Abstract
A gene regulatory network was generated in the bacterium Enterococcus faecalis in order to understand how this organism can activate its expression under different copper concentrations. The topological evaluation of the network showed common patterns described in other organisms. Integrating microarray experiments allowed the identification of sub-networks activated under low (0.05 mM CuSO4) and high (0.5 mM CuSO4) copper concentrations. The analysis indicates the presence of specific functionally activated modules induced by copper, highlighting the regulons LysR, ArgR as global regulators and CopY, Fur and LexA as local regulators. Taking advantage of the fact that E. faecalis presented a homeostatic module isolated, we produced an in vivo intervention removing this system from the cell without affecting the connectivity of the global transcriptional network. This strategy led us to find that this bacterium can reconfigure its gene expression to maintain cellular homeostasis, activating new modules principally related to glucose metabolism and transcriptional processes. Finally, these results position E. faecalis as the organism having the most complete and controllable systemic model of copper homeostasis available to date.
Introduction
Based on its importance as a micronutrient and its toxic effects in excess, much is known about the copper homeostatic mechanisms involved in maintaining suitable concentrations inside the cell (Kim et al, 2008). However, microarray experiments show that more than 90% of the transcriptional changes induced by copper code for other cellular processes, mainly related to oxidative stress responses, protein synthesis, basal metabolism and energy generation (Gonzalez et al, 2008). These findings suggest that complex transcriptional responses are the result of a series of interaction cascades and biochemical reactions that lead to the coordination and regulation of gene expression (Balazsi & Oltvai, 2005), probably controlled by regulatory mechanisms activated by copper. To address this question from a systemic and integrative level, it is crucial to have first, an appropriate biological model and second, knowledge of the homeostatic mechanisms directly activated by the metal and their relationship with other processes. Once having those two pieces of information, it is possible to produce rapid and effective identification and interpretation of putative transcriptional networks activated by copper (Kitano, 2002).
In this context, the pathogenic bacterium E. faecalis confines its resistance to copper to essentially three genes (Reyes-Jara et al, 2010): copY (transcriptional regulator), copA (copper efflux ATPase) and copZ (chaperone), organized in a single transcriptional unit (the cop operon), described as the simplest homeostatic model to date (Reyes-Jara et al, 2010). In this work, we took advantage of this resistance mechanism, as well as the availability of microarray data where the bacteria are exposed to high and low copper concentrations (as well as another metals) (Abrantes et al, 2011; Lopez et al, 2012; Reyes-Jara et al, 2010) and chose E. faecalis to construct a new transcriptional regulatory model capable of integrating the information of different metal’s microarrays. This model led us to the identification of potential transcriptional regulons involved in the cellular adaptation to copper, highlighting common subnetworks activated under different experimental conditions. Finally, by removing the cop operon from the system, we determined how bacteria can transcriptionally induce new components, identifying the capacity of the bacteria to reconfigure its network activation with the purpose of supplementing the absence of copper homeostasis.
Results
Global gene regulatory network
Unlike other bacterial models, E. faecalis does not have a database or sufficient information regarding the regulons or transcription factor binding sites present in its genome. This led us to generate a new probabilistic weight matrix for E. faecalis using the procedure of Radionov et al., which has previously been successful in the identification of transcriptional factor binding sites in other organisms (Rodionov, 2007). Using this protocol, we constructed an E. faecalis global gene regulatory network composed of a total of 608 non-redundant putative binding sites (14 E. faecalis families of transcription factors represented) distributed into 451 operons (863 genes) (Figure 1).
Figure 1.
Global gene regulatory network model for E. faecalis. The graph contains 465 nodes connected by 608 edges (putative binding sites). Orange circles indicate transcriptional factor families. Node shape represents COG class classification. Colors show conservation of the network in the Lactobacillales order. Numbers denote the total elements in each group.
The sequences and distribution of the binding sites found display homology with their corresponding reported transcription factor families (supplementary figure 1, 2). This result suggests a high homology in gene connectivity inside its bacterial order. The analysis showed that more than 70% of the network interactions (operon and binding sites) are conserved with other Lactobacillales species, mainly with L. johnsonii and L. plantarum, a result expected by E. faecalis phylogenetic proximity to these two species (Reyes et al, 2006). Interestingly we identified a core of putative conserved interactions composed of 282 operons (present in more than 4 species). Inside this core, there are genes that encode proteins involved in specific and global metabolic pathways, primarily related with the transcriptional control of processes like sugar metabolism (FruR regulon). Also, the presence of this set of conserved interactions represents the highest in silico prediction confidence, suggesting an effective and reliable connection between the transcription factor and its corresponding target operons.
To determine the topological characteristics of the model, we analyzed classical connectivity parameters described in other biological networks. The in-degree followed the classical power law distribution, with γ equal to 3.17, a value expected for this kind of network (Albert, 2005). The promoter architecture resembles that which was previously described in E. coli (Balleza et al, 2009), where more than 80% of the promotors show a single type structure (only one binding site per operon). Although the out-degree distribution of the E. faecalis network did not fit perfectly within the classical power law, this same characteristic was also previously reported in the M. tuberculosis network (Balazsi et al, 2008). Related with the transcriptional factor connectivity, the hierarchical layout places the families LysR (general metabolism), ArgR (arginine metabolism) and CRP-FNR (general metabolism) as global regulators (Schroder & Tauch, 2010) which connect nearly 70% of the operons in the network.
