Abstract
We sought to assess the relationship between the metabolic syndrome, abdominal obesity, and glucose deterioration amongst patients with type 2 diabetes. Our prospective cohort consisted of 164 adult patients with established diabetes who have a history of poor glycemic control, have just completed an intensive intervention aimed at improved control, and have demonstrated reduced HbA1c prior to enrollment. Waist circumference and presence of metabolic syndrome were assessed at baseline, and patients were followed-up (median 24 months) for assessment of the study outcome, time-to-hyperglycemic relapse, pre-defined as HbA1c >8% and >1% rise over baseline. Kaplan-Meier estimates of relapse-free survival and multivariable Cox regression models were used to quantify the independent effects of the metabolic syndrome and waist circumference on risk of glucose deterioration. Baseline waist circumference was 42.9 ± 5.5 inches. Prevalence of the metabolic syndrome was 80%. During follow-up, 39 patients (24%) experienced hyperglycemic relapse. The metabolic syndrome was not associated with time-to-relapse (p=0.15). The waist circumference component alone however was associated with increased likelihood of hyperglycemic relapse with an unadjusted hazard ratio of 3.4 (95% CI 1.2 – 9.7) and a hazard ratio of 3.2 (95% CI 1.1 – 9.1) after adjusting for age, gender, insulin use, weight change, and physical activity level. The NCEP ATPIII metabolic syndrome had limited ability to predict glucose deterioration in this type 2 diabetes cohort. Waist circumference alone, however, is a strong predictor of future glucose control and may be a parsimonious tool for risk stratification. BMI may also be a useful predictive tool.
OBJECTIVE
To assess the relationship between the metabolic syndrome, abdominal obesity, and glucose deterioration amongst patients with type 2 diabetes.
METHODS AND PROCEDURES
Our prospective cohort consisted of 164 adult patients with established type 2 diabetes who have a history of poor glycemic control, have just completed an intensive intervention aimed at improved control, and have demonstrated reduced HbA1c prior to enrollment. Waist circumference and presence of metabolic syndrome were assessed at baseline, and patients were followed-up (median 24 months) for assessment of the study outcome, time-to-hyperglycemic relapse, pre-defined as HbA1c >8% and >1% rise over baseline. Kaplan-Meier estimates of relapse-free survival and multivariable Cox regression models were used to quantify the independent effects of the metabolic syndrome and waist circumference on risk of glucose deterioration.
RESULTS
Baseline waist circumference was 42.9 ± 5.5 inches. Prevalence of the metabolic syndrome was 80%. During follow-up, 39 patients (24%) experienced hyperglycemic relapse. The metabolic syndrome was not associated with time-to-relapse (p=0.15). The waist circumference component alone however was associated with increased likelihood of hyperglycemic relapse with an unadjusted hazard ratio of 3.4 (95% CI 1.2 – 9.7) and a hazard ratio of 3.2 (95% CI 1.1 – 9.1) after adjusting for age, gender, insulin use, weight change, and physical activity level.
CONCLUSION
The NCEP ATPIII metabolic syndrome had limited ability to predict glucose deterioration in this type 2 diabetes cohort. Waist circumference alone, however, is a strong predictor of future glucose control and may be a parsimonious tool for risk stratification. BMI may also be a useful predictive tool.
Keywords: Glucose Control, Insulin Resistance, metabolic syndrome components, BMI, obesity
The metabolic syndrome is a multiplex risk factor for cardiovascular disease and type 2 diabetes that reflects the clustering of individual risk variables due to obesity and insulin resistance. While the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATPIII) metabolic syndrome correlates well with direct measures of insulin resistance and is a powerful predictor of new onset type 2 diabetes(1–5), it is unclear whether it is a useful additional diagnosis in patients with established type 2 diabetes.
Over three quarters of all patients with type 2 diabetes have the metabolic syndrome(6). These patients are at increased risk for cardiovascular events(7–10) and have an increased prevalence of the microvascular complications of diabetes(11; 12). However, there is no overall increased mortality in the subset of type 2 diabetes patients with the metabolic syndrome(13), and currently diabetes treatment guidelines do not differ for patients with a concomitant diagnosis of metabolic syndrome. This has led the American Diabetes Association to discourage use of the metabolic syndrome diagnosis (ICD9 277.7) in the type 2 diabetes population(14).
