s using Bio-kine software (Biologic, France). Five raw tracings were averaged and analyzed subsequently. Pf was calculated as previously described (23).
PDW-measurements
Recently, we successfully used a combination of stop-flow kinetics and aminonaphthalene-trisulfonic acid (ANTS) fluorescence to measure PDW in rabbit renal BBMV (16). This methodology takes advantage of the fact that the fluorescence of ANTS changes with the D2O:H2O ratio. In a separate set of experiments, fresh BBMV suspensions were brought to a concentration of 10 mg protein/ml and loaded with ANTS (10 mM) overnight (12–16 h), in the dark at 4°C. After loading, BBMV were washed five times with ice-cold 80 mosmol/kgH2O-resuspension buffer and ultracentrifugation (368,000 g) for 10 min at 4°C. After the fifth spin, pellets were resuspended using 22- and 25-gauge needles, and vesicles were brought to a final concentration of 1.0 mg protein/ml. This suspension (100 μl) was rapidly mixed with two volumes of isoosmotic resuspension buffer (2 × 100 μl) made with D2O as solvent using the previously described experimental setup. As the D2O diffuses through the membrane, the D2O:H2O ratio will change, which results in an increase in the fluorescence from the ANTS. ANTS was excited by the 75-W xenon arc lamp with the monochrometer set at 380 nm. The photomultoplier tube was equipped with a 500-nm cut-on filter to monitor the intensity of emitted light. Data were collected at 100-μs intervals for 50 ms using the Bio-kine software. Five raw tracings were averaged, normalized to initial fluorescence, and fitted to a single-exponential curve using Biokine Software (Molecular Kinetics, Pullman, WA). PDW was calculated using the following relation: PDW = 1/[τex(S/V)], where τex is the exchange time (1/k), and S/V is the surface area to volume ratio of BBMV (12). In our calculations, the diameter of BBMV was assumed to be 200 nm, as previously measured in our laboratory in neonates and adults (24).
Immunoblotting for AQP1
Immunoblots were performed as described previously (23, 24). Briefly, BBMV samples (50-μg total protein) were brought to a 40-μl volume using isolation buffer. After addition of 10 μl of 5× loading buffer (final SDS concentration of 10 mg/ml), samples were heated to 95°C for 5 min, chilled on ice, and loaded on a 10% SDS gel. Samples were transferred overnight onto PVDF membrane at 4°C.
Anti-AQP1 (Alomone Labs, Israel) was used at 1:1,000 and anti-β-actin (Sigma) at 1:5,000 dilutions. After preincubation with Blotto (5% nonfat milk and 0.05% Tween-20 in PBS), membranes were incubated with the primary antibody at room temperature for 2 h, washed four times over 40 min, and incubated with the secondary antibody at room temperature for 1 h. Detection was performed using an ECL kit (Amersham).
Membrane lipid fluidity
Membrane fluidity was assessed with a fluorescence technique using laurdan (13). BBMV (100 μg protein in 100 μL buffer) were incubated with 0.3 nmol laurdan at 37°C for >20 min, after prolonged mixing by vortex. Steady-state emissions at 440 and 490 nm were measured at an excitation wavelength of 340 nm using a spectrofluorometer (PC1, ISS, Urbana-Champaign, IL). The generalized polarization (GP) was calculated using ISSPC software using the following relation: GPlaurdan = [I440 − I490]/[I440 + I490], where I440 and I490 are the emission intensities at 440 and 490 nm, respectively. GP is inversely related to membrane fluidity, as laurdan will produce in a polar environment a higher-intensity emission at 440 nm; whereas in a crystalline phase, it will produce an even higher-intensity emission at 490 nm (19).
Statistical analysis
Data were analyzed using SigmaStat statistical software (Jandel, San Rafael, CA). Data were presented as means ± SE, unless otherwise noted. Comparisons were performed using t-tests or ANOVA (with Student-Newman-Keuls post hoc tests), as appropriate.
