Abstract
Background
Saliva contains a large number of biomolecules, some of which have putative diagnostic usefulness. A potential problem with the use of biomolecules in diagnosis is day-to-day fluctuation due to within-subject variability. This study evaluated the intraindividual variability of six salivary analytes in healthy adults and determined their normal range.
Methods
Unstimulated whole saliva (5 ml) was collected every 2 to 3 days on six occasions from 30 subjects in good oral and systemic health. Four of the samples were collected in the clinic, and two were collected by the subject at home. The concentration ranges of interleukin (IL)-1β, IL-6, matrix metalloproteinase-8, prostaglandin E2, tumor necrosis factor-alpha, interferon-alpha, and albumin were examined. Descriptive statistics were computed, and a one-way random-effects model was used to quantify within- and between-subject components of variability. Intraclass correlation coefficients (ICCs) were calculated for each subject/analyte combination.
Results
Within-subject coefficients of variation for these analytes ranged from 67.6% to 172.1% for the in-clinic samples and from 111.9% to 201.0% for the at-home samples. The ICC for the various analytes ranged from 41% to 61% for the in-clinic samples. The at-home samples exhibited significantly more variability than did those obtained in the clinic under supervision.
Conclusions
There was marked within-subject variation in the salivary concentrations of these analytes. With increased interest in salivary diagnostics, the within-subject variability, normal range, and threshold levels for abnormal levels of individual salivary analytes need to be determined if these diagnostics tests are to have clinical usefulness.
Keywords: Biologic markers, diagnostic tests, reproducibility of results, saliva, statistics
Saliva contains a large number of biomolecules, some of which may be informative of an individual’s oral or systemic health status.1–17 A number of specific salivary analytes have been investigated for their usefulness in assessing periodontal status or disease activity.18–24 This is based on the observation that whole saliva may contain biomarkers of periodontal disease originating in the gingival crevicular fluid and may permit inferences about an individual’s periodontal status.23,24
The use of saliva as a diagnostic fluid offers some potential advantages for periodontal screening and diagnosis. The collection of whole saliva is easy, not invasive, and does not require any special apparatus. It does not require highly trained personnel, as do venipuncture and clinical periodontal examination, and samples can be collected at home by the patient. A salivary diagnostic test based on a single analyte or panel of analytes might allow periodontal diagnosis and/or screening to also be performed in non-dental settings, such as medical offices. Given the current interest in possible associations between oral disease and systemic health status, such a test, if valid, might have important implications for research and clinical practice.
Salivary diagnostics could be used in the treatment of individual patients or as screening tools in epidemiologic studies. Such tests might also be used to monitor the periodontal status of individual patients after active treatment. However, validation of the usefulness of the marker(s) must be performed before salivary analysis is used to make inferences about a patient’s current or future health status. The concentration of the marker (or its presence or absence) must be related in some meaningful way to the patient’s health status. Ideally, the use of the information generated by the test should have some impact on clinical decision making.25
This study evaluated intraindividual variation in levels of selected salivary biomarkers, which have been suggested as measures of periodontal health, over a 2-week period in a group of young, healthy adults. The primary aim of this investigation was to assess the variability of these salivary analytes in the absence of significant changes in oral health status. The secondary aims were to compare the reproducibility of home saliva collection to supervised, in-clinic collection and to estimate the normal ranges for these analytes.
MATERIALS AND METHODS
Study Population and Clinical Assessments
The study was conducted from January 2008 through June 2008. Thirty volunteers were recruited from the faculty, staff, and students of the University of Kentucky. All subjects were between 18 and 45 years of age and in good general health. All subjects also demonstrated periodontal health, with a minimum of 20 teeth, bleeding on probing (BOP) in <10% of sites, probing depth (PD) ≥5 mm in <2% of sites, no PD ≥6 mm, and clinical attachment loss >2 mm in <1% of sites. Individuals were excluded if they had a fever; infectious diseases, including, but not limited to, human immunodeficiency virus; hepatitis B; and inflammatory conditions, including rheumatoid arthritis. Additional exclusion criteria were certain medications believed to affect the inflammatory response and/or periodontal status (current use of glucocorticoids, cyclooxygenase inhibitors, and bisphosphonates), as well as pregnancy and the use of tobacco products. Subjects were not excluded based on race, gender, or ethnicity. The study protocol was reviewed and approved by the Institutional Review Board at the University of Kentucky. The subjects were provided information about the nature and purpose of the study at the time of recruitment. Written and verbal informed consent were obtained from all subjects.