Metabolic syndrome research in type 2 diabetes has largely focused on cardiovascular endpoints and has not explored the impact of the syndrome on glycemic control. Prolonged hyperglycemia is known to be a powerful predictor of poor outcomes in type 2 diabetes(15), and improved long-term glucose control is an increasingly important public health concern(16). A common problem in the treatment of patients with type 2 diabetes is failure to maintain adequate glycemic control once achieved. So-called ‘glycemic relapse’ or ‘hyperglycemic relapse’ is thought to be due both to patient behavioral factors as well as physiologic factors such as beta cell deterioration and insulin resistance(17). Despite being touted as easy-to-measure surrogate of insulin resistance, it is unknown if the metabolic syndrome is associated with future glucose deterioration among patients with type 2 diabetes.
The aim of this study is to assess whether the NCEP ATPIII metabolic syndrome, or its component risk factors such as abdominal obesity, are able to predict hyperglycemic deterioration in patients with type 2 diabetes.
METHODS AND PROCEDURES
Study Design and Subjects
We co-enrolled all participants of the ongoing NIDDK-funded Glycemic Relapse Prevention Trial into a prospective, observational cohort study of the association between the NCEP ATPIII metabolic syndrome, abdominal obesity, and hyperglycemic relapse. We have previously published the study protocol of the Glycemic Relapse Prevention Trial (18).
One hundred sixty-four non-pregnant adult patients with type 2 diabetes were recruited into this study over the 30-month period June 2002 to January 2005. Enrollment criteria for the Glycemic Relapse Prevention Trial included patients aged 18 to 75 who had documented previous poor glucose control (HbA1c >8%), entered the 12-week Diabetes Improvement Program at Vanderbilt University, and completed the program demonstrating improved blood glucose control (HbA1c <8%). One patient from the trial registry was excluded from the analysis due to missing baseline data. Informed consent was obtained from each patient. The study protocol was approved by the Institutional Review Board of Vanderbilt University and was carried out in accordance with institutional guidelines.
Definition of Metabolic Syndrome and Baseline Measurements
Presence of the metabolic syndrome was determined according to the NCEP ATPIII definition, first proposed in 2001(19) and updated in 2005(20). Since all patients had diabetes, each fulfilled the glycemic component of the definition (fasting blood sugar >100 mg/dL). Patients with metabolic syndrome fit at least two of the remaining four criteria: waist circumference >40 inches for men and >35 inches for women, HDL cholesterol <40 mg/dL in men and <50 mg/dL in women, triglycerides ≥150 mg/dL, and systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or on anti-hypertensive medication or documented history of an active clinical diagnosis of hypertension.
Waist circumference was measured with the patient standing and the examiner positioned to the right of the subject. The top of the right iliac crest was identified, and a measuring tape was extended in a horizontal plane around the abdomen from this point. Before measurement was made, the tape measure was confirmed to be snug around the abdomen, without compressing the skin, in a plane parallel to the floor. Measurement was then made at normal minimal inspiration(21).
A thorough medical history was taken by a trained clinician upon enrollment that included demographic and anthropomorphic data, known co-morbidities such as hyperlipidemia or hypertension, duration of diabetes since diagnosis, and medication regimens including details of insulin use. Self-reported data was validated against the electronic medical record for all patients. In addition, patients completed the CES-D depression measurement tool(22), rated their compliance with insulin by quantifying average number of missed shots per week, and completed seven-day food diaries from which three complete days were analyzed by a registered dietitian. Patients also wore a tri-axial portable accelerometer for one week to assess activity energy expenditure. Finally, all participants had their baseline HbA1c measured via the core lab at Vanderbilt University Medical Canter. The assay used follows the National Glycohemoglobin Standardization Program that began in mid-1996 and has been given a certificate of traceability to the DCCT reference method to ensure precision and accuracy(23).
Follow-up
The cohort was followed over the 42-month period from June 2002 until December 2005. Patients were seen at months 3, 6, 12, 18, 24, 30, and 36 after baseline. At each visit, patient weight and HbA1c were measured. Patients again were asked to rate their compliance with their insulin regimens. In addition, changes in the medical history were noted and confirmed via the electronic medical record including hospitalizations and medication changes. Three patients did not return for follow-up, and they are retained in the analysis. Six patients dropped out of the study before the scheduled end of follow-up. Median follow-up time of the cohort was 24 months (IQR 12 – 30 months).