In terms of topological patterns (Alon, 2007), the network contains autoregulatory systems (n = 6), chain regulation (n = 6), single input motifs (n = 10) and feed-forward loops (n = 6). Feed-forward loops were represented principally by the transcription factor PhoP (general stress control) and PurR (nucleic acid metabolism) which also show the most density overlapping inside the network, a characteristic that probably fine tunes the control of the gene expression (Janga & Collado-Vides, 2007).
A sub-network activated by copper
Our model describes a static transcriptional configuration of elements probably involved in the response to copper. Consequently, we cross referenced the network with microarray expression data of E. faecalis exposed to copper under two treatment conditions: low level 0.05 mM (Abrantes et al, 2011) and high level 0.5 mM (high level (Reyes-Jara et al, 2010) (Figure 2). The resulting sub-network activated by this metal covered 12% of the global network, maintaining the same ratio of the total activated genes in the microarray to the total genes encoded in the genome. The topological analysis indicated that both sub-networks (low and high) keep the same proprieties of the global network.
Figure 2.
Sub-networks activated by copper. The graph shows the global gene regulatory factors affected by copper exposure from the microarray data set previously generated for E. faecalis. The left panel represents data from copper treatments (0.05 and 0.5 mM of CuSO4. The right panel denotes specificity against iron (0.5 mM of FeSO4) and zinc (4 mM ZnSO4). Dashed lines indicate activated modules I (green), II (red) and III (blue). Node colors and shapes represent the expression of each operon in the microarrays experiments and COG functions, respectively.
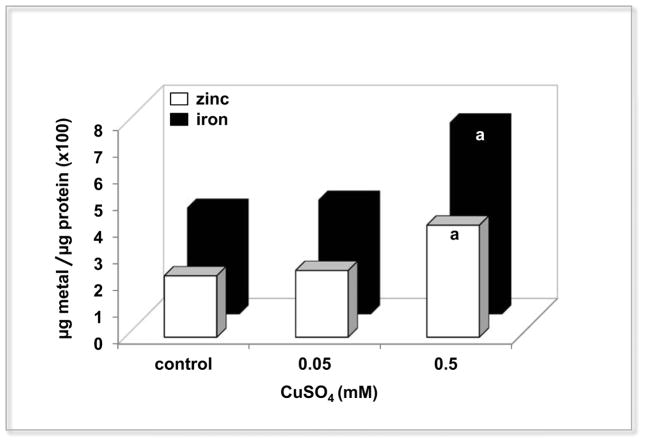
The operation of these two sub-networks can be understood in terms of the activation of three response modules: The first module (I) was activated under both copper conditions (up regulated, essentially confined to the cop operon). This module is isolated from the rest of the network, which gives a high specificity in response to copper, where the transcriptional factor CopY can directly bind copper atoms losing its affinity for the promoter activating the system (Solioz & Stoyanov, 2003). The second module (II) is the most represented in both sub-networks (connecting more than 80% of the operons). Genes in this module principally encode components directly involved in energy generation and synthesis of basic molecules, regulated by transcriptional factors ArgR, LysR and CRP-FNR. The high connectivity between its components described a complex regulation, reflected in the up and down regulation present in this module. These diverse transcriptional responses suggest that E. faecalis redirects some metabolic pathways with the objective of maintaining the cellular homeostasis, a phenotype observed in other organisms exposed to copper (Bundy et al, 2008). The last module (III) was activated only under the high levels of copper, and is involved in DNA damage (LexA regulon) and metal stress and homeostasis response (Fur regulon). The induction of LexA indicated that an excess of copper could result in the presence of oxidative stress damage. The up regulation of components related with iron (feo, fhu) and zinc (adc) uptake systems correlates directly with an increase in the intracellular content of these two metals only when the external copper concentration is 0.5 mM (Figure 3). Both micronutrients are probably needed as cofactors by oxidative stress enzymes activated by toxic levels of copper (Cornelis et al, 2011; Gaballa & Helmann, 2002).
Figure 3.
Intracellular iron and zinc content in E. faecalis exposed to copper. Data values represent the average of iron or zinc content exposed after 3 h to low (0.05 mM CuSO4) and high (0.5 mM CuSO4) levels of copper for three biological replicates with three technical absorbance measurement replicates. The letter “a” denotes a statistically significant difference between the control and the corresponding copper treatment (ANOVA test p <0.05).
Finally, the connectivity degree inside each module apparently depends on the protein function encoded. Specific functions (such as copper homeostasis, oxidative stress and iron acquisition) show a lower connectivity (isolated from the rest of the subnetwork), versus proteins related with global metabolism and energy production which present the highest transcriptional connectivity (LysR and ArgR regulons). The next step in our work was to verify the specificity of this activation. Figure 2 also shows the transcriptional response of both copper sub-networks under two other metals (0.5 mM FeSO4 and 4 mM of ZnSO4). Most of the regulons were not activated by iron or zinc, suggesting that the transcriptional families present in the sub-networks respond with a high specificity to copper. Furthermore, operons activated by copper, zinc and iron are controlled principally by LysR and ArgR transcriptional family members. These two families contain a high number of protein members encoded in the E. faecalis genome, which explains the ability of this module to respond to different stimuli. For the first time, we identified and characterized sub-networks of transcriptional interactions activated under two different conditions (low and high copper levels). Interestingly, the copper homeostasis is confined independently from the rest of the network. Taking advantage of this configuration, without affecting the global connectivity, we removed module I from the system (copΔ mutant strain), generating a new scenario that allowed us to understand how bacteria can reconfigure their network activation whose primary purpose is to control copper homeostasis under the same treatments.
