Abstract
We have previously shown that neonate rabbit tubules have a lower chloride permeability but comparable mannitol permeability compared with adult proximal tubules. The surprising finding of lower chloride permeability in neonate proximals compared with adults impacts net chloride transport in this segment, which reabsorbs 60% of the filtered chloride in adults. However, this maturational difference in chloride permeability may not be applicable to other species. The present in vitro microperfusion study directly examined the chloride and mannitol permeability using in vitro perfused rat proximal tubules during postnatal maturation. Whereas there was no maturational change in mannitol permeability, chloride permeability was 6.3 ± 1.3 × 10−5 cm/s in neonate rat proximal convoluted tubule and 16.1 ± 2.3 × 10−5 cm/s in adult rat proximal convoluted tubule (P < 0.01). There was also a maturational increase in chloride permeability in the rat proximal straight tubule (5.1 ± 0.6 × 10−5 cm/s vs. 9.3 ± 0.6 × 10−5 cm/s, P < 0.01). There was no maturational change in bicarbonate-to-chloride permeabilities (PHCO3/PCl) in the rat proximal straight tubules (PST) and proximal convoluted tubules (PCT) or in the sodium-to-chloride permeability (PNa/PCl) in the proximal straight tubule; however, there was a significant maturational decrease in proximal convoluted tubule PNa/PCl with postnatal development (1.31 ± 0.12 in neonates vs. 0.75 ± 0.06 in adults, P < 0.001). There was no difference in the transepithelial resistance measured by current injection and cable analysis in the PCT, but there was a maturational decrease in the PST (7.2 ± 0.8 vs. 4.6 ± 0.1 Ω· cm2, P < 0.05). These studies demonstrate there are maturational changes in the rat paracellular pathway that impact net NaCl transport during development.
Keywords: paracellular pathway, mannitol permeability
The adult proximal tubule reabsorbs over half of the filtered chloride (2, 5, 6, 18, 27, 28). Active transcellular chloride is via the parallel operation of the Na+/H+ exchanger and a Cl/base exchanger (5, 27). The other half of chloride transport by the proximal tubule is passive, paracellular, and in large part, dependent on the chloride permeability. Thus the characteristics of the paracellular pathway can have a substantive effect on NaCl transport by the proximal tubule.
Previous studies examining the maturational changes in paracellular permeability have been conflicting. In a study (17) where solutes were injected into the early proximal tubule of guinea pigs and the urine was collected from the ipsilateral kidney, recovery of sucrose and creatinine did not vary with age and were comparable to inulin. However, mannitol recovery increased from 92% at 1 day of age to 100% at 49 days of age (17). Whereas these studies were indirect and assessed mannitol absorption, a sugar that is not actively transported along the entire nephron, these data were consistent with a more permeable paracellular pathway in neonates than in adults.
We have examined the permeability properties of the rabbit proximal tubule (23, 29). We found that the neonate had lower proximal tubule chloride permeability, but there was no maturational difference in the mannitol permeability. In addition, neonate proximal tubules had a higher sodium-to-chloride permeability (PNa/PCl) and bicarbonate-to-chloride permeabilities (PHCO3/PCl) than that of the adult proximal tubule. These studies are consistent with a maturational change in passive paracellular ion permeability, which could affect NaCl transport.
Our previous studies were performed using rabbit proximal tubules, because, traditionally, this has been the species used for the relative ease of dissection (23, 28). It is unclear whether the findings in our studies in the rabbit had any general applicability to rodents or other species. Furthermore, the rabbit has limitations in the study of renal development, because they do not survive adrenalectomy, a procedure often used in studying postnatal development (14, 15). In the present rat proximal tubule in vitro microperfusion flux study, we examine some of the developmental permeability properties in rats to determine whether our previous studies in rabbits have any applicability to other species.
MATERIALS AND METHODS
Animals
Sprague-Dawley rats were studied between 11–60 days of age. Neonate rats (11–18 days of age) were studied before weaning, and adult rats were all >30 days of age. Neonate rats were cared for by their mothers. This species was not used previously for in vitro proximal tubule microperfusion flux studies because of the fibrous interstitial tissue making dissection extremely difficult. However, the amount of interstitial fibrosis increases substantively as rats age and proximal tubules can be dissected in rats younger than 2 mo.
