Abstract
Purpose of review
Atrial fibrillation (AF) is the most common sustained arrhythmia in the patients with kidney disease. The purpose of this review is to describe the burden of AF in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD), postulate possible mechanisms to explain this burden of disease, understand the clinical consequences of AF and review the treatment options for AF specific to patients with kidney disease.
Recent findings
Recent literature has revealed that the clinical multi-organ impact of AF in patients with CKD and ESRD is substantial. Although novel oral anticoagulants to treat AF and prevent associated complications have been tested in large trials in the general population, there is a paucity of data on the efficacy and safety of these agents in patients with advanced CKD and ESRD.
Summary
AF is a significant comorbidity in patients with CKD and ESRD with important prognostic implications. More research is needed to understand the mechanisms that contribute to the disproportionate burden of this arrhythmia in patients with kidney disease and treatment options specific to this population of high-risk patients.
Keywords: Atrial fibrillation, CKD, ESRD
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in the general population. The burden of AF is even greater in patients with concomitant kidney disease. Published studies in the last few years have highlighted the often under-recognized, yet highly prevalent relation between kidney disease and AF. Furthermore, evidence has suggested that the burden of AF will likely rise in this high-risk population, making the intersection of kidney disease and AF a highly relevant clinical problem.
Among the large population of patients with chronic kidney disease (CKD) not yet requiring dialysis, several studies have noted a high prevalence of AF, typically 2-to-3-fold higher than reported in the general population.1–5 In participants with moderate to advanced CKD in the Chronic Renal Insufficiency Cohort (CRIC), the prevalence of AF was 18%overall and >25% in participants ≥ 70 years old.5 Older age, female sex, tobacco use and history of cardiovascular disease, including heart failure, were significantly associated with prevalent AF in that study. Another study of the Medicare 5% sample with CKD found that the two-year incidence of AF diagnosed by administrative codes was 14.4% among patients with stage 3–5 CKD.6 In the Atherosclerosis Risk in Community (ARIC) Study, during 10 years of follow-up, there was a graded, increased risk of diagnosed incident AF with lower eGFR or higher level of albuminuria at cohort entry, even after adjustment for other clinical risk factors.1
Among patients with end-stage renal disease (ESRD) on dialysis,7 the prevalence of AF is estimated to be 7–20%.8 An analysis of 63,884 Medicare/Medicaid-eligible dialysis patients found that age >60 years, male sex, white race, overweight/obesity, inability to ambulate, and prior cardiovascular disease were significantly associated with prevalent AF.7 Furthermore, recent data from the United States Renal System (USRDS) reported that the prevalence of AF continues to increase among patients with ESRD.8
While these data are compelling, our estimates of the burden of AF are likely conservative given current methods of ascertainment of AF, and particularly incident AF. Most studies have relied on self-report, 12-lead electrocardiograms or administrative/ICD-9 diagnostic codes, which all may be insensitive measures given the often paroxysmal nature of AF and the fact that many patients are asymptomatic. Thus, innovative methods are needed to capture prevalent and incident AF more comprehensively and cost-effectively in large studies of patients with kidney disease.
Mechanisms that contribute to increased risk of AF in CKD
Several possible mechanisms may explain the high rate of identified AF among patients with CKD including: older age and a high burden of risk factors such as hypertension and cardiovascular disease;2 excessive inflammation which has been linked to both CKD and AF;9–16 larger left atrial and left ventricular sizes among CKD patients;2,17–23 and activation of the renin-angiotensin-aldosterone system.24,25 Other plausible pathways linking kidney disease and AF include abnormalities in mineral metabolism. Specifically, elevations in phosphorus and fibroblast growth factor (FGF)-23 have been linked to increased left ventricular mass.26–28 It is possible that alterations in these pathways may also contribute to risk of AF in patients with CKD and ESRD through effects on cardiac structure, endothelial function and vascular calcification. Further investigations are needed to explore unique kidney-specific biological pathways linking AF and kidney disease given the disproportionately high burden of disease in this population.
AF associated with increased risk of stroke and death in CKD and ESRD
Impaired kidney function and AF are both associated with thromboembolic disease and the synergetic impact of these conditions enhances the risk of complications such as ischemic stroke. AF and kidney disease lead to endothelial injury, abnormal blood flow and hypercoagulability, which result in substantial risk of thromboembolism (Figure 1).