Sub-network activated by copper in the absence of the homeostasis module
The absence of the cop operon (specifically the lack of CopY) showed virtually no effect on the basal global expression of E. faecalis growing in media without copper added. Only three genes significantly changed their transcriptional levels, none of which contained the CopY consensus binding site TACAxxTGTA in their promoter region (Magnani et al, 2008; Reyes et al, 2006). These results confirm the in silico prediction where the transcription factor CopY has a high specificity in the network, controlling only the expression of the cop operon. In terms of the physiology of the bacteria, presumably the absence of CopA (efflux ATPase) generates a basal increase in the intracellular copper content. However, non-significant changes were observed, results that indicate that the lack of the cop operon does not affect the copper homeostasis of E. faecalis under a basal condition (supplementary figure 3, 4).
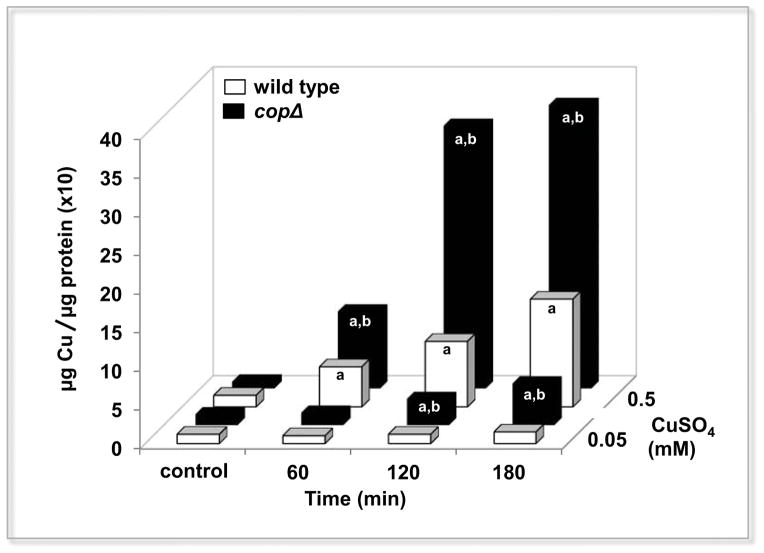
In terms of the total gene expression in the copΔ mutant exposed to copper, most of the activated genes did not respond in the wild type strain treated under the same conditions (Figure 4). Unlike the wild strain, the copΔ mutant did not activate genes which encode proteins involved in energy production (ATP), the substrate required for the correct operation of CopA. The copΔ mutant also shows down-regulation of the ABC transporters (sugars transport particularly) and up-regulation of the molybdenum system (mobABC); both systems are probably related to the copper uptake mechanism. When we analyze the intracellular copper content over time, the copΔ mutant accumulates more copper in relation to the wild type (Figure 5), reaching the highest values after two hours of high copper treatment (almost three times more than the wild type strain), theoretically showing the maximum intracellular copper concentration that E. faecalis can tolerate without affecting the viability of the bacteria (supplementary figure 5). These results indicate that the lack of cop operon generates a completely different transcriptional scenario under copper exposure, probably induced by the high copper accumulation.
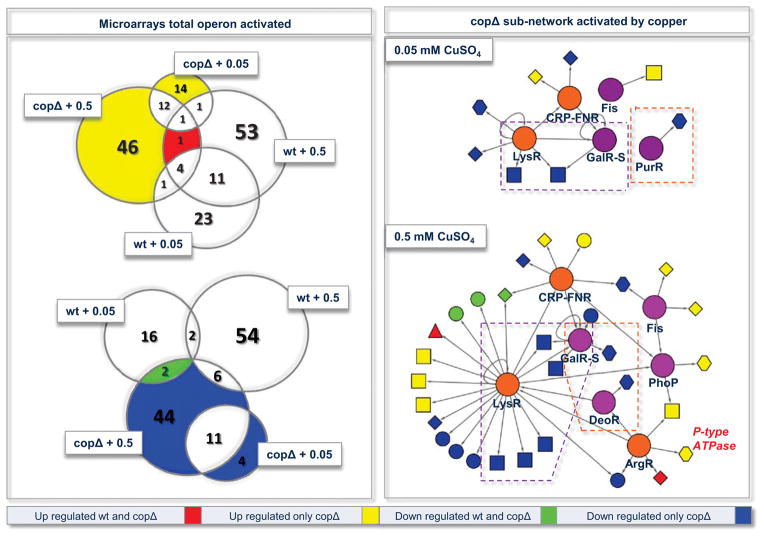
Figure 4.