In vitro microperfusion flux studies
Isolated segments of midcortical and juxtamedullary proximal convoluted tubules (PCT) and proximal straight tubules (PST) were perfused as previously described for mice and rabbits (6, 7, 22, 26, 28, 29). Briefly, tubules were dissected in HBSS containing (in mM) 137 NaCl, 5 KCl, 0.8 MgSO4, 0.33 Na2HPO4, 0.44 KH2PO4, 1 MgCl2, 10 Tris (hydroxymethyl) aminomethane hydrochloride, 0.25 CaCl2, 2 glutamine, and 2 L-lactate at 4°C without the use of collagenase. Tubules were transferred to a 1.2 ml temperature-controlled bath. The tubules were perfused using concentric glass pipettes at 38°C.
PCT were perfused at ~5 nl/min. Perfusion solutions and bathing solutions are described below. The osmolality of all solutions was adjusted to 295 mosmol/kgH2O. The pH and osmolality of the bathing solution were maintained constant by continuously changing the bath at a rate of 0.5 ml/min. Net volume absorption (JV, in nl/mm−1 · min−1) was measured as the difference between the perfusion (VO) and collection (VL) rates (nl/min) normalized per millimeter of tubular length (L). Exhaustively dialyzed [methoxy-3H]inulin was added to the perfusate at a concentration of 75 μCi/ml so that the perfusion rate could be calculated. The collection rate was measured with a 50-nl constant-volume pipette. The length (in mm) and internal diameter (in μm) were measured with an eyepiece micrometer. Tubules were incubated for at least 15 min before initiation of the control period.
In the first series of experiments, we examined mannitol permeability (Pmann) in neonate and adult rat PCT. PCT were perfused with a solution containing (in mM) 10 mannitol, 110 NaCl, 30 Na gluconate, 5 NaHCO3, 5 KCl, 1 Na2HPO4, 1.8 CaCl2, 1 MgSO4, 1 acetazolamide, and [14C]mannitol (15 μCi/ml). The bathing solution was a serum-like albumin solution containing (in mM) 115 NaCl, 25 NaHCO3, 2.3 Na2HPO4, 10 Na acetate, 1.8 mM CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, 5 alanine, and 6 g/dl BSA heated to 38°C. We previously showed that under these conditions the rate of volume absorption was not different from zero (8, 22, 26). Pmann was calculated as previously described in our laboratory (22, 26).
In the next series of experiments, we examined chloride permeability in neonate and adult proximal convoluted and straight tubules. Tubules were perfused at ~5 nl/min with a high-chloride solution simulating late proximal tubular fluid containing (in mM) 140 NaCl, 5 NaHCO3, 5 KCl, 4 Na2HPO4, 1 CaCl2, and 1 MgSO4 and bathed in an identical solution. Tubules were cooled to 18°C to inhibit active transport. Lumen-to-bath chloride permeability was determined by the addition of 36Cl to the tubular lumen (50 μCi/ml) using the following equation
where A is the area of the luminal area calculated from the internal radius and length, and are the concentration of 36Cl (in counts/min−1 · nl−1) in the perfusate and bath, and VO and VL are the perfusion and collection rates.
In the next series of experiments, we measured the relative PNa/PCl and PHCO3/PCl in proximal convoluted and straight tubules from rats. PNa/PCl and PHCO3/PCl were calculated from the passive transepithelial potential difference due to imposed ion concentration gradients exactly as previously described in our laboratory (8, 23) and using similar methodology as used by others (10, 23, 31). The transepithelial potential difference (PD; in millivolts) was measured using the perfusion pipette as the bridge into the tubular lumen. All bathing solutions contained 10−4 M ouabain to inhibit any transepithelial potential difference generated from active transport. Tubules were perfused with an ultrafiltrate-like solution. The composition of the bathing solutions used to measure dilution potentials are shown in Table 1.
Table 1.