RESULTS
Osmotic water permeability (Pf)
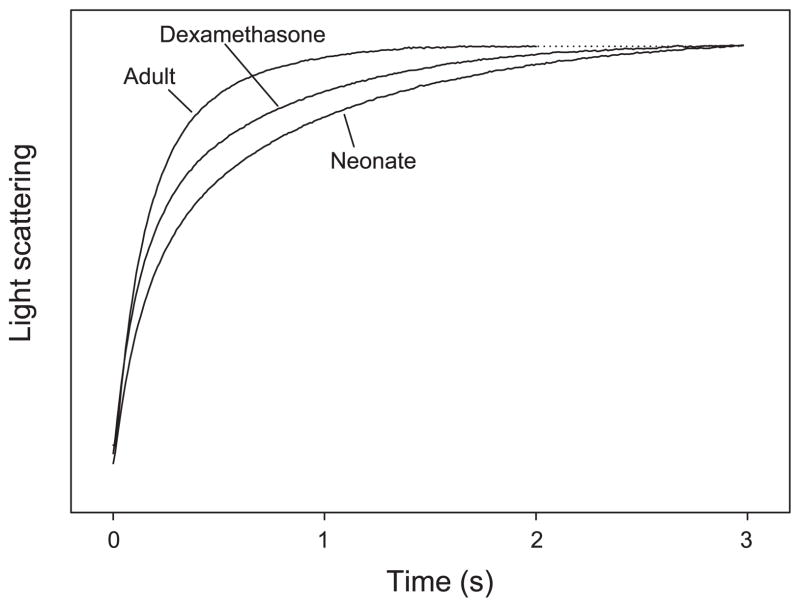
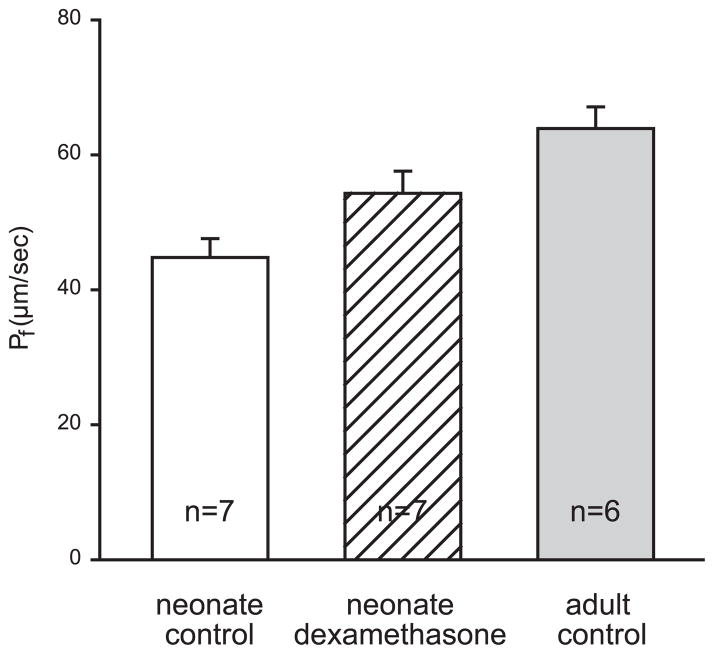
Typical tracings of osmotically induced vesicle shrinkage for neonatal, dexamethasone, and adult BBMV are shown in Fig. 1. Both maturation and dexamethasone-treatment affected Pf (Figs. 1 and 2). Pf was significantly different between all three groups (P < 0.05). Comparisons between the groups were made using ANOVA with Student-Newman-Keuls post hoc tests. Pf was lower in control neonates than in dexamethasone-treated neonates or adults. Dexamethasone treatment led to an increase in Pf, which did not reach adult levels. Dexamethasone treatment partially mimicked maturational changes.
Fig. 1.
Typical tracings for measurement of Pf in neonatal, dexamethasone, and adult brush border membrane vessicle (BBMV) at 20°C. BBMV were prepared with 80 mosmol/kgH2O resuspension buffer and exposed to a 80 mosmol/kgH2O inward osmotic gradient. Light scattering is increased as a result of vesicle shrinkage. Averaged tracings were normalized to initial fluorescence and fitted to a double-exponential curve. The dotted line indicates the asymptote for the adult tracing.
Fig. 2.
Pf for neonatal (n = 7), dexamethasone treatment (n = 7) and adult (n = 6) groups. Error bars denote means ± SE.