Figure 1.
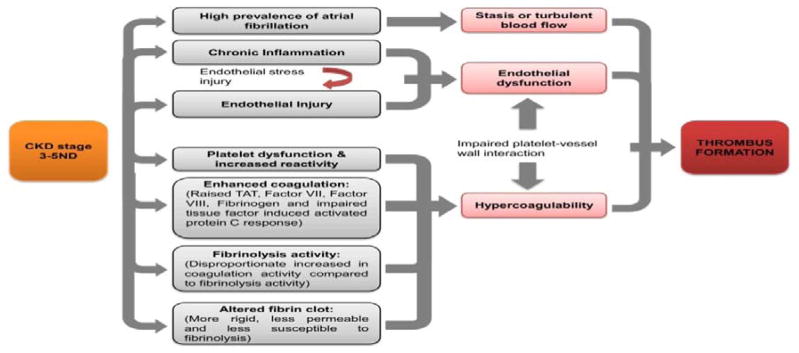
Potential mechanisms of increased thromboembolic risk in patients with chronic kidney disease stages 3–5 nondialysis (CKD 3-5ND). Factors known to be associated with CKD lead to abnormalities in all 3 factors in Virchow’s triad, enhancing the risk of thrombus formation.
(Adapated from Ng et al, Am J Kidney Dis. 2013 Sep;62(3):615–32. doi: 10.1053/j.ajkd.2013.02.381. Epub 2013 Jun 5)
Among patients with CKD, several clinical studies have reported that AF is associated with increased risk of stroke and death. In a study of 132,372 patients with nonvalvular AF, patients with CKD had 49% increased rate of stroke or systemic thromboembolism compared with patients without kidney disease.29 In a study of nearly 11,000 patients with AF, proteinuria increased the risk of thromboembolism by 54% and there was a graded, increased risk of stroke associated with a progressively lower level of eGFR.30 In the ROCKET AF (Rivaroxaban Once-daily, oral direction factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation), reduced creatinine clearance was a strong, independent predictor of stroke and systemic embolism, second only to prior stroke or transient ischemic attack, in the setting of anticoagulation.31 A model that included creatinine clearance improved net reclassification index by 8.2% (95% CI 2.5%–14%, p=0.005) compared with the CHADS(2) score. When this new prediction score that included kidney function was validated in an external model, the NRI improved by 17.4%, suggesting that stroke risk stratification in patients with AF should include kidney function.31 A large study of the 2006 5% Medicare population found that incident AF was associated with a 27% increased rate of death among patients with CKD.6 A single center study of 387 Japanese patients with AF reported higher death rates in patients with decreased eGFR and high CHADS2 score compared with patients with preserved eGFR and low CHADS2 score.32 Among patients with eGFR<60 ml/min/1.73 m2, there was a 2.8-fold (95% CI 1.3–5.8) higher rate of death in patients with CHADS2 score <2 and 6.9-fold (95% CI 3.5–13.5) higher rate of death in patients with CHADS2 score ≥ 2.32 Furthermore, change in kidney function may also be an important predictor of AF complications. A study of 617 AF patients followed over 2 years (with measures of kidney function every 6 months), found that decline in eGFR>25% over 6 months was associated with greater than 2-fold increased rate of stroke and death.33
Complications associated with AF among ESRD patients on dialysis appear to be even greater.34,35 In a study of 132,372 patients with nonvalvular AF, patients with ESRD had 83% increased rate compared with patients without kidney disease.29 In another study of dually eligible Medicare-Medicaid chronic dialysis patients, chronic AF was significant associated with 26% higher rates of ischemic strokes.36 A similar study of incident dialysis patients found that AF was one of the strongest risk factors for ischemic stroke, even stronger than age or hypertension.37 Among >17,000 dialysis patients enrolled in the international Dialysis Outcomes and Practice Patterns Study (DOPPS), AF at study enrollment was associated with higher rates of stroke (adjusted hazard ratio 1.28, 95% CI: 1.01–1.63) and death (adjusted hazard ratio 1.16, 95% CI: 1.08–1.25).35 Within the nationally comprehensive U.S. Renal Data System between 1989 and 2006, the adjusted 1-year risk of death was 45% higher in dialysis patients with AF compared with those who did not have documented AF.8
These large studies are the first step in elucidating the complex interaction between kidney disease and AF and highlight that AF is far from a “benign” arrhythmia.