Solutions used to measure and PNa/PCl and PHCO3/PCl
| Ultrafiltrate-like Solution | NaCl Dilution | NaHCO3 Dilution | |
|---|---|---|---|
| NaCl | 104 | 54 | 104 |
| NaHCO3 | 25 | 25 | 5 |
| Na2HPO4 | 4 | 4 | 4 |
| NaAc | 7.5 | 7.5 | 7.5 |
| CaCl2 | 1 | 1 | 1 |
| MgSO4 | 1 | 1 | 1 |
| KCl | 5 | 5 | 5 |
| Glucose | 5 | 5 | 5 |
| Alanine | 5 | 5 | 5 |
| Urea | 5 | 5 | 5 |
| Mannitol | * | * |
Values are in millimolars.
Mannitol was added to increase the osmolality to 295 mosmol/kgH2O.
Measurement of transepithelial resistance
Specific resistance (Rm) was measured as described by Berry (9) and as we (23) have previously reported. Briefly, proximal convoluted and straight tubules from adult and neonate rats were perfused and bathed with HBSS at 38°C. Rat PCT were perfused with a double-barreled pipette made from theta glass (Hilgenberg Glass, Hilgenberg, Germany). One barrel of the pipette was used to measure the potential difference at the perfusion end (PD0) and the other was for current injection (30 – 60 nA) with the use of a Grass S44 stimulator (Grass Instruments, Quincy, Mass) via silver wire. The current injected was measured with a Keithley 617 programmable electrometer (Keithley Instruments, Cleveland, OH). A KCl/KNO3 agarose bridge was also placed in the collecting end to measure the voltage deflection at the distal end of the rat tubule (PDL). The PD measurements were recorded on a Linseis, L6512B (Linseis, Princeton, NJ) two-channel chart recorder. The coupling resistance was determined by measuring the voltage deflections at the perfusion and collection ends with no tubule present. These voltage deflections were subtracted from the readings with the perfused tubule in place and are represented in the equations below by ΔPD0 and ΔPDL for the corrected voltage deflection at the perfusion and collection ends, respectively.
Cable analysis was used to calculate the length constant (λ, micrometers), input resistance (Ri, ohms), transepithelial resistance (RT, ohm centimeter), and the specific resistance (Rm, Ω · cm2) according to the following equations (9, 20, 23)
where L is the tubule length, ΔPD0 and ΔPDL are the corrected voltage deflections at the perfusion and collection ends, I0 is the input current, and ρ is the solution resistivity. The ρ was measured using a conductance meter (Yellow Springs Instruments, Yellow Springs, OH) and was found to be 63.3 Ω· cm2 at 38°C.
There were at least three measurements of each parameter in each period in flux studies. The mean measurement of volume absorption and permeability was used as the rate or permeability for that tubule. Student’s t-test for unpaired data was used to determine statistical significance. Data is expressed as means ± SE.
RESULTS
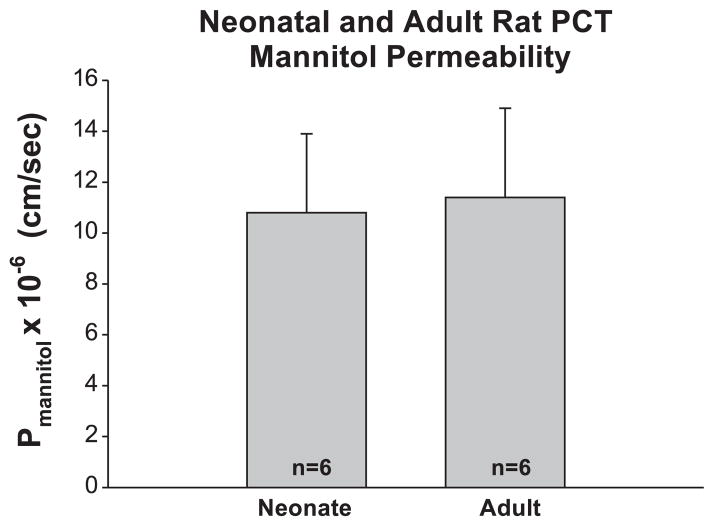
In the first series of experiments, we examined mannitol permeability in neonate and adult rat PCT. Mannitol is a sugar that is neither metabolized nor actively transported by the proximal tubule. The mean tubular lengths were 0.5 ± 0.1 and 0.7 ± 0.1 mm in adult and neonate groups, respectively (P = ns). The perfusion solution was designed to produce a volume absorption rate of zero to eliminate transport by solvent drag in tubules bathed in a serum-like albumin solution (26). The rate of volume absorption was −0.05 ± 0.05 and 0.01 ± 0.03 nl/mm−1· min−1 in the adult rat and neonate rats, respectively (P = ns). The mannitol permeabilities in the two groups are shown in Fig. 1. As can be seen, mannitol permeability in the adult and neonate groups was not different.