These results indicate that during maturation, Pf increases, which is consistent with our earlier findings of a higher Pf in adults (24). Dexamethasone accelerates the maturational increase of Pf.
Diffusional water permeability (PDW)
Figure 3 shows the results of PDW measurements for the three groups. PDW was significantly lower in both neonatal groups than in adults (P < 0.05). This is consistent with our recent findings of PDW in adults and 11-day-old neonates (16).
Fig. 3.
PDW for neonatal (n = 7), dexamethasone treatment (n = 7) and adult (n = 6) groups. Error bars denote means ± SE.
We also compared the Pf to PDW ratio between the three groups. The ratio was lower in the control neonatal groups compared with the dexamethasone-treated neonates and the adult BBMV (5.1 ± 0.3, 7.0 ± 0.1 and 6.3 ± 0.1, respectively; P < 0.001).
Immunoblot
To determine the effect of glucocorticoids on the expression of AQP1 in the proximal tubule apical membrane, we performed Western blots on the BBMV from the three groups, as previously described from our laboratory (24). As seen in Fig. 4, the expression of AQP1 in the neonatal BBMV was significantly less than that in the adult BBMV. Treatment of the neonates with dexamethasone for 3 days increased the amount of AQP1 expression to a value that was between that of the neonatal and adult BBMV. Densitometry of the expression of AQP1 factored for β-actin expression was neonates: 407 ± 299; dexamethasone: 4,435 ± 1,183; adults: 11,201 ± 424; P < 0.05 for differences between all three groups. Thus glucocorticoids have an effect on the expression of AQP1.
Fig. 4.
A: immunoblot of aquaporin 1 (AQP1) protein expression in BBMV. Lanes were loaded with 50 μg of protein. Expression was compared using densitometry. B: densitometry results. AQP1: β-actin ratios: neonates: 407 ± 299; dexamethasone treatment: 4,435 ± 1,183; adults: 11,201 ± 424. P < 0.05 for differences between all three groups, and there were 3 samples for each group.
Membrane fluidity
Characteristics of water transport in BBMV are not only determined by the expression of AQP1. Changes in the membrane composition may have occurred and contributed to our findings of increased water permeability in dexamethasone and adult BBMV compared with neonatal BBMV. To examine possible changes in membrane fluidity, we measured the steady-state laurdan fluorescence. Generalized polarization of laurdan (GPlaurdan), which is inversely related to membrane fluidity, was found to be significantly lower in neonatal BBMV than both dexamethasone and adult BBMV (Fig. 5). In the latter two, GP was not different. Therefore, neonatal BBMVs were found to be more fluid than the other two groups. This is consistent with the finding that PDW of neonatal BBMV, with lower AQP1 protein expression, is not different from dexamethasone and adult BBMV at higher temperatures, since increased membrane fluidity has been linked directly to higher water permeability (33).
Fig. 5.
Generalized polarization (GP) of steady-state laurdan fluorescence. BBMV (100 μg protein) were incubated with 0.33 nmol laurdan at 37°C for 20 min before measurements. Excitation wavelength was 340 nm. GP was calculated from the emission intensities at 440 and 490 nm. The lower GP of neonatal BBMV corresponds to a higher membrane fluidity.
DISCUSSION
The present study examined the role of glucocorticoids in the maturation of renal BBM water transport. Glucocorticoids were shown to accelerate maturational changes of BBM water transport in rabbit neonates. Treatment with dexamethasone on days 4–7 of life resulted in an increase of Pf, PDW, and Pf/PDW ratio. This was accompanied by an increase in AQP1 protein expression. This increased expression of AQP1 was paralleled by a decrease in membrane fluidity, indicating that glucocorticoids had multiple effects on water transport.
The osmotic water permeability of the proximal tubule apical membrane was clearly affected by the administration of dexamethasone. The Pf of the BBMV from the dexamethasone-treated neonates was greater than that of BBMV from the control neonates and less than the adult. We have previously shown similar results in the maturational changes in brush border membrane water permeability characteristics (16, 24). In our previous study of osmotic water permeability, we used fluorescence quenching of an entrapped fluorophore to measure the shrinkage of the brush border membrane vesicles (24). We had found that maturational changes in Pf included an increase in Pf and AQP1 expression, a decreased Ea, and an increase in mercury sensitivity (24). The present study also demonstrates a maturational increase in Pf and AQP1 expression.