Intake Examination and Eligibility Screening
The subject-participation period was 2 weeks. The first visit took place between the hours of 8:00 am and 10:00 am, at which time the subjects completed a medical history and data-collection questionnaire that elicited information regarding health status, demographic profile, recent oral trauma, recent dental procedures, and acute illness/infection. A comprehensive periodontal and oral examination was conducted. Potential subjects with active oral infections were excluded from participation. One calibrated examiner conducted all examinations. PDs were measured at six locations per tooth (mesial-buccal, midbuccal, distal-buccal, mesial-lingual, mid-lingual, and distal-lingual) using a probe.|| After the measurement of PDs, all sites were observed for BOP. The degree of bleeding was estimated and recorded (0 = no bleeding; 1 = light bleeding; 2 = moderate bleeding; and 3 = heavy bleeding) for each probed site. Clinical attachment levels were measured at interproximal sites only.
Collection and Handling of Salivary Samples
After the intake and screening process, 5ml unstimulated whole saliva was collected from those subjects meeting the eligibility requirements. At initial intake and subsequent visits, unstimulated whole saliva was collected according to a modification of the method described by Navazesh.26 Subjects were asked to avoid oral hygiene measures (i.e., flossing, brushing, and mouthrinses), eating, drinking, or gum chewing for 1 hour before collection. Subjects rinsed their mouth with tap water, after which they expectorated ≥5ml whole saliva into sterile tubes containing a protease inhibitor solution.¶
Samples were immediately placed on ice and transferred to the laboratory. After collection of the samples, aliquots were prepared, and the samples were frozen at −80°C until analysis.
Subjects returned to the clinic for saliva collection three additional times between the hours of 8:00 am and 10:00 am (on Friday of week 1 and Monday and Friday of week 2); hereafter, these are referred to as the in-clinic samples. Subjects were also instructed to collect 5 ml saliva at home between the hours of 8:00 am and 10:00 am on Wednesday of weeks 1 and 2, using the same protocol as was used for the in-clinic sample collection; hereafter, these are referred to as the at-home samples. The collection tubes containing these samples were to be placed in the home freezer and returned to one of the investigators (JS) on the following day, after which they were handled in the same manner as the in-clinic samples. At each of the clinic visits, 5 ml whole saliva was collected, and a questionnaire was completed to determine any change in oral or systemic health status, including incidence of oral trauma, dental procedures, general infection, and other changes in health status. Six saliva specimens were obtained per subject, and all samples were centrifuged prior to analysis.
All subjects reporting oral trauma, dental procedures, or infections during the 2-week experimental period were exited from the study. Such subjects were allowed to restart the study after a 2-week healing period, if they desired.
Salivary Analysis
Salivary concentrations of interleukin (IL)-1β, IL-6, tumor necrosis factor-alpha (TNF-α), and interferon-alpha (IFN-α) were measured using human multiplex assays.# Enzyme immunosorbent assays were used to determine levels of salivary matrix metalloproteinase (MMP)-8,** prostaglandin E2 (PGE2),†† and albumin.‡‡ All assays were performed in duplicate.
Statistical Analysis
Standard measures of central tendency and variability of the data were calculated. The biomarker concentration variables were log-transformed for analysis because the data exhibited right-skewed distributions. Descriptive statistics included the mean, median, standard deviation, minimum (min), maximum (max), and within-subject (individual) coefficient of variation (CVI). The CVI estimates the amount of variation seen between repeated measures taken from the same individual at different time points.