Fig. 1.
Mannitol permeability (Pmannitol) in neonate and adult perfused proximal convoluted tubules (PCT). Tubules were perfused with a solution to yield a net volume absorption (Jv) of zero so there would be no solvent drag and all transport would be passive. As can be seen, there is no maturational change in Pmannitol in rat PCT.
In the next series of experiments, we examined whether there was a maturational change in chloride permeability in rat PCT. Neonate tubules were slightly longer than adult tubules (0.7 ± 0.1 vs. 0.5 ± 0.1 mm, P < 0.05). Neonate PCT was perfused and bathed in identical solutions at 18°C. Neonate PCT had a Jv of 0.09 ± 0.07 nl/mm−1· min−1 and adult PCT had a Jv of −0.04 ± 0.10 nl/mm−1· min−1, both not different from zero. The 36Cl permeabilities, however, were significantly different as shown in Fig. 2. Adult PCT had a chloride permeability far greater than that in neonates (P < 0.01).
Fig. 2.
A: 36Cl permeability in neonate and adult perfused PCT. Tubules were perfused and bathed in a high Cl solution simulating late proximal tubular fluid at 18°C to inhibit active transport. PCl was significantly higher in adult PCT than in neonates. B: 36Cl permeability in neonate and adult perfused proximal straight tubules (PST). PCl was significantly higher in adult PST than in neonates.
We also measured chloride permeability in neonate and adult PST. We studied six neonate and eight adult tubules. Neonates and adult PST had a length of 0.5 ± 0.1 mm. The rate of volume absorption was 0.16 ± 0.13 nl/mm−1· min−1 in neonate tubules and 0.02 ± 0.07 nl/mm−1· min−1 in adult PST, both not different from zero. Chloride permeability was 5.1 ± 0.6 × 10−5 cm/s in the neonate and 9.3 ± 0.6 × 10−5 cm/s in the adult proximal straight tubule (P < 0.01). Thus there is a maturational increase in chloride permeability in this segment as in the convoluted tubule.