AF and renal outcomes
While it is generally accepted that CKD increases the risk of developing AF, few studies have evaluated the potential bidirectional relationship between AF and CKD. One cohort study of Japanese participants in the Niigata Preventative Medicine Study found that participants with preserved kidney function (defined as eGFR > 60 ml/min/1.73 m2 and absence of dipstick detectable proteinuria) and with AF at entry was associated with 80% higher adjusted rate of developing eGFR<60 ml/min/1.73 m2 and a 116% higher adjusted rate of developing proteinuria that could be detected by urine dipstick testing.38 We recently extended the work from this study to a large integrated healthcare delivery system in Northern California.39 In a study of 206,229 adults with confirmed CKD, we identified outpatient and inpatient AF using validated approaches. Our primary outcome was progression to ESRD, defined as the receipt of chronic dialysis or a kidney transplant. Over a mean follow-up time of 5 years, there was a 67% increased rate of ESRD among CKD patients who developed incident AF compared to those without AF, even after statistical adjustment for demographic characteristics, household income, educational status, entry eGFR level, comorbid conditions, outpatient blood pressure level, albuminuria, hemoglobin level and medication use,.39 In adjusted models stratified by age, gender, race and baseline eGFR level, we found a consistently higher adjusted rate of ESRD associated with incident AF in all of the targeted patient subgroups, except for baseline eGFR <30 ml/min/1.73 m2. Adjustment of interim hospitalizations for HF and MI only slightly attenuated the association between incident AF and ESRD. While previous literature has shown that CKD is associated with a high incidence and prevalence of AF,1–5 our novel results support that AF may contribute to an accelerated progression of CKD to ESRD independent of other known risk factors.
Several possible mechanisms may contribute to how AF could increase the risk of ESRD. AF promotes systemic inflammation, 12–16 which has been strongly associated with progression of ESRD in patients with CKD.40,41 Given that AF can also induce fibrosis within the myocardium,42 it is possible that this same fibrosis process is activated within the kidney as well, perhaps through a systemic pro-fibrotic tendency (although there is not definitive evidence for this mechanism). AF also contributes to decline of left ventricular systolic and diastolic function over time,43,44 which may promote progression of CKD through altered hemodynamics, 44,45 venous congestion and activation of the renin-angiotensin-aldosterone system.24,25 It is also possible that AF may be prothrombotic, leading to renal micro-infarcts, similar to silent cerebral infarcts that have been noted in patients with AF.46 It is also plausible that some of the medications used to treat AF may contribute to decline in renal function (e.g., diuretics).
AF and kidney transplant
The burden and consequences of AF in kidney transplant recipients remains largely understudied. The USRDS estimates that ~7% of all cardiovascular hospitalizations are primarily due to AF in the first 2 years after kidney transplantation.47 Among 304 kidney transplant recipients at a single center in Italy, 6.9% had incident AF in the post-operative period after surgery (median time of 3 days).48 The cumulative post-operative AF risk was highest on the day of surgery (2.5%), increased steeply up to day 3 (5.3%) and reached 9.5% on the 19th day of admission. 48 The highest risk of incident AF after kidney transplant was among kidney transplant recipients who were older, men and white with a higher burden of hypertension and coronary heart disease. Another analysis examined the prevalence of pre-transplant AF and associated outcomes after transplant among over 62,000 first kidney transplant recipients.49 Of those, 6.4% were diagnosed with AF prior to kidney transplant. Over a mean follow-up of 4.9 years, pre-transplant AF was associated with 46% increased rate of death, 41% increase in rate of graft failure and 36% increase in rate of stroke after transplant compared to patients who did not have AF prior to kidney transplant.49 It seems clear that pre- and post-transplant AF has serious implications on outcomes post transplantation. These studies are a preliminary step to further characterize the burden and consequences of AF in kidney transplant recipients. Further investigations are necessary to fully understand the unique interactions of peri-kidney transplant care and AF