Linear mixed models with compound symmetric covariance matrices were used to test and compare different mean structures for log assay concentrations, including the same mean value for each collection day (constant mean model), a different mean value for each collection day (full model), different mean values for data collected in the clinic versus at home (two-means model), and a repeated-measures analysis of variance (ANOVA) to determine whether log assay values, on average, increased or decreased in a linear fashion over time (linear trend model). Linear mixed models were also fit, using as a response variable the log concentration of each biomarker scaled by albumin. A one-way random-effects model was used to quantify within- and between-subject components of variability separately for the at-home and in-clinic data, and intraclass correlation coefficients (ICCs) were calculated for each subject/analyte combination. Calculations were performed using two statistical packages.§§,||||
RESULTS
The study sample consisted of 16 men and 14 women aged 18 to 45 years. The subjects were medically and periodontally healthy, with a high level of dental care knowledge and a relatively high level of periodontal health (Table 1). Two subjects reported infections during the study period and were exited. After they were symptom-free for 2 weeks, they desired to repeat the study and were allowed to do so.
Table 1.
Subject Characteristics
| Parameter | Value |
|---|---|
| Mean age (years; mean ± SD) | 31.4 ± 6.80 |
| Male (%; mean) | 53.3 |
| White (%; mean) | 93.3 |
| African American (%; mean) | 0 |
| Asian (%; mean) | 3.3 |
| Hispanic (%; mean) | 3.3 |
| Periodontal index (% sites; mean ± SD) | |
| PD ≥5 mm | 0.80 ± 0.06 |
| PD ≥4 mm | 2.60 ± 1.43 |
| BOP | 4.06 ± 3.22 |
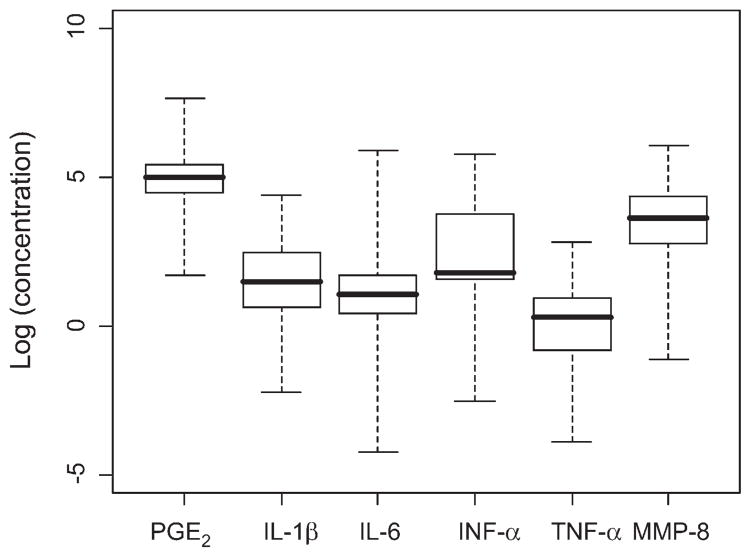
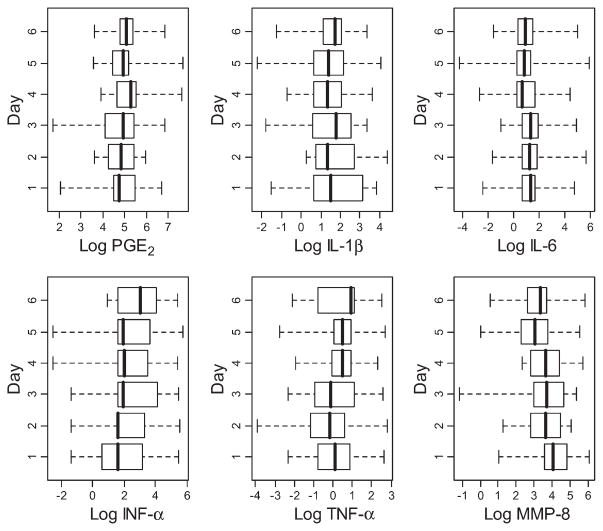
Within- and between-subject analyses were performed, as were standard descriptive statistics relating to the spread of the data. Data were analyzed in stratified and non-stratified form. In the non-stratified form, the data from the in-clinic and at-home samples were pooled, whereas these groups were analyzed separately in the stratified analysis. Concentrations of the analytes across all subjects and sampling points are shown in Figure 1. Figure 2 provides a similar depiction of concentrations of individual analytes, but separated based on the day of collection. These results demonstrate a broad range of values for each of the analytes within the subject population throughout each of the sampling time points. Table 2 shows the ranges of CVI values observed in the current study.
Figure 1.