Definition of Outcome and Study Covariates
The primary study outcome was time-to-hyperglycemic relapse. Hyperglycemic relapse was predefined as a subsequent HbA1c >8% and at least a one percentage-point rise over baseline. For example, an individual with baseline HbA1c of 7.2% would experience hyperglycemic relapse if at follow-up the HbA1c was 8.2% or greater.
Weight change was defined as the difference between the weight at last follow-up and baseline weight. Physical activity level was defined by the formula (total energy intake)/(total energy intake – energy expenditure from activity) via accelerometer data(24). Adherence to medication regimen was calculated as the average proportion of scheduled insulin doses missed per week.
Statistical Analysis
Baseline characteristics of the study patients are presented based on presence or absence of metabolic syndrome. Frequencies and proportions are reported for categorical variables, and either means with standard deviations or medians with interquartile ranges are reported for continuous variables based on normality of distribution. Fisher’s Exact tests were used to compare categorical variables while Wilcoxon rank-sum tests were used for comparing continuous variables.
Cumulative probability of hyperglycemic relapse-free curves were generated using Kaplan-Meier estimates, and log-rank statistics were used to assess difference by metabolic syndrome status. Subjects were censored at the earlier of time of last contact or at 36 months follow-up. To evaluate the effect of the NCEP ATPIII metabolic syndrome and its components on time-to-hyperglycemic relapse, hazard ratios and 95 percent confidence intervals (95% CI) were calculated using the Cox proportional hazards regression model. The maximum number of independent variables included in the regression model (three) was determined based on the 10 events-per-variable rule(25).
We first built a model adjusting for age, sex, and insulin use (Model 1). A second model was then applied using propensity score adjustment (Model 2). This method simultaneously adjusts for many confounding factors while preserving analytical power by combining factors into one score, preventing over-fitting(26; 27). Our propensity score was the predicted probability of having metabolic syndrome as a function of age, sex, weight change, physical activity level, and insulin use. Other covariates such as duration of diabetes, other co-morbid illnesses, and other medication use were tested in the propensity score model and had no effect on the overall results.
To further characterize the accuracy of obesity measures for the prediction of relapse, we constructed adjusted Receiver Operating Characteristic (ROC) curves for waist circumference and BMI, calculated optimum cutpoints, and quantified the area under each ROC curve (AUC). The continuous prediction variable used was the calculated probability of hyperglycemic relapse at 18 months. We then compared the area under these curves, allowing quantification of the overall value of these measures as predictive tools.
All analyses used a 5% two-sided significance level. Calculations were performed using STATA software, version 8.2 and R version 2.1.0 (www.r-project.org).
RESULTS
Baseline waist circumference was 42.9 ± 5.5 inches. A total of 131 patients (80%) had metabolic syndrome at baseline. There were no statistically significant differences in the age, race, gender, education level, duration of diabetes since diagnosis, physical activity level, or incidence of depression between the exposure groups at baseline (Table 1). Oral hypoglycemic use did not differ between the groups. Insulin use was different between groups, with 60% of patients with metabolic syndrome and 28% of those without requiring insulin at baseline (p=0.001). In addition, amongst those using insulin, the number of units used was greater in patients with metabolic syndrome (p=0.04).
Table 1.