Treatment of AF in kidney disease
There are clear and evidence-based guidelines for the use of anticoagulation in the prevention of thromboembolic stroke in the general population.50 Yet, despite the tremendous burden of AF and associated adverse consequences in patients with CKD and ESRD, the role of anticoagulation in patients with kidney disease is less defined. Patients with kidney disease uniquely have increased risk for thromboembolism and a paradoxical increased risk of bleeding, making decisions on anticoagulation challenging. A previous analysis identified serum creatinine concentration of >1.5 mg/dL as an independent predictor of major bleeding events.51 Observational studies and clinical trials have reported heightened risk of hemorrhagic stroke and other bleeding events in patients with reduced kidney function.52–55 Reduced kidney function is also associated with larger hematoma volumes56 and poorer survival after intracerebral hemorrhage.57 Impaired platelet adhesion, decreased storage and section of platelet-activating mediators, disturbances in platelet aggregation and presence of uremic toxins are just a few of the postulated mechanisms to explain the increased bleeding risk in patients with CKD and ESRD.58,59
Clinical trials of anticoagulation in patients with AF have largely excluded patients with advanced CKD or ESRD.60 In the ESRD population, observational studies of anticoagulation have yielded conflicting results, with some noting better61 and others worse,62–64 outcomes with the use of warfarin. In the CKD population, there are limited data on the safety of warfarin. However, the few studies in this area have suggested that warfarin use is associated with lower risk of stroke.65,66 Among 516 stage 3 CKD patients in the Stroke Prevention in Atrial Fibrillation 3 trial, rates of ischemic stroke/systemic embolism was reduced by 76% in participants treated with adjusted-dose warfarin compared to participants treated with aspirin/low-dose warfarin.65 There was no difference in major hemorrhage in the 2 groups.65 Yet the mean eGFR was 50 ml/min/1.73 m2 among the CKD participants in this trial; thus, it remains unknown if the same conclusions can be made at more advanced stages of CKD.
Over the last several years, several novel oral anticoagulants have been tested in large randomized trials for prevention of stroke in patients with AF.55,67–69 These novel anticoagulants include two direct thrombin inhibitors (ximelagatran and dabigatran) and two factor Xa inhibitors (apixaban and rivaroxaban) and have the benefit of not requiring regular anticoagulation monitoring and frequent dose adjustments. Yet, all have substantial renal clearance with prolonged half-life in patients with CKD (Table 1). Three of these novel anticoagulants have been recently been approved for clinical use (dabigatran, apixaban and rivaroxaban) and have been shown to be non-inferior or superior to adjusted-dose warfarin for stroke prevention, and, in some cases, reduced risk of major hemorrhagic complications. While major clinical trials of these agents have excluded patients with advanced CKD, many have included patients with moderate CKD (Table 2). Overall, evidence from these trials support the use of these novel agents in patients with moderate CKD (Table 2). There remains a paucity of data on the efficacy and safety of these agents in more advanced stages of CKD and ESRD.
Table 1.
Key pharmacological characteristics of novel oral anticoagulants
| Feature | Drug | |||
|---|---|---|---|---|
| Dabigatran etexilate | Apixaban | Rivaroxaban | Edoxaban | |
| Coagulation target | Thrombin | Factor Xa | Factor Xa | Factor Xa |
| Prodrug | Yes | No | No | No |
| Bioavailability (%) | 6 | 70 | 80 | Not known |
| Protein binding (%) | 35 | 90 | 90 | 55 |
| Dosing frequency* | Twice daily | Twice daily | Once daily | Once daily |
| Half-life (h) | 12–14 | 12 | 7–11 | 8–10 |
| Renal clearance (%) | 80 | 25 | 35 | 40 |
| Routine monitoring | No | No | No | No |
| Drug interactions | P-glycoprotein | CYP3A4 and P-glycoprotein | CYP3A4 and P-glycoprotein | CYP3A4 and P-glycoprotein |
| Approved for ESRD | No | No | No | No |
For patients with atrial fibrillation. Abbreviation: ESRD, end-stage renal disease. Adapted with permission from Wolters Kluwer Health © Eikelboom, J. W. & Weitz, J. I. New anticoagulants. Circulation 121 (13), 1523–1532 (2010).
Adapted from Wolters Kluwer Health © Eikelboom, J. W. & Weitz, J. I. New anticoagulants. Circulation 121(13), 1523–1532 (2010)
Table 2.