Sub-networks activated by copper in the copΔ strain. The left panel shows the intersection between all the copper microarray experiments used in this work (Venn diagrams). The right panel is a connectivity graph showing what is activated by copper in the E. faecalis copΔ strain (low 0.05 and high 0.5 mM of CuSO4). Colors represent the intersection expression data between the wild type and copΔ microarray copper experiments. Transcriptional factors in orange and purple indicate common (also present in wild type sub-network) or new (only in copΔ) activated elements, respectively.
Figure 5.
Intracellular copper time course content in E. faecalis wild type and copΔ mutant strains exposed to copper. Values obtained at low (0.05 mM CuSO4) and high (0.5 mM CuSO4) levels of copper calculated at each time represent the average of three biological replicates with three technical replicate absorbance measurements. The letter a denotes statistically significant differences between the control and 0.05 mM or 0.5 mM of CuSO4 treatment, letter b denotes statistically significant differences between the wild type and copΔ at the same time exposure (ANOVA test p <0.05).
After characterizing the global transcriptional response of the copΔ strain exposed to copper, the next step was to identify the possible activated sub-networks. Figure 4 also shows two new groups of regulons not activated in the wild type strain. A fourth module (IV) composed of several new transcriptional factors; the present of new regulators besides increasing the copper signal, also redirects transcriptional pathways that could eventually impact the metabolism of the bacteria in order to adapt the system to internal high copper concentrations. Finally, a fifth module (V) was found to be associated with repression of the mechanism involved in sugar uptake (ABC transporters). Module V is controlled principally by the transcription factors GalR-S (galactose uptake transport), PurR (purine metabolism), and DeoR (sugar metabolism). The repression of this last module under high copper, suggest that sugar uptake systems are related to the metal resistance. To address this, we checked the E. faecalis copΔ mutant’s resistance to 1 mM of CuSO4 (toxic exposure level) under different sugar concentrations (Figure 6). The data show that a low concentration of sugar increases cellular viability, probably produced by a reduction in the copper content. This result describes a direct relationship between sugar availability and copper resistance. Accordingly, the down-regulation of a sugar ABC transporter suggests a direct or indirect participation of this system in the copper uptake process, which explains the reduction of the metal in a scenario lacking of sugar.
Figure 6.
Homeostatic response of the copΔ strain under different sugar concentrations exposed to toxic copper concentrations (1 mM CuSO4). A. Copper intracellular concentration. The letter “a” corresponds to statistically significant differences between the control and copper treatment, the letter “b” indicate statistically significant differences between both growth sugar conditions (ANOVA test p <0.05). B. Cellular viability as expressed as the percent of OD change between 1% glucose (used in the previous experiments) and the new glucose conditions. Each value represents the average of three biological replicates with three technical replicates. Black arrows indicate the exact point where the copper concentration was calculated.
Discussion
Analysis of gene regulatory networks has contributed to our knowledge about how organisms can transduce signals at a system level (Balazsi & Oltvai, 2005). To date, nothing has been known about the transcriptional regulatory mechanisms involved in the activation or repression of gene expression after copper exposure. In this context, the work presented here shows the first report of a global gene regulatory network able to integrate different copper gene expression data in the pathogen E. faecalis.
The global gene regulatory network allowed us to identify putative connections between transcriptional factors and operons encoded in E. faecalis. The model constructed demonstrated a high level of similarity with other bacterial networks, including a core of genes and connections highly conserved in other species within the Lactobacillales order, possibly acquired by the common ancestor of this order (Coenye & Vandamme, 2003). Most of the unique transcriptional interactions in the network (only present in E. faecalis) belong to hypothetical proteins (n = 398). These elements provide valuable information about putative unique transcriptional configurations, which probably contribute to the physiological differentiation between E. faecalis and the others species of the Lactobacillales order. A high number of transcriptional factors belonging to the families LysR, ArgR and CRP-FNR encoded in the E. faecalis genome maintain the correct operation of the network, ensuring the transcriptional control of several genes regulated by this group of global factors (Alon, 2007). The presence of putative origons, like CopY, DnaA (transcription initiation) and Fur, indicate that the system can directly transduce a stimulus without the presence of a second regulator or sub-network, establishing isolated units in the network conferring a highly specific response (Balazsi et al, 2008).
The transcriptional activation of the network allowed us to identify two subnetworks, whose operation depended principally on four modules of response activated according to the levels of copper. Low concentration of the metal (0.05 mM CuSO4) can be classified as elementary perturbation (Balazsi et al, 2005) because it induces a small numbers of genes, linked to the control of copper homeostasis (module I) and energy production (module II). In particular, the activation of module II (mainly linked with ATP production) suggests an energy requirement which explains the activation of the sensors LysR and ArgR, necessary for the operation of the module I (CopA ATPase copper efflux).
High levels of copper (0.5 mM CuSO4), on the other hand, can be classified as a complex response. Besides the activation of module I, the number of operons activated in module II increase more than two-fold compared to low copper exposure. This increase of activated elements suggests necessity of other substrates probably linked with the activation of module III (protection against oxidative stress). The induction of this module suggests that the network can respond to putative secondary effects induced by the metal, probably related with the generation of oxidative molecules.