Box plots of concentrations of salivary biomarkers on log scale. The data are presented for all subjects at all sampling points. The boxes denote the interquartile range, the whiskers represent the entire range of values observed, and the dark lines indicate medians.
Figure 2.
Box plots of concentrations of salivary biomarkers (on log scale) stratified by day of collection for all subjects. The data are presented for all subjects at all sampling points. The boxes denote the interquartile range, the whiskers represent the entire range of values observed, and the dark lines indicate medians.
Table 2.
Range of CVI Values for Selected Salivary Analytes
| Analyte | Salivary CVI (%) |
|---|---|
|
| |
| PGE2 | 21.2 to 102.7 |
| IL-1β | 39.8 to 150.2 |
| IL-6 | 20.6 to 111.9 |
| TNF-α | 38.0 to 148.1 |
| MMP-8 | 44.8 to 127.8 |
| Albumin (salivary) | 11.12 to 150.70 |
The one-way random-effects ANOVA model can be used to estimate the ICC, which expresses the correlation between repeated measures taken from the same subject at different times. Table 3 similarly depicts point estimates of the ICCs for the in-clinic and at-home groups, based on the values presented in the previous tables.
Table 3.
ICC Point Estimates for In-Clinic and At-Home Samples
| Assay | In-Clinic Samples | At-Home Samples |
|---|---|---|
|
| ||
| IL-1β | 0.46 | 0.40 |
| IL-6 | 0.59 | 0.52 |
| TNF-α | 0.39 | 0.24 |
Table 4 depicts the proportion of subject-analyte combinations having a maximum concentration that was more than twice as large as the corresponding minimum concentration for the analyte. This max/min >2 parameter is informative of the magnitude of the range; i.e., when the maximum value exceeds the minimum value by a factor ≥2, it suggests a relatively wide data spread.
Table 4.
Comparison of Within- and Between-Subject Variance for Samples
| Assay | Within-Subject σ2 | Between-Subject σα2 | σ2/(σ2 + σα2) |
|---|---|---|---|
| In-clinic | |||
| IL-1β | 0.90 (95% CI: 0.77 to 1.04) | 0.78 (95% CI: 0.56 to 1.11) | 0.54 |
| IL-6 | 0.72 (95% CI: 0.62 to 0.83) | 1.03 (95% CI: 0.77 to 1.38) | 0.41 |
| TNF-α | 1.03 (95% CI: 0.89 to 1.19) | 0.65 (95% CI: 0.42 to 0.99) | 0.61 |
|
| |||
| At-home | |||
| IL-1β | 1.03 (95% CI: 0.80 to 1.33) | 0.70 (95% CI: 0.38 to 1.28) | 0.60 |
| IL-6 | 1.05 (95% CI: 0.81 to 1.35) | 1.14 (95% CI: 0.77 to 1.67) | 0.48 |
| TNF-α | 1.46 (95% CI: 1.13 to 1.88) | 0.47 (95% CI: 0.06 to 3.32) | 0.76 |
CI = confidence interval.
DISCUSSION
The data from this exploratory study provide some information regarding the normal range of salivary analytes and suggest significant within-subject variation in this group of subjects for the salivary analytes examined. In addition, the data provide support for the need to further define the normal range of a healthy population for salivary analytes being proposed to be used in diagnostic testing.
Diagnostic tests must be validated for reproducibility and precision during the feasibility stage.27 Thereafter, external validation of the test in various populations should be performed, although this is infrequently done in practice. Van den Bruel et al.28 examined a group of diagnostic studies published in 1993 and found that none of the original articles reported any form of validation; follow-up validation studies could be identified for only seven of the 11 original studies. Precision implies that “the same test applied to the same, unchanged patient must produce the same result.”29 However, even in the absence of significant changes in an individual’s health status, serialclinical laboratory test results usually vary over time because of a combination of preanalytical influences, analytical random error, and inherent biologic variation (BV).
BV (intraindividual variability) can be due to cyclical variations (circadian, monthly, or seasonal) or variations over the lifespan of the individual (e.g., age-related hormonal changes). However, “for many analytes, this is not a major problem because they do not have marked clinically important cyclical rhythms.”30 Most analytes fluctuate around a homeostatic set-point. This intraindividual random variation is known as within-subject variation and is differentiated from between-subject variation. The latter is due to the fact that different individuals have different set-points. The determination of the magnitude of such variations is of great importance to the interpretation of laboratory results in clinical medicine and research.