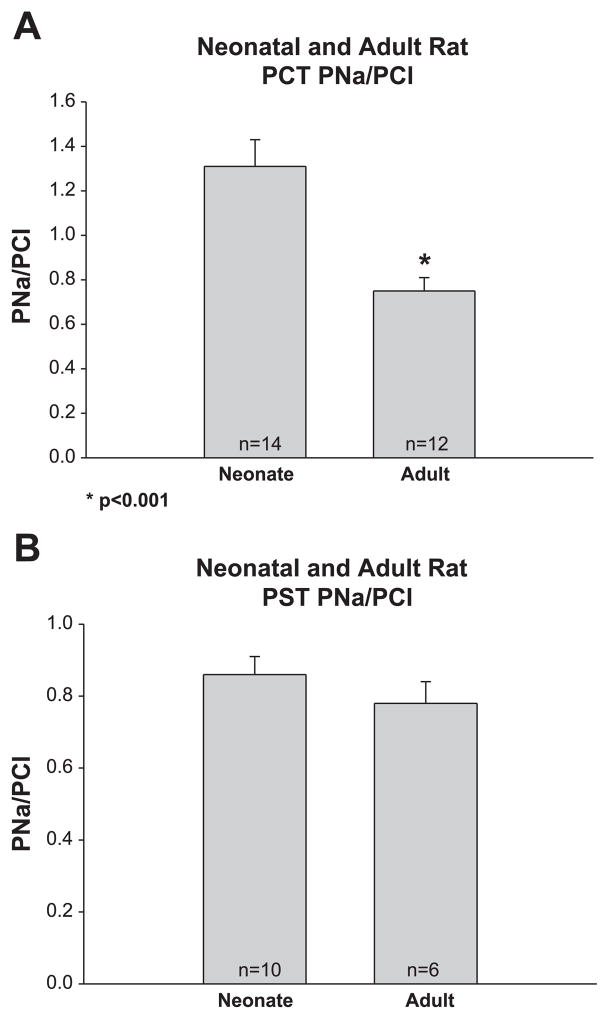
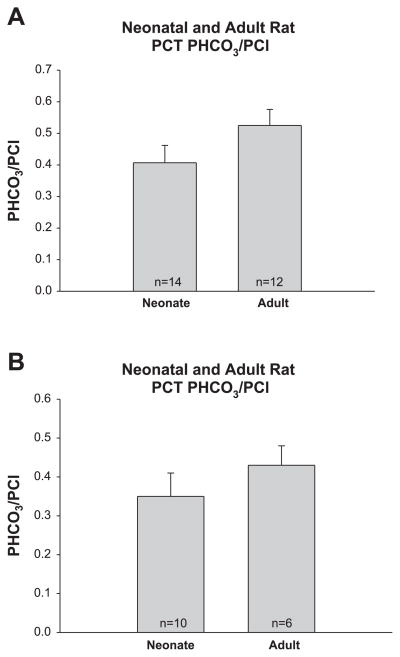
In the next series of experiments, we measured the potential difference generated by imposed ionic gradients to determine the PNa/PCl in neonate and adult PCT. As shown in Fig. 3 the PNa/PCl was almost twofold greater in the neonate than that of the adult proximal convoluted tubule, whereas there was no difference in the PNa/PCl in the proximal straight tubule. There was no difference in PHCO3/PCl in neonate and adult proximal convoluted and straight tubules as shown in Fig. 4.
Fig. 3.
A: PNa/PCl in neonate and adult PCT. PNa/PCl was measured using dilution potentials with bathing solutions that contained 10−4 M ouabain to inhibit active transport. PNa/PCl was significantly higher in neonate than adult tubules as has previously been found in the rabbit. B: PNa/PCl in neonate and adult PST. PNa/PCl was comparable in neonate and adult PST.
Fig. 4.
A: bicarbonate-to-chloride permeabilities (PHCO3/PCl) in neonate and adult PCT. PHCO3/PCl was measured using dilution potentials with bathing solutions that contained 10−4 M ouabain to inhibit active transport. PHCO3/PCl was comparable in neonate and adult rat PCT. B: PHCO3/PCl in neonate and adult PST. PHCO3/PCl was comparable in neonate and adult rat PST.
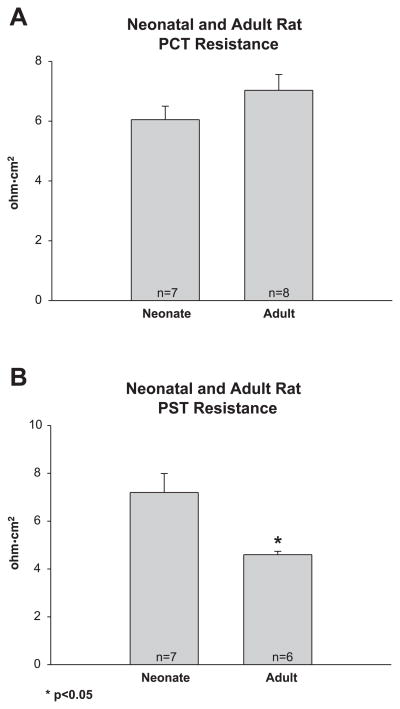
In the final series of experiments, we used current injection and cable analysis to measure transepithelial resistance. As shown in Fig. 5, there was no difference in the resistance in neonate and adult PCT. However, the resistance of the neonate proximal straight tubule was greater than that of the adult segment.