Patients characteristics by presence versus absence of the metabolic syndrome
| Metabolic Syndrome N = 131 |
No Metabolic Syndrome N = 32 |
P value | |
|---|---|---|---|
| Age (years) | 55.9 ± 10.4 | 52.2 ± 11.9 | 0.13 |
| Female gender (%) | 46 | 34 | 0.32 |
| Non-white race (%) | 25 | 22 | 0.82 |
| High school education (%) | 87 | 97 | 0.20 |
| Physical Activity Level | 1.35 ± 0.25 | 1.38 ± 0.17 | 0.07 |
| CES-D Depression score | 11.9 ± 8.7 | 11.6 ± 7.2 | 0.81 |
| Duration of diabetes (years) | 5 (0.6 – 10.0) | 3 (0.46 – 8.5) | 0.24 |
| Baseline HbA1c (%) | 6.70 ± 0.65 | 6.73 ± 0.82 | 0.72 |
| Oral Hypoglycemic use (%) | 70 | 81 | 0.27 |
| Insulin use (%) | 60 | 28 | 0.001 |
| † Waist circumference (in.) | 44.1 ± 4.9 | 38.0 ± 6.2 | <0.001 |
| † BMI (kg/m2) | 35.3 ± 6.5 | 29.0 ± 6.1 | <0.001 |
| † HDL (mg/dL) | 40.8 ± 10.1 | 50.3 ± 12.7 | <0.001 |
| † Triglycerides (mg/dL) | 195 (138 – 256) | 108 (87 – 137) | <0.001 |
| † Systolic blood pressure (mmHg) | 126.4 ± 16.3 | 124.0 ± 13.0 | 0.32 |
| † Diastolic blood pressure (mmHg) | 72.6 ± 11.0 | 72.6 ± 10.2 | 0.95 |
| † Hypertension (%) | 79 | 22 | <0.001 |
| † Metabolic Syndrome Score‡ | 3.96 ± 0.79 | 1.69 ± 0.47 | <0.001 |
Data are expressed as means ± SD or medians (interquartile range) or percentages. P values obtained using Fisher’s Exact test for categorical variables and Wilcoxon rank-sum tests for continuous variables.
Variables used to define metabolic syndrome or variables known to be highly correlated with defining variables.
Number of components (1–5) of metabolic syndrome present.
Patients with the metabolic syndrome (mean follow-up 23.6 ± 10.7 months) and without the metabolic syndrome (21.9 ± 10.3 months) were observed for a similar period of time. Patterns and number of missed follow-up visits were not different between the groups. A total of 39 of the 163 (24%) patients experienced hyperglycemic relapse, 34 of which were in the metabolic syndrome group.
Metabolic syndrome as a predictor of hyperglycemic relapse
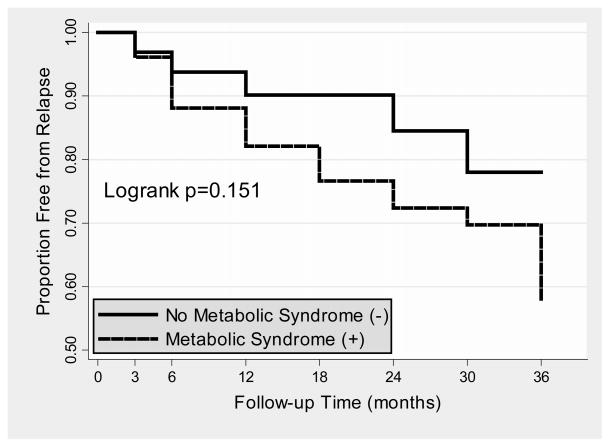
Kaplan-Meier estimates of cumulative probability of being free-from-hyperglycemic relapse according to metabolic syndrome status are shown in Figure 1. The log-rank test indicated no difference between the curves (p=0.15).
Figure 1.
Kaplan Meier estimates of relapse-free survival over follow-up, by metabolic syndrome status
Legend: P value derived from unadjusted logrank test.
The results from three different Cox proportional hazards models, each based on the presence or absence of metabolic syndrome, are shown in Table 2. In univariate analysis, the NCEP ATPIII metabolic syndrome displayed a trend toward an increased the risk of relapse, with an unadjusted hazard ratio of 1.97 (95% CI 0.77 – 5.03). Adjusted for age, gender, and insulin use, the hazard ratio for the metabolic syndrome was 1.87 (0.72 – 4.85). Adjusted for propensity score, the metabolic syndrome had a hazard ratio of 1.82 (95% CI 0.71 – 4.70).
Table 2.