The within-subject or intraindividual coefficient of variation (CVI) has classically been used to assess BV of diagnostic analytes. Data on within- and between-subject variability with regard to serum analytes is available in reference value databases.31,32 In a recently published comprehensive database of serum analytes, the CVI values range from 0.7 for sodium to 52.6 for C-reactive protein.33 Narrower ranges are seen for analytes that are under strict physiologic control (e.g., chloride, potassium, sodium, and calcium ions). Even disregarding those analytes that must be maintained within narrow ranges to maintain homeostasis, the CVI values for most serum analytes used to assess health status are substantially lower than the values reported in the current study for these salivary analytes. The values obtained in the current study are approximately two to 50 times greater than those of common serum analytes. This was confirmed by the observed ranges of CVI (within-subject or intraindividual coefficient of variation) values for the analytes.
Generally, there is much more variability observed for the salivary analytes in our study than is usually seen in reference databases for the serum analytes. However, direct comparisons are not possible for most of the markers we studied because serum reference ranges are not available for most of the analytes we examined in the current study, with the exception of albumin. There is a good deal of data on the CVI of albumin in serum because plasma albumin is commonly assessed in clinical laboratory diagnosis. 32 The within-subject variability in serum albumin samples is given as 3.1% in the database compiled by Ricós et al.33 This is in contrast to the albumin in the current study, which varied from 11.12% to 150.70%. Of the 30 subjects in the current study, the observed CVI values for albumin were >20% in 93% of the subjects. Reference serum albumin values also were recently reported for various disease states, with CVI values ranging from 2.8% in patients with type I diabetes to 6.7% in patients after myocardial infarction.31 These are all considerably lower than the values observed in our study. These findings suggest that, at least for this protein, a substantially greater natural within-subject variation in concentration occurs in saliva, in the absence of overt oral clinical changes in the subjects.
ICC is used todescribe the correlation between repeated measures in the same subject. The one-way random-effects model ANOVA can be used to estimate the ICC. In the current case, the model for log concentration for patient i at time j is expressed as: log concentrationij = β0 + αi + eij. The parameter αi represents random patient effects, assumed to be normal with a mean of zero and unknown variance σα 2. The parameter σα 2 measures the between-subject variability in mean assay concentration. The error terms are assumed to be normal with a mean of zero and unknown variance σ2. The parameter σ2 measures the within-subject variability in assay concentration. If this parameter is equal to 0, then all repeated measurements obtained for a given subject would be equal (i.e., the assay would have perfect repeatability). For a reproducible assay, the value of σ2 should be low relative to the between-subject variability (σα2).
Acommon parameter used to express this relationship is the ratio σ2/(σ2 + σα 2), which denotes the fraction of the total variability attributable to within-subject deviations in assay values. This parameter is equal to 1 − ICC. The results of this analysis for in-clinic and at-home samples are shown in Table 4.
Ideally, the ICC should equal 1 for a perfectly reproducible test. As the value of the ICC becomes smaller, the reproducibility of the test is lower, and the within-subject variability is greater. It was stated that acceptable reproducibility is assumed when the ICC is ≥0.60,34 a value that was not reached by any of the analytes in our study. The ICC values for the analytes in our study ranged from 0.39 to 0.59 for the inclinic samples and from 0.24 to 0.52 for the at-home samples.
The max/min ratio is also informative with regard to within-subject variation. Ninety-three percent of the subject-analyte combinations exhibited max/min ratios >2. It was suggested that the maximum allowable imprecision for log-Gaussian distributions is “dependent upon the ratio of the upper to lower reference limits. When this ratio is below 1.5, the goals are close to those proposed by Gowans for Gaussian distributions and can be considered valid. Fortunately, ratios below 1.5 are found for nearly all analytes.”35 None of the analytes measured in the current study met that criterion.
The at-home collection of salivary samples exhibited significantly more variability than did those obtained in the clinic under supervision. This may have been due to inconsistency in post-collection handling or to a lack of compliance with regard to pre-collection behavior (e.g., restrictions on oral hygiene behavior prior to collection). Because of the limited dataset, this is, at best, a very preliminary observation that will require a better understanding to consider the optimal implementation of this point-of-care strategy.