Overview of phase III randomized trials of new oral anticoagulants
| Study (n) | Agents | Design features | Exclusion criteria related to CKD | Dose adjustment related to CKD | Stage 3 CKD (%) | Mean time in therapeutic range (INR 2– 3) | Main results |
|---|---|---|---|---|---|---|---|
| RE-LY68 | Dabigat ran 150 mg or 110 mg twice daily vs. warfarin | Warfarin given open- label | eCrCl<30 ml/min | None | 10% eCrCl 30–49 ml/min | 64% | Stroke, non-CNS embolism and cardiovascular mortality reduced by dabigatran 150 mg vs. warfarin; major haemorrhage reduced by dabigatran 110 mg vs. warfarin; intracranial bleeding reduced by both doses of dabigatran vs. warfarin; no significant differences in total mortality |
| AVERROES67 (5,599) | Apixaban 5 mg twice daily vs. aspirin | Double- blind; restricted to those deemed unsuitable for warfarin | Serum creatinine >221 umol/L or eCrCr<25 ml/min | 2.5 mg twice daily if serum creatinine ≥ 133 umol/L plus age ≥ 80 years or weight ≤60 kg | 30% eCrCl 30–59 ml/min | NA | Stroke and non-CNS embolism reduced by apixaban vs aspirin; major haemorrhage and intracranial bleeding comparable with both agents; no significant difference in cardiovascular or total mortality |
| ROCKET AF69 (14, 264) | Rivaroxaban 20 mg per day vs. warfarin | Double- blind; restricted to those at high risk of stroke | eCrCl<30 ml/min | 15 mg per day if CrCl<50 ml/min | 21% eCrCl 30–49 ml/min | 55% | Rivaroxaban noninferior to warfarin for stroke and non-CNS embolism; major haemorrhage comparable with both agents; intracranial bleeding reduced by rivaroxaban vs. warfarin; no significant difference in cardiovascular or total mortality |
| ARISTOTLE55 | Apixaban 5 mg twice daily vs. warfarin | Double- blind | Serum creatinine >221 umol/L or eCrCl<25 ml/min | 2.5 mg twice daily if serum creatinine ≥ 133 umol/L plus age ≥ 80 years or weight ≤ 60 kg | 15% eCrCl 30–50 ml/min | 62% | Stroke, non-CNS embolism, major haemorrhage, intracranial bleeding and total mortality reduced by apixaban vs. warfarin; no significant difference in cardiovascular mortality |
Abbreviations: CKD=chronic kidney disease; CNS=central nervous system; eCrCl=estimated creatinine clearance; INR=international normalized ratio; NA=not available
Hart, R. G. (2012) Anticoagulants in atrial fibrillation patients with chronic kidney disease et al. Nat. Rev. Nephrol. doi:10.1038/nrneph.2012.160
Conclusions
The burden of AF in patients with kidney disease is disproportionately high and continues to rise. AF afflicts patients with all stages of kidney disease, including advanced stages of CKD, ESRD and kidney transplant recipients. Recent work has highlighted that AF and kidney disease synergistically lead to serious complications. Additional studies are necessary to understand the distinct kidney-specific pathophysiological pathways that contributes to the development of AF as well as the unique considerations in preventing and treating AF specific to patients with a broad range of kidney disease.
KEY POINTS.
Atrial fibrillation is common in patients with CKD and ESRD and is associated with significant morbidity and mortality.
The biological pathways linking atrial fibrillation and kidney disease remain incompletely understood.
Patients with kidney disease have unique considerations in the treatment of atrial fibrillation and further studies are needed to study anticoagulation in patients with advanced CKD and ESRD.
Acknowledgments
Dr. Bansal recieves support from K23 DK088865 from the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK). Dr. Go receives support from 5 U01 DK060902 from the NIDDK and 5 U19 HL091179 and 5 RC2 HL101589 from the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health, Department of Health and Human Services. Dr. Hsu receives support from K24 DK92291from the NIDDK.
Footnotes
None of the authors have any conflicts of interest or financial disclosures.