Cox proportional hazards analyses of the metabolic syndrome, or its individual components, and hyperglycemic relapse.
| Hazard Ratio (95% confidence interval)
|
|||
|---|---|---|---|
| Unadjusted Model
|
Model 1†
|
Model 2‡
|
|
| Metabolic Syndrome | 1.96 (0.77 – 5.03) | 1.87 (0.72 – 4.85) | 1.82 (0.71 – 4.70) |
| Waist Circumference | 3.44 (1.22 – 9.71)* | 3.18 (1.11 – 9.08)* | 3.13 (1.11 – 8.91)* |
| Low HDL | 1.75 (0.89 – 3.45) | 1.66 (0.84 – 3.28) | 1.56 (0.81 – 3.15) |
| High Triglycerides | 1.11 (0.58 – 2.14) | 1.54 (0.78 – 3.05) | 1.30 (0.65 – 2.45) |
| Hypertension | 0.91 (0.47 – 1.76) | 0.85 (0.42 – 1.71) | 0.82 (0.41 – 1.64) |
p<0.05
Model 1: adjusted for age (nonlinear), sex, and insulin use
Model 2: adjusted for propensity score (derived from imputed dataset using age, sex, insulin use, weight change, and physical activity)
Metabolic syndrome components considered individually
Table 2 also shows results of with the metabolic syndrome components considered individually. The HDL, triglycerides, and hypertension components of the syndrome were not associated with a significantly increased risk of hyperglycemic relapse. The waist circumference component was associated with an increased risk of relapse, with an unadjusted hazard ratio of 3.44 (95% CI 1.22 – 9.71). Adjusted for age, gender, and insulin use, waist circumference remained significantly associated with increased risk for hyperglycemic relapse, hazard ratio 3.18 (95% CI 1.11 – 9.08). The hazard ratio of waist circumference for relapse was 3.13 (95% CI 1.11 – 8.91) after propensity score adjustment.
A series of separate analyses adjusting for duration of diabetes, co-morbidities related to obesity, and other medication use did alter the association between metabolic syndrome and hyperglycemic relapse or waist circumference and hyperglycemic relapse.
Waist circumference vs. BMI
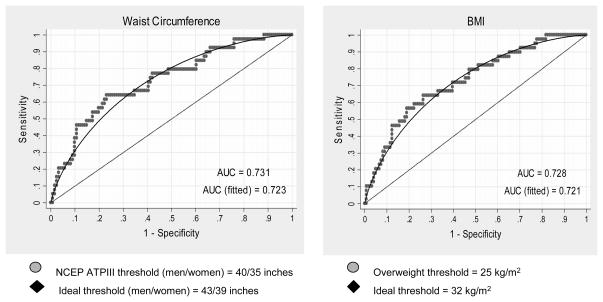
The adjusted AUC for waist circumference was 0.728 (see Figure 2). The optimum cutpoint for predicting hyperglycemic relapse was 43 inches for men (sensitivity 81.3%, specificity 50.0%) and 39 inches for women (sensitivity 82.6%, specificity 34.7%).
Figure 2. Adjusted Receiver Operator Characteristic (ROC) curves for the association of obesity measures and hyperglycemic relapse.
Legend: BMI refers to Body Mass Index. AUC refers to area under the receiver operating characteristic curve. NCEP ATP III is the National Cholesterol Education Program Adult Treatment Panel III guidelines.
There is no difference in area under these two curves (p=0.78). Ideal thresholds for identifying glucose deterioration are calculated from unadjusted data.
A similar curve was constructed replacing waist circumference with BMI. The adjusted AUC was 0.731 (see Figure 2). The optimum cutpoint for predicting hyperglycemic relapse was 32 kg/m2. There was no difference in overall diagnostic accuracy using waist circumference compared to BMI (P=0.78).
DISCUSSION
In this study of previously poorly controlled type 2 diabetics who have achieved improved glycemic control, the metabolic syndrome was not significantly associated with subsequent hyperglycemic relapse. A single measure of central adiposity, waist circumference, was however a significant predictor of hyperglycemic relapse. The association of waist circumference with relapse persisted despite adjustment for known predictors of hyperglycemic relapse(28). To our knowledge, this is the first study exploring the relationship between the metabolic syndrome and future glycemic control in the type 2 diabetes population. Poor glucose control is a well-known risk factor for complications and mortality in these patients(15).
These results lend additional support to the statements by the American Diabetes Association (14) and others(29) that the metabolic syndrome, as presently defined by the NCEP ATPIII, is not a useful additional diagnosis in patients with type 2 diabetes. Prior studies have shown that the metabolic syndrome is predictive of cardiovascular events(7–10) in patients with type 2 diabetes and is associated with the microvascular complications of diabetes(11; 12). However, presence of the metabolic syndrome does not increase mortality in this population(13), and perhaps more importantly, treatment guidelines for the metabolic syndrome are in general less strict than those for patients with type 2 diabetes. Thus, an additional diagnosis of the metabolic syndrome may not provide the clinician with additional information, and some argue may distract the clinician from proper treatment of hyperglycemia(14). These prior studies have largely focused on cardiovascular endpoints. This study is the first to suggest that the metabolic syndrome is not a useful diagnostic tool for the prediction of future glucose deterioration.