Fig. 5.
A: transepithelial resistance in neonate and adult PCT. Transepithelial resistance was measured using current injection and cable analysis. There was no difference in transepithelial resistance between adults and neonate tubules. B: transepithelial resistance in neonate and adult PST. Neonate PST had a higher transepithelial resistance than adult tubules.
DISCUSSION
The proximal tubule receives the glomerular ultrafiltrate and reabsorbs two-thirds of this fluid in a nearly isosmotic fashion. There is preferential reabsorption of organic solutes and bicarbonate over chloride ions, which leaves the late proximal tubule fluid with a composition significantly different from the initial ultrafiltrate (19, 24). The luminal fluid delivered to the late proximal tubule has a higher chloride concentration and lower bicarbonate concentration than the peritubular plasma.
This transport process provides the opportunity for passive chloride transport along the full length of the proximal tubule. The early proximal tubule has a lumen negative potential difference generated from sodium-dependent active reabsorption of glucose. This leaves the lumen with a negative potential difference and a driving force for either chloride absorption or sodium secretion across the paracellular pathway. The relative paracellular flux is dependent on the PNa/PCl. The higher the PNa/PCl, the more sodium will be recycled and the less net NaCl chloride will be reabsorbed. The neonate rat proximal convoluted tubule has a higher PNa/PCl, which does not facilitate passive chloride transport to the same extent as that of the adult tubule by this mechanism.
The late proximal tubule also has the potential for passive chloride transport due to the fact that the luminal chloride concentration is higher than that of the peritubular plasma. The luminal bicarbonate concentration, on the other hand, is far lower than that in the peritubular plasma, which could result in net bicarbonate secretion. Factors that determine the relative fluxes are the relative concentration gradients and the relative permeabilities of these two ions. In both adults and neonate proximal convoluted and straight tubules, the PHCO3/PCl was far less than one indicating that chloride diffusion will occur to a far greater extent than back diffusion of bicarbonate. The net passive chloride flux is dependent on the chloride permeability. As in the rabbit, we find that the chloride permeability in both the rat proximal convoluted and straight tubule is less in the neonate than in adult (23, 27, 28).
In this study, we characterized the maturational changes that occur in the permeability properties of the rat proximal tubule. Our results in the rat proximal straight tubule differ somewhat from what we have described in this segment in the rabbit (23). Whereas there was a maturational increase in chloride permeability and decrease in tubular resistance in the proximal straight tubule of both species, the neonate rabbit proximal straight tubule had a higher PNa/PCl and higher PHCO3/PCl than the adult segment, whereas these differences were not seen in the rat. The PNa/PCl and PHCO3/PCl can affect passive solute flux as described above, and thus there are important species differences that could affect passive solute flux.
There were differences in the maturational changes that occurred in the rat proximal straight tubule and convoluted tubule. Whereas both segments showed a maturational increase in chloride permeability, only the proximal convoluted tubule had a difference in PNa/PCl during development. Surprisingly, there was a maturational decrease in the resistance only in the proximal straight tubule, despite changes in chloride permeability in both segments. The reason why there was a maturational change in the proximal tubular chloride permeability but not in resistance in the rat proximal convoluted tubule is not immediately apparent. There must be another ion or ions that account for the current flow in the resistance experiments in the PCT. Because the PNa/PCl in the PST is identical in the neonate and adult tubules, the neonate PNa is also likely lower in the neonate than the adult. Thus when resistance is measured, the resistance was found to be higher in the neonate tubules. However, in the PCT, the PNa/PCl was higher in the neonate tubules, indicating that although the PCl was lower in the neonate tubules, the PNa was probably not different between the neonate and adult tubules. Thus sodium may be the ion that carries the current in the PCT experiments, and, therefore, the resistance measurements were not different between the adult and neonate tubules.
This study, showing a maturational change in proximal tubule chloride permeability, may be of clinical significance. Premature neonates have significant renal salt wasting requiring NaCl supplements to prevent hyponatremia (1, 3, 4, 12). Because the adult proximal tubule reabsorbs one-third of the filtered chloride via passive diffusion across the paracellular pathway (24), a lower chloride permeability in the neonate proximal tubule may be a factor responsible for this renal salt wasting.