References
- 1.Alonso A, Lopez FL, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011 Jun 28;123(25):2946–2953. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ananthapanyasut W, Napan S, Rudolph EH, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010 Feb;5(2):173–181. doi: 10.2215/CJN.03170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baber U, Howard VJ, Halperin JL, et al. Association of Chronic Kidney Disease With Atrial Fibrillation Among Adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 2011 Feb 1;4(1):26–32. doi: 10.1161/CIRCEP.110.957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horio T, Iwashima Y, Kamide K, et al. Chronic kidney disease as an independent risk factor for new-onset atrial fibrillation in hypertensive patients. J Hypertens. 2010 Aug;28(8):1738–1744. doi: 10.1097/HJH.0b013e32833a7dfe. [DOI] [PubMed] [Google Scholar]
- 5.Soliman EZ, Prineas RJ, Go AS, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC) Am Heart J. 2010 Jun;159(6):1102–1107. doi: 10.1016/j.ahj.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson SE, Shroff GR, Li S, Herzog CA. Impact of chronic kidney disease on risk of incident atrial fibrillation and subsequent survival in medicare patients. Journal of the American Heart Association. 2012 Aug;1(4):e002097. doi: 10.1161/JAHA.112.002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Wetmore JB, Mahnken JD, Rigler SK, et al. The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int. 2012 Mar;81(5):469–476. doi: 10.1038/ki.2011.416. (Annotation: Important paper identifying predictors of AF in a large, nationally representative U.S. dialysis population) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011 Feb;22(2):349–357. doi: 10.1681/ASN.2010050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta J, Mitra N, Kanetsky PA, et al. Association between Albuminuria, Kidney Function, and Inflammatory Biomarker Profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012 Dec;7(12):1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Upadhyay A, Larson MG, Guo CY, et al. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol Dial Transplant. 2011 Mar;26(3):920–926. doi: 10.1093/ndt/gfq471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003 Jan 7;107(1):87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 12.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001 Dec 11;104(24):2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 13.Hatzinikolaou-Kotsakou E, Tziakas D, Hotidis A, et al. Relation of C-reactive protein to the first onset and the recurrence rate in lone atrial fibrillation. Am J Cardiol. 2006 Mar 1;97(5):659–661. doi: 10.1016/j.amjcard.2005.09.104. [DOI] [PubMed] [Google Scholar]
- 14.Dudley SC, Jr, Hoch NE, McCann LA, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005 Aug 30;112(9):1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 15.Friedrichs K, Klinke A, Baldus S. Inflammatory pathways underlying atrial fibrillation. Trends in molecular medicine. 2011 Oct;17(10):556–563. doi: 10.1016/j.molmed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Negi S, Sovari AA, Dudley SC., Jr Atrial fibrillation: the emerging role of inflammation and oxidative stress. Cardiovascular & hematological disorders drug targets. 2010 Dec 1;10(4):262–268. doi: 10.2174/187152910793743850. [DOI] [PubMed] [Google Scholar]
- 17.Bansal N, Keane M, Delafontaine P, et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: the CRIC study. Clin J Am Soc Nephrol. 2013 Mar;8(3):355–362. doi: 10.2215/CJN.06020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckardt KU, Scherhag A, Macdougall IC, et al. Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. J Am Soc Nephrol. 2009 Dec;20(12):2651–2660. doi: 10.1681/ASN.2009060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999 Jul;34(1):125–134. doi: 10.1016/s0272-6386(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 20.Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis. 2005 Aug;46(2):320–327. doi: 10.1053/j.ajkd.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Foley RN, Curtis BM, Randell EW, Parfrey PS. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol. 2010 May;5(5):805–813. doi: 10.2215/CJN.07761109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE. Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J Am Soc Nephrol. 2000 May;11(5):912–916. doi: 10.1681/ASN.V115912. [DOI] [PubMed] [Google Scholar]