The metabolic syndrome and insulin resistance
There are several factors which are thought to influence glucose deterioration in patients with type 2 diabetes. One category includes behavioral factors such as physical activity, diet, and medication compliance. These variables are the primary consideration of the Glycemic Relapse Prevention Trial and are controlled for in this study. Another category includes physiologic factors such as insulin resistance and beta-cell failure.
The underlying pathophysiology of the metabolic syndrome is thought to be insulin resistance(30). In general, clinical definitions of the metabolic syndrome seek to identify individuals that are sufficiently insulin resistant to be at risk for a variety of adverse conditions, most notably cardiovascular disease(31). However the NCEP ATPIII metabolic syndrome is a specific, but not sensitive, method for identifying insulin resistance(1; 32). Studies have shown that there are simpler methods for identifying insulin resistance with similar or improved accuracy as compared to the metabolic syndrome. For example, McLaughlin et al. showed that using the triglyceride component alone with a slightly lower cutpoint (>130 mg/dL) could identify insulin resistant individuals with similar accuracy as the NCEP ATPIII metabolic syndrome(33). Cheal et al. showed that BMI highly correlated with insulin resistance and that other factors such as hypertension were much less highly correlated(1). It is likely that the NCEP ATPIII metabolic syndrome, by using arbitrary cutpoints to dichotomize risk variables that represent both causes (abdominal obesity) and consequences (high triglycerides) of insulin resistance, is an inefficient grouping that may dilute the predictive power of its individual components(34).
Waist circumference and insulin resistance
Our results indicate that waist circumference alone may be a parsimonious, yet powerful tool for the prediction of glucose deterioration in patients with type 2 diabetes. This finding echoes a recent article by Sierra-Johnson et al. studying the relationship between the metabolic syndrome and its components to a direct measure of insulin resistance(35).
In this study, 256 non-Hispanic white subjects underwent frequently sampled intravenous glucose tolerance tests for the determination of insulin resistance. Accuracy for identifying insulin resistance was expressed as area under the receiver operating curve (AUC). The NCEP ATPIII metabolic syndrome, considered as an ordinal variable counting metabolic syndrome components (ranging from 0–5), had a diagnostic accuracy of AUC=0.768. Waist circumference considered alone had an accuracy of AUC=0.813.
Indeed, recent evidence implicates total and regional adipose tissue load as among the most potent predictors of insulin resistance(36). Therefore it may not be surprising that waist circumference, the only surrogate measure of body fat included the current NCEP ATPIII metabolic syndrome definition, is highly predictive.
Study limitations
There are several potential study limitations. First, our study is limited by the smaller sample size. A larger study may have sufficient power to demonstrate an association between the metabolic syndrome and hyperglycemic relapse. However, this finding would be less important given that waist circumference alone is more predictive than the entire NCEP ATPIII metabolic syndrome.
Second, our study sample was limited in racial and ethnic diversity, and had a high level of education compared to the general diabetes population. As such, predictors of glucose deterioration should be evaluated among more diverse populations in future research.
Third, the study was conducted at a university medical center, and thus our findings may not be generalizable to other patient populations. These patients have all accepted referral to the Vanderbilt Diabetes Improvement Program prior to enrollment in the study because of failure to achieve glycemic control in the primary care setting. These patients are in general heavier, require more insulin, and are at higher risk for deterioration of glucose control. However, this also makes them a convenient group to study.
Finally, our relapse rates are lower than those reported in previous studies(28). This is likely due to the setting of this study within an ongoing interventional trial.
Future directions
The mechanism by which obesity leads to hyperglycemic relapse can not be concluded from this study. In particular, this study does not permit the inference that insulin resistance predicts hyperglycemic relapse. While waist circumference is highly correlated with insulin resistance, and attempts have been made to control for behavioral variables associated with waist circumference, a study utilizing a direct measure of insulin resistance would be required to assess the relative contribution of this mechanism to relapse.