Our findings must be placed in the context of certain inherent limitations of the study. First, only healthy subjects were studied, which prevents a comparison of the ranges and any discrimination in levels that would be noted between healthy and diseased populations. Another limitation relates to the number of subjects studied. The sample size was small. However, this is reasonable, considering that this was designed as an exploratory study. Future studies will require larger sample sizes.
An additional limitation relates to the effect of preanalytical influences on observed within-subject variation. This category includes subject preparation (fasting state, degree of hydration, exercise, and use of caffeine or other stimulants) and sample collection and handling. It was shown that unstimulated salivary flow rate, salivary protein, and osmolality track hydration status.36,37 It was suggested that saliva viscosity may be an indicator of dehydration in the elderly. 38 The protocol was designed to reduce the preanalytical influences by collecting samples 1) from subjects who were not taking stimulants, diuretics, or drugs that cause xerostomia; 2) during the morning to account for potential circadian rhythms; and 3) after breakfast when subjects should have received sufficient hydration. Despite these precautions, it is inevitable that variations in hydration status, oral hygiene, and other factors may have caused transient changes in salivary flow rates and, thus, influenced analyte concentrations.
Although the importance of these factors is acknowledged, the use of salivary diagnostics in clinical medicine and research will likely occur in a context where such variables are difficult to control. Such constraints are seen with some other biomedical assays that require certain preanalytical behavior on the part of the patient/subject (e.g., fasting plasma glucose); therefore, they do not present insurmountable problems to the use of salivary analysis. Thus, some preanalytical variation is inherent in all salivary diagnostics tests.
CONCLUSIONS
Whole saliva collected over a 2-week period showed marked within-subject variation in the concentrations of IL-1β and −6, MMP-8, PGE2, TNF-α, IFN-α, and albumin in a group of periodontally and systemically healthy adult subjects. This variability seemed greater when collection was unsupervised. Before salivary diagnostics and point-of-care systems using saliva can be used as diagnostic and screening tools, the normal variability of salivary analytes needs to be considered to create accurate thresholds enabling the definition of abnormal values. Future investigations should include examination of additional analytes and establish this type of data for larger and more diverse populations to quantify the variability of salivary analytes, as was done with serum analytes.
Acknowledgments
This study was supported by a grant U01 DE017793, from the National Institutes of Health, Bethesda, Maryland, and funds from the Center for Oral Health Research, University of Kentucky College of Dentistry. The authors acknowledge the invaluable assistance of Mr. Jason Stevens, Center for Oral Health Research, University of Kentucky, in the laboratory analysis portion of the project.
Footnotes
PCP-UNC 15, Hu-Friedy, Chicago, IL.
SIGMAFAST, Sigma, St. Louis, MO.
Luminex, Invitrogen, Carlsbad, CA.
R&D Systems, Minneapolis, MN.
Assay Designs, Ann Arbor, MI.
Alpco Diagnostics, Salem, NH.
R 2.7.1 statistical package, R Foundation for Statistical Computing, Vienna, Austria.
S-PLUS 8.0, Insightful, Seattle, WA.
The authors report no conflicts of interest related to this study.