Kaskel et al. (17) have shown that there is no developmental difference in the width of the paracellular pathway or the length of the zona occludens. There was, however, an almost twofold maturational increase in the length of the paracellular pathway (17). These anatomical factors may indeed be relatively minor compared with other factors that affect ion transport. The gatekeepers of the paracellular pathway have been identified as the tight junction proteins occludin and a family of proteins called claudins. Claudin 2 and occludin have been identified as tight junction proteins in the proximal tubule (11, 13, 21, 25). Using immunoblot and immunohistochemistry, we have found in the rat that claudin 2 has a fourfold greater abundance in neonate proximal tubules than in adult tubules (16). Claudin 2 is a cation-selective claudin (30), which may account for the higher PNa/PCl measured in the PCT of neonates. Occludin, on the other had, has a fourfold greater expression in adult tubules than in neonate tubules (16). As speculation, it is possible that changes in claudin 2, occludin, or other proximal tubule tight junction proteins may account for the changes in paracellular properties found in this study.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-41612 (to M. Baum).
References
- 1.Al-Dahhan J. Sodium homeostasis in term and preterm neonates. Arch Dis Child. 1983;58:335–342. doi: 10.1136/adc.58.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpern RJ, Howlin KJ, Preisig PA. Active and passive components of chloride transport in the rat proximal convoluted tubule. J Clin Invest. 1985;76:1360–1366. doi: 10.1172/JCI112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aperia A, Broberger O, Elinder G, Herin P, Zetterstrom R. Postnatal development of renal function in pre-term and full-term infants. Acta Paediatr Scand. 1981;70:183–187. doi: 10.1111/j.1651-2227.1981.tb05539.x. [DOI] [PubMed] [Google Scholar]
- 4.Aperia A, Broberger O, Thodenius K, Zetterstrom R. Renal response to an oral sodium load in newborn full-term infants. Acta Paediatr Scand. 1972;61:670–676. doi: 10.1111/j.1651-2227.1972.tb15965.x. [DOI] [PubMed] [Google Scholar]
- 5.Aronson PS, Giebisch G. Mechanisms of chloride transport in the proximal tubule. Am J Physiol Renal Physiol. 1997;273:F179–F192. doi: 10.1152/ajprenal.1997.273.2.F179. [DOI] [PubMed] [Google Scholar]
- 6.Baum M, Berry CA. Evidence for neutral transcellular NaCl transport and neutral basolateral chloride exit in the rabbit convoluted tubule. J Clin Invest. 1984;74:205–211. doi: 10.1172/JCI111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum M, Loleh S, Saini N, Seikaly M, Dwarakanath V, Quigley R. Correction of proximal tubule phosphate transport defect in Hyp mice in vivo and in vitro with indomethacin. Proc Natl Acad Sci USA. 2003;100:11098–11103. doi: 10.1073/pnas.1834060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baum M, Quigley R. Thyroid hormone modulates rabbit proximal straight tubule paracellular permeability. Am J Physiol Renal Physiol. 2004;286:F477–F482. doi: 10.1152/ajprenal.00248.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry CA. Lack of effect of peritubular protein on passive NaCl transport in the rabbit proximal tubule. J Clin Invest. 1983;71:268–281. doi: 10.1172/JCI110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry CA, Rector FC., Jr Relative sodium-to-chloride permeability in the proximal convoluted tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1978;235:F592–F604. doi: 10.1152/ajprenal.1978.235.6.F592. [DOI] [PubMed] [Google Scholar]
- 11.Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol. 2001;281:F966–F974. doi: 10.1152/ajprenal.2001.281.5.F966. [DOI] [PubMed] [Google Scholar]