Our results indicate that BMI may be at least as accurate in predicting hyperglycemic relapse as waist circumference. The superiority of waist circumference over a more general measure of adiposity have been thoughtfully questioned by others(37). Indeed, BMI is highly correlated with waist circumference (r>0.8, p<0.001) and offers several advantages, most notably that it is easier for clinicians to measure(29). In addition, BMI appears to be at least as good of a predictor of insulin resistance(38). A larger study should be undertaken with sufficient power to discern a difference in the predictive value of these variables. Results of such a study could potentially be used by diabetes care providers to stratify their type 2 diabetes patients into low-risk and high-risk for potential relapse, with higher risk groups considered for more intense behavioral and pharmacotherapy.
Abbreviations
- HbA1c
hemoglobin A1C
- CEP ATPIII
National Cholesterol Education Program Adult Treatment Panel III
- BMI
Body Mass Index
Footnotes
None of the authors for this paper have conflicts of interest to disclose.
References
- 1.Cheal KL, Abbasi F, Lamendola C, McLaughlin T, Reaven GM, Ford ES. Relationship to Insulin Resistance of the Adult Treatment Panel III Diagnostic Criteria for Identification of the Metabolic Syndrome. Diabetes. 2004;53:1195–1200. doi: 10.2337/diabetes.53.5.1195. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM. The Metabolic Syndrome as Predictor of Type 2 Diabetes: The San Antonio Heart Study. Diabetes Care. 2003;26:3153–3159. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 3.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Archives of Internal Medicine. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 4.Hanley AJ, Karter AJ, Williams K, et al. Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: the Insulin Resistance Atherosclerosis Study. Circulation. 2005;112:3713–3721. doi: 10.1161/CIRCULATIONAHA.105.559633. [DOI] [PubMed] [Google Scholar]
- 5.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. American Journal of Epidemiology. 2002;156:1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 6.Marchesini G, Forlani G, Cerrelli F, et al. WHO and ATPIII proposals for the definition of the metabolic syndrome in patients with Type 2 diabetes. Diabetic Medicine. 2004;21:383–387. doi: 10.1111/j.1464-5491.2004.01115.x. [DOI] [PubMed] [Google Scholar]
- 7.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular Morbidity and Mortality Associated With the Metabolic Syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 8.Bonora E, Targher G, Formentini G, et al. The Metabolic Syndrome is an independent predictor of cardiovascular disease in Type 2 diabetic subjects. Prospective data from the Verona Diabetes Complications Study. Diabetic Medicine. 2004;21:52–58. doi: 10.1046/j.1464-5491.2003.01068.x. [DOI] [PubMed] [Google Scholar]
- 9.Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. Prevalence of atherosclerotic vascular disease among subjects with the metabolic syndrome with or without diabetes mellitus: the METS-GREECE Multicentre Study. Current Medical Research & Opinion. 2004;20:1691–1701. doi: 10.1185/030079904x5599. [DOI] [PubMed] [Google Scholar]
- 10.Guzder R, Gatling W, Mullee M, Byrne C. Impact of metabolic syndrome criteria on cardiovascular disease risk in people with newly diagnosed type 2 diabetes. Diabetologia. 2006;49:49–55. doi: 10.1007/s00125-005-0063-9. [DOI] [PubMed] [Google Scholar]
- 11.Isomaa B, Henricsson M, Almgren P, et al. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia. 2001;44:1148–1154. doi: 10.1007/s001250100615. [DOI] [PubMed] [Google Scholar]
- 12.Costa LA, Canani LH, Lisboa HR, Tres GS, Gross JL. Aggregation of features of the metabolic syndrome is associated with increased prevalence of chronic complications in Type 2 diabetes. Diabetic Medicine. 2004;21:252–255. doi: 10.1111/j.1464-5491.2004.01124.x. [DOI] [PubMed] [Google Scholar]
- 13.Bruno G, Merletti F, Biggeri A, et al. Metabolic syndrome as a predictor of all-cause and cardiovascular mortality in type 2 diabetes: the Casale Monferrato Study. Diabetes Care. 2004;27:2689–2694. doi: 10.2337/diacare.27.11.2689. [DOI] [PubMed] [Google Scholar]