The diffusional water permeability of the proximal tubule apical membrane was not affected by the administration of dexamethasone. The maturational changes in the diffusional water permeability that we had previously found were also similar to those seen in the present study (16). Both of these studies used the same techniques for measuring the PDW and found that the adult membranes had a higher PDW (16). The Pf/PDW ratio was calculated from the values for Pf and PDW from corresponding preparations. The results indicate that there is a significant increase in the ratio that occurs with development and that dexamethasone accelerates the maturational changes. This corresponds to an increase in the fraction of water that moves through water channels instead of through the lipid bilayer.
In addition to the physiological effects of glucocorticoids on water transport characteristics, we measured their effect on AQP1 expression. We found that 3 days of glucocorticoid treatment increased the expression of AQP1 in the BBMV to a level that was not different from that of the adult BBMV. Other investigators have studied glucocorticoids and their potential role in the developmental expression of AQP1 in the kidney (11, 32). These studies report a stimulating effect of glucocorticoids on AQP1 expression at both mRNA and protein levels, but there have been no reports of functional studies on isolated renal membrane vesicles. Glucocorticoids also induced the expression of AQP1 in lung endothelium (11) and AQP3 in airway epithelial cells (30). The functional effect of the latter was confirmed in stopped-flow experiments, in which Pf of the cell membrane was shown to increase (30).
The genetic mechanisms underlying the induction of AQP1 expression by dexamethasone have been studied in a mouse erythroleukemia cell line (15). This effect was shown to be mediated by two glucocorticoid response elements 0.5 kilo-bases upstream from the transcription initiation site. Although they suspected that this may play a central role in maturational changes, the authors remain cautious in their conclusions, because the induction by other compounds, including dimethyl sulfoxide, was found to be much greater (15). These compounds may mimic the effects of factors present in vivo that are involved in the maturational changes.
The laurdan measurements of membrane fluidity showed that both adult and dexamethasone BBMV were less fluid than neonatal BBMV. This is consistent with reported effects of aging (14) and dexamethasone (2, 13, 17) on renal and intestinal BBM fluidity. It is interesting that 3-day dexamethasone treatment increases AQP1 protein expression to adult levels and has an effect on fluidity, indicating that dexamethasone has differential effects on factors that determine BBMV water transport.
Although GP of laurdan fluorescence was not different between dexamethasone and adult BBMV, it cannot be concluded that the membrane composition of dexamethasone BBMV and adult BBMV are identical. Dexamethasone and normal maturation may affect the actual lipid and protein composition differently. Therefore, dexamethasone BBMV cannot simply be thought of as adult BBMV with lower AQP1 content.
In conclusion, dexamethasone increases the protein expression of AQP1 in neonatal BBMV, resulting in a higher Pf despite a decrease in membrane fluidity. This indicates that glucocorticoids accelerate maturation of water transport across the BBM and suggests a physiological role of glucocorticoids in the developmental changes. These results indicate that glucocorticoids have multiple effects on the maturation of the proximal tubule cell membranes.
Acknowledgments
We thank Drs. Moshe Levi and Hubert Zajicek (Department of Veterans Affairs Medical Center, Dallas, Texas) for assistance and expertise with measuring membrane fluidity.
GRANTS
Jaap Mulder was supported by the Rijksuniversiteit Groningen, the Netherlands and the Dutch Kidney Foundation. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-41612 (to M. Baum).