According to this transcriptional response, we can classify the modules in three different ways: 1) independent: activated independently of the fluctuations of copper (module I); 2) dependent: activated under high concentration of the metal, probably linked to secondary effects (module III); and 3) mixed: activated dependent and independently of the availability of copper (module II). This transcriptional operation indicates that E. faecalis is able to adjust its gene regulatory machinery according to the concentrations of copper, describing at this level two thresholds of activation (high and low), characteristics that confer a highly specific response. This division in the transcriptional activation also has been observed in other models. By analyzing the putative dynamic response of the sub-network in the regulatory transcriptional network model of M. tuberculosis (Balazsi et al, 2008), the authors found that growth arrest can activate different regulons (early, intermediate and late), depending on the time course rather than the environmental conditions (hypoxia or stationary phase). In terms of copper, a similar response was previously described for homeostasis genes in E. coli. Beside the Cop proteins (primary mechanism), this bacterium contains other specific homeostatic mechanisms in which activation depends primarily on high copper concentrations (Cus and Cue system) (Yamamoto & Ishihama, 2005).
Having identified and characterized the sub-networks activated by copper, we took advantage of the fact that the homeostatic module is isolated from the rest of the network, producing for the first time an in vivo intervention of a global transcriptional network. No common elements were found between the copΔ mutant and wild type strains, suggesting two completely different cellular states against copper exposure. In a wild type state, the activation of energy metabolic genes apparently depends on the ATP requirement of CopA, whereas the lack of this protein in the mutant generates a significant increase in the intracellular copper which probably is activating two new modules.
The emergence of module IV, linked with the activation of a novel set of regulators, explains the new transcriptional configuration compared with the wild type strains, reaffirming the fact that E. faecalis can reconfigure its gene expression to keep cellular homeostasis. The presence of these elements describes a classical chain transcriptional regulation process (Alon, 2007). According to the model, copper first is sensed by the transcription factors GalR-S and LysR, which subsequently repress the expression over these new set of regulators. The impact of the lack of direct copper homeostasis apparently is reduced by the down-regulation of mechanisms involved in sugar capture probably also linked with copper uptake (module V). In the bacterium Vibrio vulnificus, it has been demonstrated that glucose bioavailability through CRP-FNR can modulate the expression of siderophores (Kim et al, 2012), molecules also related with copper uptake systems (Chaturvedi et al, 2012). The repression of module V also can be understood as a decrease in the metabolism of glucose, which suggests that E. faecalis could intentionally generate a metabolic arrest with the purpose of decreasing its capacity to capture copper by other systems (indirect effect) (Valcourt et al, 2012).
While the two new sub-networks activated present mostly new components, it is important to note that some genes, probably controlled by the family of transcriptional factors LysR, are capable of responding to high concentrations of copper in both the wild type and copΔ strains. This group of genes becomes part of an invariant response inside the sub-networks, over-expressed independently of the presence of the copper homeostasis module. This core of genes is composed mainly of proteins involved in the uptake of amino acids (ABC transporters), whose activation show high specificity for copper as no changes were present in the zinc and iron microarrays.
In general, the understanding of the design of this kind of network is far from complete (Balazsi et al, 2008). It is important to note that the current model covers only 30% of the putative interactions and elements (transcription factors and genes) present in the E. faecalis genome. Also, this model does not consider other mechanisms that also affect transcription, such as small RNAs (Shioya et al, 2011). Considering these limitations, the generation of a global gene regulatory network model able to integrate information of transcriptional responses from different stimuli (including the complex circuit of interactions between transcription factors and genes) allows us to understand how the cell can transduce extracellular copper signals into coordinated gene expression.
Our experimental approach not only determined the transcriptional response of E. faecalis to copper under two homeostatic scenarios, but also improved the general understanding about the capacity of living organisms to adapt their transcriptional regulation under extreme conditions. Moreover, we could generate a hypothetical homeostasis scenario in the copΔ, where the bacteria significantly increase their copper content, reaching the limit of saturation of the system without affecting the cellular viability. Currently, the most common gene regulatory network model organisms are Escherichia coli, Saccharomyces cerevisiae and Mycobacterium tuberculosis (Balazsi et al, 2005; Balazsi et al, 2008; Lee et al, 2002). The characterization of these models, based on the topology and structure of their networks, allowed the determination of subnetworks specifically activated under different environmental conditions. However, all present several independent mechanisms related with cell protection against metal toxicity, making a more direct interpretation of the copper effects on bacterial cells difficult. Additionally, none of them provide a high number of global gene transcription experiments under the condition of copper treatment, which position E. faecalis as one of the most complete and controllable copper homeostasis models available to date.
Furthermore, the information generated not only provides important data for understanding how a species can modulate its transcription in the presence of different copper concentrations, it also can be useful for understanding the behavior of gene expression in other situations. For example, this model could potentially be used to identify new sub-networks activated under other stress conditions or even when E. faecalis is acting as a pathological agent. Finally, the bioinformatic strategy used to identify putative binding sites can also be applied to other bacterial genomes sequenced to construct new global gene regulatory networks.