References
- 1.Al-Hashimi I, Haghighat N, Fox PC. Salivary electrophoresis in the diagnosis of Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:542–547. doi: 10.1016/s1079-2104(98)90288-8. [DOI] [PubMed] [Google Scholar]
- 2.Borrow R, Fox AJ, Cartwright K, Begg NT, Jones DM. Salivary antibodies following parenteral immunization of infants with a meningococcal serogroup A and C conjugated vaccine. Epidemiol Infect. 1999;123:201–208. doi: 10.1017/s0950268899002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso EM, Arregger AL, Tumilasci OR, Contreras LN. Diagnostic value of salivary cortisol in Cushing’s syndrome. Clin Endocrinol (Oxf) 2009;70:516–521. doi: 10.1111/j.1365-2265.2008.03381.x. [DOI] [PubMed] [Google Scholar]
- 4.Deutschbein T, Unger N, Mann K, Petersenn S. Diagnosis of secondary adrenal insufficiency in patients with hypothalamic-pituitary disease: Comparison between serum and salivary cortisol during the high-dose short synacthen test. Eur J Endocrinol. 2009;160:9–16. doi: 10.1530/EJE-08-0600. [DOI] [PubMed] [Google Scholar]
- 5.Nunes ML, Vattaut S, Corcuff JB, et al. Late-night salivary cortisol for diagnosis of overt and subclinical Cushing’s syndrome in hospitalized and ambulatory patients. J Clin Endocrinol Metab. 2009;94:456–462. doi: 10.1210/jc.2008-1542. [DOI] [PubMed] [Google Scholar]
- 6.Shibayama Y, Higashi T, Shimada K, et al. Simultaneous determination of salivary testosterone and dehydroepiandrosterone using LC-MS/MS: Method development and evaluation of applicability for diagnosis and medication for late-onset hypogonadism. J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Nov 5; doi: 10.1016/j.jchromb.2008.10.051. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Carroll T, Raff H, Findling JW. Late-night salivary cortisol measurement in the diagnosis of Cushing’s syndrome. Nat Clin Pract Endocrinol Metab. 2008;4:344–350. doi: 10.1038/ncpendmet0837. [DOI] [PubMed] [Google Scholar]
- 8.Kim TH, Lee KJ, Yeo M, Kim DK, Cho SW. Pepsin detection in the sputum/saliva for the diagnosis of gastroesophageal reflux disease in patients with clinically suspected atypical gastroesophageal reflux disease symptoms. Digestion. 2008;77:201–206. doi: 10.1159/000143795. [DOI] [PubMed] [Google Scholar]
- 9.Mantella RC, Butters MA, Amico JA, et al. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33:773–781. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houali K, Wang X, Shimizu Y, et al. A new diagnostic marker for secreted Epstein-Barr virus encoded LMP1 and BARF1 oncoproteins in the serum and saliva of patients with nasopharyngeal carcinoma. Clin Cancer Res. 2007;13:4993–5000. doi: 10.1158/1078-0432.CCR-06-2945. [DOI] [PubMed] [Google Scholar]
- 11.Samaranayake L. Saliva as a diagnostic fluid. Int Dent J. 2007;57:295–299. doi: 10.1111/j.1875-595x.2007.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 12.Delpisheh A, Kelly Y, Rizwan S, Brabin BJ. Salivary cotinine, doctor-diagnosed asthma and respiratory symptoms in primary schoolchildren. Matern Child Health J. 2008;12:188–193. doi: 10.1007/s10995-007-0229-9. [DOI] [PubMed] [Google Scholar]
- 13.Fu E, Chinowsky T, Nelson K, et al. SPR imaging-based salivary diagnostics system for the detection of small molecule analytes. Ann N Y Acad Sci. 2007;1098:335–344. doi: 10.1196/annals.1384.026. [DOI] [PubMed] [Google Scholar]
- 14.Helmerhorst EJ. Whole saliva proteolysis: Wealth of information for diagnostic exploitation. Ann N Y Acad Sci. 2007;1098:454–460. doi: 10.1196/annals.1384.013. [DOI] [PubMed] [Google Scholar]
- 15.Malamud D. Salivary diagnostics: The future is now. J Am Dent Assoc. 2006;137:284, 286. doi: 10.14219/jada.archive.2006.0158. [DOI] [PubMed] [Google Scholar]
- 16.Wong DT. Salivary diagnostics powered by nanotechnologies, proteomics and genomics. J Am Dent Assoc. 2006;137:313–321. doi: 10.14219/jada.archive.2006.0180. [DOI] [PubMed] [Google Scholar]