References
- 1.Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, Guggino WB, Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol Renal Fluid Electrolyte Physiol. 1993;265:F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- 2.Arar M, Levi M, Baum M. Maturational effects of glucocorticoids on neonatal brush-border membrane phosphate transport. Pediatr Res. 1994;35:474–478. [PubMed] [Google Scholar]
- 3.Baum M, Biemesderfer D, Gentry D, Aronson PS. Ontogeny of rabbit renal cortical NHE3 and NHE1: effect of glucocorticoids. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;268:F815–F820. doi: 10.1152/ajprenal.1995.268.5.F815. [DOI] [PubMed] [Google Scholar]
- 4.Baum M, Quigley R. Prenatal glucocorticoids stimulate neonatal juxtamedullary proximal convoluted tubule acidification. Am J Physiol Renal Fluid Electrolyte Physiol. 1991;261:F746–F752. doi: 10.1152/ajprenal.1991.261.5.F746. [DOI] [PubMed] [Google Scholar]
- 5.Bondy C, Chin E, Smith BL, Preston GM, Agre P. Developmental gene expression and tissue distribution of the CHIP28 water-channel protein. Proc Natl Acad Sci USA. 1993;90:4500–4504. doi: 10.1073/pnas.90.10.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butkus A, Alcorn D, Earnest L, Moritz K, Giles M, Wintour EM. Expression of aquaporin-1 (AQP1) in the adult and developing sheep kidney. Biol Cell. 1997;89:313–320. [PubMed] [Google Scholar]
- 7.Devuyst O, Burrow CR, Smith BL, Agre P, Knepper MA, Wilson PD. Expression of aquaporins-1 and -2 during nephrogenesis and in autosomal dominant polycystic kidney disease. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;271:F169–F183. doi: 10.1152/ajprenal.1996.271.1.F169. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein A. Water Movement Through Lipid Bilayers, Pores, and Plasma Membranes: Theory and Reality. New York: Wiley; 1987. [Google Scholar]
- 9.Henning SJ. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol Endocrinol Metab Gastrointest Physiol. 1978;235:E451–E456. doi: 10.1152/ajpendo.1978.235.5.E451. [DOI] [PubMed] [Google Scholar]
- 10.Hummelink R, Ballard PL. Endogenous corticoids and lung development in the fetal rabbit. Endocrinology. 1986;118:1622–1629. doi: 10.1210/endo-118-4-1622. [DOI] [PubMed] [Google Scholar]
- 11.King LS, Nielsen S, Agre P. Aquaporin-1 water channel protein in lung: ontogeny, steroid-induced expression, and distribution in rat. J Clin Invest. 1996;97:2183–2191. doi: 10.1172/JCI118659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawaczeck R. Water permeability through biological membranes by isotopic effects of fluorescence and light scattering. Biophys J. 1984;45:491–494. doi: 10.1016/S0006-3495(84)84184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levi M, Shayman JA, Abe A, Gross SK, McCluer RH, Biber J, Murer H, Lotscher M, Cronin RE. Dexamethasone modulates rat renal brush border membrane phosphate transporter mRNA and protein abundance and glycosphingolipid composition. J Clin Invest. 1995;96:207–216. doi: 10.1172/JCI118022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medow MS, Segal S. Age-related changes in fluidity of rat renal brushborder membrane vesicles. Biochem Biophys Res Commun. 1987;142:849–856. doi: 10.1016/0006-291x(87)91491-4. [DOI] [PubMed] [Google Scholar]
- 15.Moon C, King LS, Agre P. Aqp1 expression in erythroleukemia cells: genetic regulation of glucocorticoid and chemical induction. Am J Physiol Cell Physiol. 1997;273:C1562–C1570. doi: 10.1152/ajpcell.1997.273.5.C1562. [DOI] [PubMed] [Google Scholar]
- 16.Mulder J, Baum M, Quigley R. Diffusional water permeability (PDW) of adult and neonatal rabbit renal brush border membrane vesicles. J Membr Biol. 2002;187:167–174. doi: 10.1007/s00232-001-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neu J, Ozaki CK, Angelides KJ. Glucocorticoid-mediated alteration of fluidity of brush border membrane in rat small intestine. Pediatr Res. 1986;20:79–82. doi: 10.1203/00006450-198601000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen S, Pallone T, Smith BL, Christensen EI, Agre P, Maunsbach AB. Aquaporin-1 water channels in short and long loop descending thin limbs and in descending vasa recta in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;268:F1023–F1037. doi: 10.1152/ajprenal.1995.268.6.F1023. [DOI] [PubMed] [Google Scholar]