- 14.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 15.UKPDS: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 16.Nathan DM. Clinical practice. Initial management of glycemia in type 2 diabetes mellitus. New England Journal of Medicine. 2002;347:1342–1349. doi: 10.1056/NEJMcp021106. [DOI] [PubMed] [Google Scholar]
- 17.Graber AL, Davidson P, Brown AW, McRae JR, Woolridge K. Dropout and relapse during diabetes care. Diabetes Care. 1992;15:1477–1483. doi: 10.2337/diacare.15.11.1477. [DOI] [PubMed] [Google Scholar]
- 18.Huizinga MM, Shintani A, Michon S, et al. A randomized controlled trial to prevent glycemic relapse in longitudinal diabetes care: Study protocol ( NCT00362193) Implement Sci. 2006;1:24. doi: 10.1186/1748-5908-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 20.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and Management of the Metabolic Syndrome. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 21.National Institutes of Health, National Heart, Lung, and Blood Institute. . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Obes Res. 1998;6(suppl 2):S51–S210. [PubMed] [Google Scholar]
- 22.Radloff L, Teri L. Use of the CES-D with Older Adults. Clinical Gerontologist. 1977;5:119–136. [Google Scholar]
- 23.American Diabetes Association Position Statement. Tests of Glycemia in Diabetes. Diabetes Care. 2004;27(suppl 1):S91–S93. doi: 10.2337/diacare.27.2007.s91. [DOI] [PubMed] [Google Scholar]
- 24.Report of a Join FAO/WHO/UNU Expert Consultation. Vol. 724. Geneva: WHO; 1985. Energy and Protein Requirements. Technical Report Series. [PubMed] [Google Scholar]
- 25.Perduzzi P, Concato J, Kemper E, Holford T, Feinstein A. A simulation study of the number of events per variable in logistic regression analysis. Journal of Clinical Epidemiology. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 26.D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Joffe M, Rausenbaum P. Invited commentary: propensity scores. American Journal of Epidemiology. 1999;150:327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 28.Elasy TA, Graber AL, Wolff K, Brown A, Shintani A. Glycemic relapse after an intensive outpatient intervention for type 2 diabetes. Diabetes Care. 2003;26:1645–1646. doi: 10.2337/diacare.26.5.1645. [DOI] [PubMed] [Google Scholar]
- 29.Reaven GM. The metabolic syndrome: requiescat in pace. Clinical Chemistry. 2005;51:931–938. doi: 10.1373/clinchem.2005.048611. [DOI] [PubMed] [Google Scholar]
- 30.Reaven G. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 31.Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinology & Metabolism Clinics of North America. 2004;33:283–303. doi: 10.1016/j.ecl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Liao Y, Kwon S, Shaughnessy S, et al. Critical Evaluation of Adult Treatment Panel III Criteria in Identifying Insulin Resistance With Dyslipidemia. Diabetes Care. 2004;27:978–983. doi: 10.2337/diacare.27.4.978. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Annals of Internal Medicine. 2003;139:802–809. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 34.Blaha MJ, Elasy TA. Clinical Use of the Metabolic Syndrome: Why the Confusion? Clinical Diabetes. 2006;24:125–131. [Google Scholar]
- 35.Sierra-Johnson J, Johnson BD, Allison TG, Bailey KB, Schwartz GL, Turner ST. Correspondance Between the Adult Treatment Panel III Criteria for Metabolic Syndrome and Insulin Resistance. Diabetes Care. 2006;29:668–672. doi: 10.2337/diacare.29.03.06.dc05-0970. [DOI] [PubMed] [Google Scholar]
- 36.Rattarasarn C. Physiological and pathophysiological regulation of regional adipose tissue in the development of insulin resistance and type 2 diabetes. Acta Physiologica. 2006;186(2):87–101. doi: 10.1111/j.1748-1716.2005.01521.x. [DOI] [PubMed] [Google Scholar]
- 37.Reaven G. All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diabetes & Vascular Disease Research. 2005;2:105–112. doi: 10.3132/dvdr.2005.017. [DOI] [PubMed] [Google Scholar]
- 38.Farin HM, Abbasi F, Reaven GM. Body mass index and waist circumference correlate to the same degree with insulin-mediated glucose uptake. Metabolism: Clinical & Experimental. 2005;54:1323–1328. doi: 10.1016/j.metabol.2005.04.021. [DOI] [PubMed] [Google Scholar]