- 17.Herr AE, Hatch AV, Throckmorton DJ, et al. Micro-fluidic immunoassays as rapid saliva-based clinical diagnostics. Proc Natl Acad Sci USA. 2007;104:5268–5273. doi: 10.1073/pnas.0607254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis – A review. J Clin Periodontol. 2000;27:453–465. doi: 10.1034/j.1600-051x.2000.027007453.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman E, Lamster IB. The diagnostic applications of saliva – A review. Crit Rev Oral Biol Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 20.Lamster IB, Kaufman E, Grbic JT, Winston LJ, Singer RE. Beta-glucuronidase activity in saliva: Relationship to clinical periodontal parameters. J Periodontol. 2003;74:353–359. doi: 10.1902/jop.2003.74.3.353. [DOI] [PubMed] [Google Scholar]
- 21.Fox PC. Salivary monitoring in oral diseases. Ann N Y Acad Sci. 1993;694:234–237. doi: 10.1111/j.1749-6632.1993.tb18356.x. [DOI] [PubMed] [Google Scholar]
- 22.Christodoulides N, Floriano PN, Miller CS, et al. Labon-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci. 2007;1098:411–428. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- 23.Frodge BD, Ebersole JL, Kryscio RJ, Thomas MV, Miller CS. Bone remodeling biomarkers of periodontal disease in saliva. J Periodontol. 2008;79:1913–1919. doi: 10.1902/jop.2008.080070. [DOI] [PubMed] [Google Scholar]
- 24.Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: A cross-sectional study. J Am Dent Assoc. 2006;137:322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- 25.Van den Bruel A, Cleemput I, Aertgeerts B, Ramaekers D, Buntinx F. The evaluation of diagnostic tests: Evidence on technical and diagnostic accuracy, impact on patient outcome and cost-effectiveness is needed. J Clin Epidemiol. 2007;60:1116–1122. doi: 10.1016/j.jclinepi.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–77. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 27.Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making. 1991;11:88–94. doi: 10.1177/0272989X9101100203. [DOI] [PubMed] [Google Scholar]
- 28.Van den Bruel A, Aertgeerts B, Buntinx F. Results of diagnostic accuracy studies are not always validated. J Clin Epidemiol. 2006;59:559–566. doi: 10.1016/j.jclinepi.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Sackett DL, Haynes R, Guyatt G, Tugwell P. The selection of diagnostic tests. In: Sackett DL, Haynes R, Guyatt G, Tugwell P, editors. Clinical Epidemiology: A Basic Science for Clinical Medicine. Vol. 58 Boston: Little, Brown, and Company; 1991. [Google Scholar]
- 30.Fraser CG. Biologic Variation: From Principles to Practice. Vol. 9 Washington, DC: American Academy for Clinical Chemistry Press; 2001. [Google Scholar]
- 31.Ricós C, Iglesias N, García-Lario JV, et al. Within-subject biological variation in disease: Collated data and clinical consequences. Ann Clin Biochem. 2007;44:343–352. doi: 10.1258/000456307780945633. [DOI] [PubMed] [Google Scholar]
- 32.Ricós C, Alvarez V, Cava F, et al. Current databases on biological variation: Pros, cons and progress. Scand J Clin Lab Invest. 1999;59:491–500. doi: 10.1080/00365519950185229. [DOI] [PubMed] [Google Scholar]
- 33.Ricós C, Cava F, García-Lario JV, et al. The reference change value: A proposal to interpret laboratory reports in serial testing based on biological variation. Scand J Clin Lab Invest. 2004;64:175–184. doi: 10.1080/00365510410004885. [DOI] [PubMed] [Google Scholar]
- 34.Chinn S. Statistics in respiratory medicine. 2. Repeatability and method comparison. Thorax. 1991;46:454–456. doi: 10.1136/thx.46.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricós C, Doménech MV, Perich C. Analytical quality specifications for common reference intervals. Clin Chem Lab Med. 2004;42:858–862. doi: 10.1515/CCLM.2004.140. [DOI] [PubMed] [Google Scholar]
- 36.Oliver SJ, Laing SJ, Wilson S, Bilzon JL, Walsh NP. Saliva indices track hypohydration during 48h of fluid restriction or combined fluid and energy restriction. Arch Oral Biol. 2008;53:975–980. doi: 10.1016/j.archoralbio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Walsh NP, Laing SJ, Oliver SJ, Montague JC, Walters R, Bilzon JL. Saliva parameters as potential indices of hydration status during acute dehydration. Med Sci Sports Exerc. 2004;36:1535–1542. doi: 10.1249/01.mss.0000139797.26760.06. [DOI] [PubMed] [Google Scholar]
- 38.Yoshihara A, Hirotomi T, Takano N, Kondo T, Hanada N, Miyazaki H. Serum markers of chronic dehydration are associated with saliva spinability. J Oral Rehabil. 2007;34:733–738. doi: 10.1111/j.1365-2842.2007.01732.x. [DOI] [PubMed] [Google Scholar]