Materials and methods
Gene regulatory networks
The global gene regulatory network was generated using a classical probabilistic weight matrix strategy (Rodionov, 2007; Stormo, 2000). For a total of 28 transcriptional regulatory protein families encoded by the E. faecalis genome, it was possible to assign a putative DNA binding site weight matrix using the information recollected from RegulonDB (Salgado et al, 1999), TractorDB (Perez et al, 2007) and DBTBS (Makita et al, 2004) databases. The algorithms Consensus (Benitez-Bellon et al, 2002) and Motif Sampler/Scanner (Thijs et al, 2002) were run independently; all the assigned matrixes were screened within 300 pb upstream and 50 pb downstream of each operon contained in the E. faecalis V583 genome (Paulsen et al, 2003). We set the parameter of prior probability of one motif copy to 0.5 in MotifSampler/MotifScanner. Also, we filtered Consensus results and retained the 10th percentile of high scores. If both procedures indicated the same DNA binding site for a operon, it was considered as a valid prediction. We accepted only DNA motifs (putative binding sites) which presented a similarity higher than 70% with the initial matrix. This information generated a global transcriptional network that contains different operons connected by the presence of a common putative transcriptional binding site. Finally, the global gene regulatory network model was compiled into a genebank format file (EfaecalisGTN.gbk, supplementary file 1), containing mainly information about transcription factor binding sites, operons, genes and proteins descriptions.
OrthoMCL algorithm (Li et al, 2003) was used to generate homology clusters between all the operons contained in the E. faecalis network and 8 genomes of Lactobacillales order species (Lactobacillus johnsonii NCC 533, Lactococcus lactis subsp. lactis Il1403, Lactobacillus plantarum WCFS1, Oenococcus oeni PSU-1, Pediococcus pentosaceus ATCC 25745, Streptococcus agalactiae 2603V/R, Streptococcus pneumoniae R6, Streptococcus pyogenes MGAS315). Then, we annotated each cluster component containing a gene of E. faecalis as a network element in order to propagate the net. Finally, each promoter region of the homologous operons contained in a cluster were screened with the corresponding putative DNA binding sites founded in E. faecalis. Only sequences with a global identity over the 50th percentile with the E. faecalis DNA binding site were accepted as a positive match, finally obtaining clusters of homologous operons which only contain similar DNA binding sites on their promoter region.
All the topology analyses were performed by R software using iGraph. The consensus transcriptional factor family binding site logos were created by WebLogo using all the putative binding sites for each E. faecalis family. (http://weblogo.berkeley.edu/) (Crooks et al, 2004). All graph displays, network analyses and crossing information between all the microarray data and the E. faecalis transcription network (activated metal sub-networks) were performed by Cytoscape software (Shannon et al, 2003). Active operons in each microarray were selected only when 50% of the operon’s genes changed their expression levels significantly in the same direction (up regulated or down regulated). Annotation previously reported (Lopez et al, 2012; Reyes-Jara et al, 2010) was used for assign gene names, Id codes, transcription factor families and COG class classifications.
cop operon mutant construction
An E. faecalis cop operon deletion mutant (copΔ) was generated using the PheS* system as described previously (Kristich et al, 2007). Briefly, fragments of ca. 450 bp located downstream and upstream of target gene were amplified by PCR using the primers GCTCTAGAGTACAGGCGGCAATACAG (up_f) and CGGGATCCCTCGTTAAATTCCTCCTTCA (up_r) for the upstream fragment CGGGATCCTTGTCAGCGAATAACAAAAA (down_f) and GGAATTCTGCTACCTAAGCGTCCTC (down_r) for the downstream fragment. The resulting amplicon (956 pb) was cloned into the pCJK-47 plasmid. The construct generated was electroporated into E. faecalis CK111 and then transferred to E. faecalis OG1RF strain via conjugation. MM9YEG agar supplemented with 10 mM p-Cl-Phe and X-gal (200 μg/mL) was used to identify plasmid loss. copΔ mutants were screened by PCR (up_f and down_r primers), and confirmed by sequencing of the junction area. Pulsed field gel electrophoresis (PFGE) was used to confirm the mutant was in the correct background.
Microarrays
E. faecalis V583 ORF transcriptional changes (microarray data) under high level copper exposure (0.5 mM CuSO4), low level copper exposure (0.05 mM CuSO4), zinc exposure (4 mM ZnSO4) and iron exposure (0.5 mM FeSO4) were recollected from GEO databank (GSE20453, GSE30949, GSE30947, GSE34432). For the copΔ mutant microarray experiment, the copΔ strain was grown in N media until mid-log phase with 0.5 mM or 0.05 mM of CuSO4, replicating the same conditions used in the wild type microarray reported (Reyes-Jara et al, 2010). RNA isolation, cDNA sample preparation and chip hybridization were performed as previously described (Reyes-Jara et al, 2010). Briefly, a cDNA pool of four biological replicates for each treatment was hybridized onto an E. faecalis V583 chip-array printed with 3,114 open reading frames (two technical replicates, catalog number A7980-00-01, NimbleGen Systems, Inc.). Mutant basal gene expression changes were obtained comparing the wild-type and copΔ data (without copper exposure). The copΔ strain’s copper expression data were generated comparing the copper data set (0.5 mM or 0.05 mM of CuSO4) with the untreated copΔ data. DNASTAR Array 5.0 software was used to identify significant differences in the levels of expression between the average value of each gene and its corresponding reference (Student’s t-test P < 0.05, GEO accession number GSE45131).