- 19.Parasassi T, De Stasio G, Ravagnan G, Rusch RM, Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys J. 1991;60:179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prabhu S, Levi M, Dwarakanath V, Arar M, Biber J, Murer H, Baum M. Effect of glucocorticoids on neonatal rabbit renal cortical sodium-inorganic phosphate messenger RNA and protein abundance. Pediatr Res. 1997;41:20–24. doi: 10.1203/00006450-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Quigley R, Baum M. Developmental changes in rabbit juxtamedullary proximal convoluted tubule water permeability. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;271:F871–F876. doi: 10.1152/ajprenal.1996.271.4.F871. [DOI] [PubMed] [Google Scholar]
- 22.Quigley R, Baum M. Water transport in neonatal and adult rabbit proximal tubules. Am J Physiol Renal Physiol. 2002;283:F280–F285. doi: 10.1152/ajprenal.00341.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quigley R, Gupta N, Lisec A, Baum M. Maturational changes in rabbit renal basolateral membrane vesicle osmotic water permeability. J Membr Biol. 2000;174:53–58. doi: 10.1007/s002320001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quigley R, Harkins EW, Thomas PJ, Baum M. Maturational changes in rabbit renal brush border membrane vesicle osmotic water permeability. J Membr Biol. 1998;164:177–185. doi: 10.1007/s002329900403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quigley R, Mulder J, Baum M. Ontogeny of water transport in the rabbit proximal tubule. Pediatr Nephrol. 2003;18:1089–1094. doi: 10.1007/s00467-003-1241-y. [DOI] [PubMed] [Google Scholar]
- 26.Schafer JA. Transepithelial osmolality differences, hydraulic conductivities, and volume absorption in the proximal tubule. Annu Rev Phys Chem. 1990;52:709–726. doi: 10.1146/annurev.ph.52.030190.003425. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz SM, Hostetler B, Ling S, Mone M, Watkins JB. Intestinal membrane lipid composition and fluidity during development in the rat. Am J Physiol Gastrointest Liver Physiol. 1985;248:G200–G207. doi: 10.1152/ajpgi.1985.248.2.G200. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz SM, Ling SD, Hostetler B, Draper JP, Watkins JB. Lipid composition and membrane fluidity in the small intestine of the developing rabbit. Gastroenterology. 1984;86:1544–1551. [PubMed] [Google Scholar]
- 29.Smith B, Baumgarten R, Nielsen S, Raben D, Zeidel ML, Agre P. Concurrent expression of erythroid and renal aquaporin CHIP and appearance of water channel activity in perinatal rats. J Clin Invest. 1993;92:2035–2041. doi: 10.1172/JCI116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka M, Inase N, Fushimi K, Ishibashi K, Ichioka M, Sasaki S, Marumo F. Induction of aquaporin 3 by corticosteroid in a human airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 1997;273:L1090–L1095. doi: 10.1152/ajplung.1997.273.5.L1090. [DOI] [PubMed] [Google Scholar]
- 31.Wang ZM, Yasui M, Celsi G. Glucocorticoids regulate the transcription of Na(+)-K(+)-ATPase genes in the infant rat kidney. Am J Physiol Cell Physiol. 1994;267:C450–C455. doi: 10.1152/ajpcell.1994.267.2.C450. [DOI] [PubMed] [Google Scholar]
- 32.Wintour EM, Earnest L, Alcorn D, Butkus A, Shandley L, Jeyaseelan K. Ovine AQP1: cDNA cloning, ontogeny, and control of renal gene expression. Pediatr Nephrol. 1998;12:545–553. doi: 10.1007/s004670050502. [DOI] [PubMed] [Google Scholar]
- 33.Worman HJ, Brasitus TA, Dudeja PK, Fozzard HA, Field M. Relationship between lipid fluidity and water permeability of bovine tracheal epithelial cell apical membranes. Biochemistry. 1986;25:1549–1555. doi: 10.1021/bi00355a014. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto T, Sasaki S, Fushimi K, Ishibashi K, Yaoita E, Kawasaki K, Fujinaka H, Marumo F, Kihara I. Expression of AQP family in rat kidneys during development and maturation. Am J Physiol Renal Physiol. 1997;272:F198–F204. doi: 10.1152/ajprenal.1997.272.2.F198. [DOI] [PubMed] [Google Scholar]
- 35.Ye R, Verkman AS. Simultaneous optical measurement of osmotic and diffusional water permeability in cells and liposomes. Biochemistry. 1989;28:824–829. doi: 10.1021/bi00428a062. [DOI] [PubMed] [Google Scholar]