- 12.Engelke S. Sodium balance in very low-birth-weight infants. J Pediatr. 1978;93:837–841. doi: 10.1016/s0022-3476(78)81097-x. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S, Valencia L, Muriel P, Ponce L, Reyes JL. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int. 2000;57:2386–2402. doi: 10.1046/j.1523-1755.2000.00098.x. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Dwarakanath V, Baum M. Maturation of the Na/H antiporter (NHE3) in the proximal tubule of the hypothroid adrenalectomized rat. Am J Physiol Renal Physiol. 2004;287:F521–F527. doi: 10.1152/ajprenal.00005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta N, Tarif SR, Seikaly M, Baum M. Role of glucocorticoids in the maturation of the rat renal Na+/H+ antiporter (NHE3) Kidney Int. 2001;60:173–181. doi: 10.1046/j.1523-1755.2001.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddad M, Lin F, Dwarakanath V, Cordes K, Baum M. Developmental changes in proximal tubule tight junction proteins. Pediatr Res. 2005;57:453–457. doi: 10.1203/01.PDR.0000151354.07752.9B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaskel FJ, Kumar AM, Lockhart EA, Evan A, Spitzer A. Factors affecting proximal tubular reabsorption during development. Am J Physiol Renal Fluid Electrolyte Physiol. 1987;252:F188–F197. doi: 10.1152/ajprenal.1987.252.1.F188. [DOI] [PubMed] [Google Scholar]
- 18.Kokko JP, Baum M. Handbook of Physiology. Oxford University Press; 1992. Chloride transport; pp. 739–766. [Google Scholar]
- 19.Liu FY, Cogan MG. Axial heterogeneity in the rat proximal convoluted tubule. I. Bicarbonate, chloride, and water transport. Am J Physiol Renal Fluid Electrolyte Physiol. 1984;247:F816–F821. doi: 10.1152/ajprenal.1984.247.5.F816. [DOI] [PubMed] [Google Scholar]
- 20.Lutz MD, Cardinal J, Burg MB. Electrical resistance of renal proximal tubule perfused in vitro. Am J Physiol. 1973;225:729–734. doi: 10.1152/ajplegacy.1973.225.3.729. [DOI] [PubMed] [Google Scholar]
- 21.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quigley R, Baum M. Effects of epidermal growth factor and transforming growth factor-alpha on rabbit proximal tubule solute transport. Am J Physiol Renal Fluid Electrolyte Physiol. 1994;266:F459–F465. doi: 10.1152/ajprenal.1994.266.3.F459. [DOI] [PubMed] [Google Scholar]
- 23.Quigley R, Baum M. Developmental changes in rabbit proximal straight tubule paracellular permeability. Am J Physiol Renal Physiol. 2002;283:F525–F531. doi: 10.1152/ajprenal.00005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rector FC., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1983;244:F461–F471. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- 25.Reyes JL, Lamas M, Martin D, del Carmen NM, Islas S, Luna J, Tauc M, Gonzalez-Mariscal L. The renal segmental distribution of claudins changes with development. Kidney Int. 2002;62:476–487. doi: 10.1046/j.1523-1755.2002.00479.x. [DOI] [PubMed] [Google Scholar]
- 26.Salmon RF, Baum M. Intracellular cystine loading inhibits transport in the rabbit proximal convoluted tubule. J Clin Invest. 1990;85:340–344. doi: 10.1172/JCI114443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah M, Quigley R, Baum M. Maturation of rabbit proximal straight tubule chloride/base exchange. Am J Physiol Renal Physiol. 1998;274:F883–F888. doi: 10.1152/ajprenal.1998.274.5.F883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah M, Quigley R, Baum M. Maturation of proximal straight tubule NaCl transport: role of thyroid hormone. Am J Physiol Renal Physiol. 2000;278:F596–F602. doi: 10.1152/ajprenal.2000.278.4.F596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheu JN, Baum M, Bajaj G, Quigley R. Maturation of rabbit proximal convoluted tubule chloride permeability. Pediatr Res. 1996;39:308–312. doi: 10.1203/00006450-199602000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 31.Warnock DG, Yee VJ. Anion permeabilities of the isolated perfused rabbit proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol. 1982;242:F395–F405. doi: 10.1152/ajprenal.1982.242.4.F395. [DOI] [PubMed] [Google Scholar]