Supplementary Material
Acknowledgments
This work was funded by Fondo Nacional de Desarrollo Científico y Tecnológico, FONDECYT N°1110427 (MG) and Fondo Nacional de Desarrollo de Areas Prioritarias, FONDAP N°15090007, Center for Genome Regulation (CGR) (ML, MG, AM), Basal Grant CMM (MG, AM), CONICYT and MECESUP UCH0713 PhD fellowships (ML).
Footnotes
Conflict of interest
All the authors of this work declare that they have no conflict of interest.
Contributor Information
Mauricio Latorre, Email: mlatorre@uchile.inta.cl.
Jessica Galloway-Peña, Email: jessica.galloway-pena@uth.tmc.edu.
Jung Hyeob Roh, Email: jung.h.roh@uth.tmc.edu.
Marko Budinich, Email: mbudinich@dim.uchile.cl.
Angélica Reyes-Jara, Email: areyes@uchile.inta.cl.
Barbara E. Murray, Email: barbara.e.murray@uth.tmc.edu.
Alejandro Maass, Email: amaass@dim.uchile.cl.
Mauricio González, Email: mgonzale@uchile.inta.cl.
References
- Abrantes MC, de Lopes MF, Kok J. Impact of manganese, copper and zinc ions on the transcriptome of the nosocomial pathogen Enterococcus faecalis V583. PLoS One. 2011;6:e26519. doi: 10.1371/journal.pone.0026519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert R. Scale-free networks in cell biology. J Cell Sci. 2005;118:4947–4957. doi: 10.1242/jcs.02714. [DOI] [PubMed] [Google Scholar]
- Alon U. Network motifs: theory and experimental approaches. Nat Rev Genet. 2007;8:450–461. doi: 10.1038/nrg2102. [DOI] [PubMed] [Google Scholar]
- Balazsi G, Barabasi AL, Oltvai ZN. Topological units of environmental signal processing in the transcriptional regulatory network of Escherichia coli. Proc Natl Acad Sci U S A. 2005;102:7841–7846. doi: 10.1073/pnas.0500365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazsi G, Heath AP, Shi L, Gennaro ML. The temporal response of the Mycobacterium tuberculosis gene regulatory network during growth arrest. Mol Syst Biol. 2008;4:225. doi: 10.1038/msb.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazsi G, Oltvai ZN. Sensing your surroundings: how transcription-regulatory networks of the cell discern environmental signals. Sci STKE. 2005;2005:pe20. doi: 10.1126/stke.2822005pe20. [DOI] [PubMed] [Google Scholar]
- Balleza E, Lopez-Bojorquez LN, Martinez-Antonio A, Resendis-Antonio O, Lozada-Chavez I, Balderas-Martinez YI, Encarnacion S, Collado-Vides J. Regulation by transcription factors in bacteria: beyond description. FEMS Microbiol Rev. 2009;33:133–151. doi: 10.1111/j.1574-6976.2008.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez-Bellon E, Moreno-Hagelsieb G, Collado-Vides J. Evaluation of thresholds for the detection of binding sites for regulatory proteins in Escherichia coli K12 DNA. Genome Biol. 2002;3:RESEARCH0013. doi: 10.1186/gb-2002-3-3-research0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy JG, Sidhu JK, Rana F, Spurgeon DJ, Svendsen C, Wren JF, Sturzenbaum SR, Morgan AJ, Kille P. ‘Systems toxicology’ approach identifies coordinated metabolic responses to copper in a terrestrial non-model invertebrate, the earthworm Lumbricus rubellus. BMC Biol. 2008;6:25. doi: 10.1186/1741-7007-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol. 2012;8:731–736. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenye T, Vandamme P. Extracting phylogenetic information from whole-genome sequencing projects: the lactic acid bacteria as a test case. Microbiology. 2003;149:3507–3517. doi: 10.1099/mic.0.26515-0. [DOI] [PubMed] [Google Scholar]
- Cornelis P, Wei Q, Andrews SC, Vinckx T. Iron homeostasis and management of oxidative stress response in bacteria. Metallomics. 2011;3:540–549. doi: 10.1039/c1mt00022e. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaballa A, Helmann JD. A peroxide-induced zinc uptake system plays an important role in protection against oxidative stress in Bacillus subtilis. Mol Microbiol. 2002;45:997–1005. doi: 10.1046/j.1365-2958.2002.03068.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Reyes A, Suazo M, Jo W, Vulpe CD. Expression of copper-related genes in response to copper load. Am J Clin Nutr. 2008;88 doi: 10.1093/ajcn/88.3.830S. in press. [DOI] [PubMed] [Google Scholar]
- Janga SC, Collado-Vides J. Structure and evolution of gene regulatory networks in microbial genomes. Res Microbiol. 2007;158:787–794. doi: 10.1016/j.resmic.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- Kim CM, Kim SJ, Shin SH. Cyclic AMP-receptor protein activates aerobactin receptor IutA expression in Vibrio vulnificus. J Microbiol. 2012;50:320–325. doi: 10.1007/s12275-012-2056-y. [DOI] [PubMed] [Google Scholar]