- 23.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996 Mar;27(3):347–354. doi: 10.1016/s0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 24.Iravanian S, Dudley SC., Jr The renin-angiotensin-aldosterone system (RAAS) and cardiac arrhythmias. Heart rhythm : the official journal of the Heart Rhythm Society. 2008 Jun;5(6 Suppl):S12–17. doi: 10.1016/j.hrthm.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin-angiotensin-aldosterone system (RAAS) for primary prevention of non-valvular atrial fibrillation: A systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2012 Mar 13; doi: 10.1016/j.ijcard.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011 Nov 1;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009 May 19;119(19):2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto KT, Robinson-Cohen C, de Oliveira MC, et al. Dietary phosphorus is associated with greater left ventricular mass. Kidney Int. 2013 Jan 2; doi: 10.1038/ki.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012 Aug 16;367(7):625–635. doi: 10.1056/NEJMoa1105594. (Annotation: Large, observational study examining association of CKD with risk of stroke/systemic embolism in patients with CKD and ESRD) [DOI] [PubMed] [Google Scholar]
- 30.Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009 Mar 17;119(10):1363–1369. doi: 10.1161/CIRCULATIONAHA.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Piccini JP, Stevens SR, Chang Y, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013 Jan 15;127(2):224–232. doi: 10.1161/CIRCULATIONAHA.112.107128. (Annotation: Large, post-hoc analysis of a clinical trial that provides additional evidence that among patients with AF, impaired kidney function is associated with worse outcomes) [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa K, Hirai T, Takashima S, et al. Chronic kidney disease and CHADS(2) score independently predict cardiovascular events and mortality in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2011 Mar 15;107(6):912–916. doi: 10.1016/j.amjcard.2010.10.074. [DOI] [PubMed] [Google Scholar]
- 33.Guo Y, Wang H, Zhao X, et al. Sequential changes in renal function and the risk of stroke and death in patients with atrial fibrillation. Int J Cardiol. 2013 Oct 12;168(5):4678–4684. doi: 10.1016/j.ijcard.2013.07.179. [DOI] [PubMed] [Google Scholar]
- 34.Genovesi S, Vincenti A, Rossi E, et al. Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis. 2008 Feb;51(2):255–262. doi: 10.1053/j.ajkd.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 35.Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010 Jun;77(12):1098–1106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 36.Wetmore JB, Ellerbeck EF, Mahnken JD, et al. Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol. 2013 Mar;23(3):112–118. doi: 10.1016/j.annepidem.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wetmore JB, Ellerbeck EF, Mahnken JD, et al. Stroke and the “Stroke Belt” in Dialysis: Contribution of Patient Characteristics to Ischemic Stroke Rate and Its Geographic Variation. J Am Soc Nephrol. 2013 Aug 29; doi: 10.1681/ASN.2012111077. (Annotation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J. 2009 Oct;158(4):629–636. doi: 10.1016/j.ahj.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 39*.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident Atrial Fibrillation and Risk of End-Stage Renal Disease in Adults with Chronic Kidney Disease. Circulation. 2012 Dec 28; doi: 10.1161/CIRCULATIONAHA.112.123992. (Annotation: Important paper suggesting a possible bi-directional association between kidney disease and AF in a large, community based population) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF Receptors 1 and 2 Predict ESRD in Type 2 Diabetes. J Am Soc Nephrol. 2012 Jan 19; doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pecoits-Filho R, Heimburger O, Barany P, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003 Jun;41(6):1212–1218. doi: 10.1016/s0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 42.Burstein B, Qi XY, Yeh YH, Calderone A, Nattel S. Atrial cardiomyocyte tachycardia alters cardiac fibroblast function: a novel consideration in atrial remodeling. Cardiovascular research. 2007 Dec 1;76(3):442–452. doi: 10.1016/j.cardiores.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Grogan M, Smith HC, Gersh BJ, Wood DL. Left ventricular dysfunction due to atrial fibrillation in patients initially believed to have idiopathic dilated cardiomyopathy. Am J Cardiol. 1992 Jun 15;69(19):1570–1573. doi: 10.1016/0002-9149(92)90705-4. [DOI] [PubMed] [Google Scholar]
- 44.Naito M, David D, Michelson EL, Schaffenburg M, Dreifus LS. The hemodynamic consequences of cardiac arrhythmias: evaluation of the relative roles of abnormal atrioventricular sequencing, irregularity of ventricular rhythm and atrial fibrillation in a canine model. Am Heart J. 1983 Aug;106(2):284–291. doi: 10.1016/0002-8703(83)90194-1. [DOI] [PubMed] [Google Scholar]
- 45.Chen SC, Su HM, Hung CC, et al. Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011 Dec;6(12):2750–2758. doi: 10.2215/CJN.04660511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke; a journal of cerebral circulation. 2008 Nov;39(11):2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.http://www.usrds.org.