- Kitano H. Systems biology: a brief overview. Science. 2002;295:1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- Kristich CJ, Chandler JR, Dunny GM. Development of a host-genotype-independent counter selectable marker and a high-frequency conjugative delivery system and their use in genetic analysis of Enterococcus faecalis. Plasmid. 2007;57:131–144. doi: 10.1016/j.plasmid.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Rinaldi NJ, Robert F, Odom DT, Bar-Joseph Z, Gerber GK, Hannett NM, Harbison CT, Thompson CM, Simon I, Zeitlinger J, Jennings EG, Murray HL, Gordon DB, Ren B, Wyrick JJ, Tagne JB, Volkert TL, Fraenkel E, Gifford DK, et al. Transcriptional regulatory networks in Saccharomyces cerevisiae. Science. 2002;298:799–804. doi: 10.1126/science.1075090. [DOI] [PubMed] [Google Scholar]
- Li L, Stoeckert CJ, Jr, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez G, Latorre M, Reyes-Jara A, Cambiazo V, Gonzalez M. Transcriptomic response of Enterococcus faecalis to iron excess. Biometals. 2012;25:737–747. doi: 10.1007/s10534-012-9539-5. [DOI] [PubMed] [Google Scholar]
- Magnani D, Barre O, Gerber SD, Solioz M. Characterization of the CopR regulon of Lactococcus lactis IL1403. J Bacteriol. 2008;190:536–545. doi: 10.1128/JB.01481-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita Y, Nakao M, Ogasawara N, Nakai K. DBTBS: database of transcriptional regulation in Bacillus subtilis and its contribution to comparative genomics. Nucleic Acids Res. 2004;32:D75–77. doi: 10.1093/nar/gkh074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen IT, Banerjei L, Myers GS, Nelson KE, Seshadri R, Read TD, Fouts DE, Eisen JA, Gill SR, Heidelberg JF, Tettelin H, Dodson RJ, Umayam L, Brinkac L, Beanan M, Daugherty S, DeBoy RT, Durkin S, Kolonay J, Madupu R, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003;299:2071–2074. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- Perez AG, Angarica VE, Vasconcelos AT, Collado-Vides J. Tractor_DB (version 2.0): a database of regulatory interactions in gamma-proteobacterial genomes. Nucleic Acids Res. 2007;35:D132–136. doi: 10.1093/nar/gkl800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Jara A, Latorre M, Lopez G, Bourgogne A, Murray BE, Cambiazo V, Gonzalez M. Genome-wide transcriptome analysis of the adaptive response of Enterococcus faecalis to copper exposure. Biometals. 2010;23:1105–1112. doi: 10.1007/s10534-010-9356-7. [DOI] [PubMed] [Google Scholar]
- Reyes A, Leiva A, Cambiazo V, Mendez MA, Gonzalez M. Cop-like operon: structure and organization in species of the Lactobacillale order. Biol Res. 2006;39:87–93. doi: 10.4067/s0716-97602006000100010. [DOI] [PubMed] [Google Scholar]
- Rodionov DA. Comparative genomic reconstruction of transcriptional regulatory networks in bacteria. Chem Rev. 2007;107:3467–3497. doi: 10.1021/cr068309+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado H, Santos A, Garza-Ramos U, van Helden J, Diaz E, Collado-Vides J. RegulonDB (version 2.0): a database on transcriptional regulation in Escherichia coli. Nucleic Acids Res. 1999;27:59–60. doi: 10.1093/nar/27.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder J, Tauch A. Transcriptional regulation of gene expression in Corynebacterium glutamicum: the role of global, master and local regulators in the modular and hierarchical gene regulatory network. FEMS Microbiol Rev. 2010;34:685–737. doi: 10.1111/j.1574-6976.2010.00228.x. [DOI] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioya K, Michaux C, Kuenne C, Hain T, Verneuil N, Budin-Verneuil A, Hartsch T, Hartke A, Giard JC. Genome-wide identification of small RNAs in the opportunistic pathogen Enterococcus faecalis V583. PLoS One. 2011;6:e23948. doi: 10.1371/journal.pone.0023948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solioz M, Stoyanov JV. Copper homeostasis in Enterococcus hirae. FEMS Microbiol Rev. 2003;27:183–195. doi: 10.1016/S0168-6445(03)00053-6. [DOI] [PubMed] [Google Scholar]
- Stormo GD. DNA binding sites: representation and discovery. Bioinformatics. 2000;16:16–23. doi: 10.1093/bioinformatics/16.1.16. [DOI] [PubMed] [Google Scholar]
- Thijs G, Marchal K, Lescot M, Rombauts S, De Moor B, Rouze P, Moreau Y. A Gibbs sampling method to detect overrepresented motifs in the upstream regions of coexpressed genes. J Comput Biol. 2002;9:447–464. doi: 10.1089/10665270252935566. [DOI] [PubMed] [Google Scholar]
- Valcourt JR, Lemons JM, Haley EM, Kojima M, Demuren OO, Coller HA. Staying alive: metabolic adaptations to quiescence. Cell Cycle. 2012;11:1680–1696. doi: 10.4161/cc.19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Ishihama A. Transcriptional response of Escherichia coli to external copper. Mol Microbiol. 2005;56:215–227. doi: 10.1111/j.1365-2958.2005.04532.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.