- 48.La Manna G, Boriani G, Capelli I, et al. Incidence and Predictors of Postoperative Atrial Fibrillation in Kidney Transplant Recipients. Transplantation. 2013 Aug 6; doi: 10.1097/TP.0b013e3182a2b492. [DOI] [PubMed] [Google Scholar]
- 49*.Lenihan CR, Montez-Rath ME, Scandling JD, Turakhia MP, Winkelmayer WC. Outcomes after kidney transplantation of patients previously diagnosed with atrial fibrillation. Am J Transplant. 2013 Jun;13(6):1566–1575. doi: 10.1111/ajt.12197. (Annotation: Important study of a large, national population of kidney transplant recipients that highlights the important of pre-existing AF on post-transplant outcomes) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McManus DD, Rienstra M, Benjamin EJ. An update on the prognosis of patients with atrial fibrillation. Circulation. 2012 Sep 4;126(10):e143–146. doi: 10.1161/CIRCULATIONAHA.112.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McMahan DA, Smith DM, Carey MA, Zhou XH. Risk of major hemorrhage for outpatients treated with warfarin. J Gen Intern Med. 1998 May;13(5):311–316. doi: 10.1046/j.1525-1497.1998.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimizu Y, Maeda K, Imano H, et al. Chronic kidney disease and drinking status in relation to risks of stroke and its subtypes: the Circulatory Risk in Communities Study (CIRCS) Stroke; a journal of cerebral circulation. 2011 Sep;42(9):2531–2537. doi: 10.1161/STROKEAHA.110.600759. [DOI] [PubMed] [Google Scholar]
- 53.Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011 Oct;32(19):2387–2394. doi: 10.1093/eurheartj/ehr342. [DOI] [PubMed] [Google Scholar]
- 54.Eikelboom JW, Connolly SJ, Gao P, et al. Stroke risk and efficacy of apixaban in atrial fibrillation patients with moderate chronic kidney disease. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2012 Aug;21(6):429–435. doi: 10.1016/j.jstrokecerebrovasdis.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011 Sep 15;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 56.Molshatzki N, Orion D, Tsabari R, et al. Chronic kidney disease in patients with acute intracerebral hemorrhage: association with large hematoma volume and poor outcome. Cerebrovascular diseases (Basel, Switzerland) 2011;31(3):271–277. doi: 10.1159/000322155. [DOI] [PubMed] [Google Scholar]
- 57.Rhoney DH, Parker D, Jr, Millis SR, Whittaker P. Kidney dysfunction at the time of intracerebral hemorrhage is associated with increased in-hospital mortality: a retrospective observational cohort study. Neurological research. 2012 Jun;34(5):518–521. doi: 10.1179/1743132812Y.0000000041. [DOI] [PubMed] [Google Scholar]
- 58.Gordge MP, Faint RW, Rylance PB, Neild GH. Platelet function and the bleeding time in progressive renal failure. Thrombosis and haemostasis. 1988 Aug 30;60(1):83–87. [PubMed] [Google Scholar]
- 59.Michalak E, Walkowiak B, Paradowski M, Cierniewski CS. The decreased circulating platelet mass and its relation to bleeding time in chronic renal failure. Thrombosis and haemostasis. 1991 Jan 23;65(1):11–14. [PubMed] [Google Scholar]
- 60.Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int. 2006 Dec;70(11):2021–2030. doi: 10.1038/sj.ki.5001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abbott KC, Trespalacios FC, Taylor AJ, Agodoa LY. Atrial fibrillation in chronic dialysis patients in the United States: risk factors for hospitalization and mortality. BMC Nephrol. 2003 Jan 24;4:1. doi: 10.1186/1471-2369-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elliott MJ, Zimmerman D, Holden RM. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007 Sep;50(3):433–440. doi: 10.1053/j.ajkd.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011 Nov;6(11):2662–2668. doi: 10.2215/CJN.04550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009 Oct;20(10):2223–2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hart RG, Pearce LA, Asinger RW, Herzog CA. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011 Nov;6(11):2599–2604. doi: 10.2215/CJN.02400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai HM, Aronow WS, Kalen P, et al. Incidence of thromboembolic stroke and of major bleeding in patients with atrial fibrillation and chronic kidney disease treated with and without warfarin. International journal of nephrology and renovascular disease. 2009;2:33–37. doi: 10.2147/ijnrd.s7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011 Mar 3;364(9):806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 68.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 69.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011 Sep 8;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]


