Abstract
IMPORTANCE
Although abuse of prescription opioids (POs) is a significant public health problem, few experimental studies have investigated the treatment needs of this growing population.
OBJECTIVE
To evaluate, following brief stabilization with a combination of buprenorphine hydrochloride and naloxone hydrochloride dihydrate, the relative efficacy of 1-, 2-, and 4-week buprenorphine tapering regimens and subsequent naltrexone hydrochloride therapy in PO-dependent outpatients.
DESIGN, SETTING, AND PARTICIPANTS
A double-blind, 12-week randomized clinical trial was conducted in an outpatient research clinic. Following a brief period of buprenorphine stabilization, 70 PO-dependent adults were randomized to receive 1-, 2-, or 4-week tapers followed by naltrexone therapy.
INTERVENTION
During phase 1 (weeks 1–5 after randomization), participants visited the clinic daily; during phase 2 (weeks 6–12), visits were reduced to thrice weekly. Participants received behavioral therapy and urine toxicology testing throughout the trial.
MAIN OUTCOMES AND MEASURES
The percentage of participants negative for illicit opioid use, retention, naltrexone ingestion, and favorable treatment response (ie, retained in treatment, opioid abstinent, and receiving naltrexone at the end of the study).
RESULTS
Opioid abstinence at the end of phase 1 was greater in the 4-week compared with the 2- and 1-week taper conditions (P = .02), with 63% (n = 14), 29% (n = 7), and 29% (n = 7) of participants abstinent in the 4-, 2-, and 1-week conditions, respectively. Abstinence at the end of phase 2 was also greater in the 4-week compared with the 2- and 1-week conditions (P = .03), with 50% (n = 11), 16% (n = 4), and 20% (n = 5) of participants abstinent in the 4-, 2-, and 1-week conditions, respectively. There were more treatment responders in the 4-week condition (P = .03), with 50% (n = 11), 17% (n = 4), and 21% (n = 5) of participants in the 4-, 2-, and 1-week groups considered responders at the end of treatment, respectively. Retention and naltrexone ingestion also were superior in the 4-week vs briefer tapers (both P = .04). Experimental condition (ie, taper duration) was the strongest predictor of treatment response, followed by buprenorphine stabilization dose.
CONCLUSIONS AND RELEVANCE
This study represents a rigorous experimental evaluation of outpatient buprenorphine stabilization, brief taper, and naltrexone maintenance for treatment of PO dependence. Results suggest that a meaningful subset of PO-dependent outpatients may respond positively to a 4-week taper plus naltrexone maintenance intervention.
Abuse of prescription opioids (POs), such as oxycodone, hydrocodone, and hydromorphone, is a significant US public health problem.1–4 Costs related to abuse of POs are estimated at $8 billion annually5,6 and include emergency department visits, overdoses, criminal activity, and psychiatric and medical consequences.1,7–10 Despite this, there is a dearth of empirical information regarding treatments for PO dependence.4,9,11 Generally, the most efficacious approach for treating opioid dependence involves maintenance treatment with agonist medications, such as methadone hydrochloride and buprenorphine hydrochloride.12–14 However, although agonist maintenance is the recommended treatment for most opioid-dependent patients, detoxification represents an important treatment option for several reasons.15,16 First, some opioid-dependent patients may prefer detoxification to extended maintenance.4,16–19 Second, access to maintenance treatment can be limited, especially in many rural areas that are struggling with high rates of PO abuse.20–24 Finally, some patients may possess demographic or drug use characteristics that are suggestive of lower baseline severity,25–28 which has been associated with favorable response to detoxification.29–31
The aim of this double-blind, randomized clinical trial was to evaluate an outpatient detoxification treatment for PO-dependent adults. More specifically, following a brief period of stabilization with a combination of buprenorphine and naloxone hydrochloride dihydrate, the relative efficacy of 1-, 2-, and 4-week buprenorphine tapering regimens was examined. A recent review32 suggested a positive association between buprenorphine taper duration and treatment outcomes; however, there have been few prospective, parametric evaluations, and results of the existing studies have been mixed. Two studies,33,34 for example, found that a buprenorphine taper duration of approximately 1 month produced greater end-of-taper abstinence than did briefer durations. However, a larger-scale study35 reported that a 7-day taper produced better rates of opioid abstinence on the final day of treatment than did a 28-day taper, although no differences remained at 1- and 3-month follow-up. Taken together, there is broad consensus that more needs to be learned about how to taper buprenorphine to discontinuation without individuals resuming opioid use.
Methods
Participants
The study took place at an outpatient research clinic in Burlington, Vermont. Participants were recruited through newspapers and flyers between October 5, 2007, and November 4, 2009. Participants had to be aged 18 years or older, meet DSM-IV criteria for opioid dependence, provide an opioid-positive urine sample, be willing to undergo detoxification, report a PO as their primary drug of abuse, and be using the PO illicitly. Individuals requiring opioids for pain were excluded, as were those who were pregnant or nursing or had a significant and unstable psychiatric (eg, active psychosis) or medical (eg, acute cardiovascular disease) illness that could interfere with consent or participation. The study was approved by the local institutional review board, and participants provided written informed consent.
Study Design
The aim was to compare, following a brief stabilization period, 3 durations of buprenorphine taper in PO-dependent adults. Because detoxification is often associated with relapse,36 the tapering period was followed by maintenance on naltrexone hydrochloride, a competitive antagonist that blocks opioid reinforcement and reduces opioid use.37,38 All participants received individual behavioral therapy based on the Community Reinforcement Approach, which has been demonstrated as efficacious with alcohol-, cocaine-, and opioid-dependent outpatients.39–41
Participants underwent a brief buprenorphine stabilization period and were then randomly assigned to a 1-, 2-, or 4-week taper (Figure 1). Stratification variables included stabilization dose, illicit opioid abstinence during stabilization, past-month cocaine use, sex, current alcohol dependence, and current chronic pain operationalized as (1) endorsement of the first question of the Brief Pain Inventory42 (ie, whether one has pain other than everyday kinds of pain) and (2) duration of pain longer than 3 months.43 During phase 1 (weeks 1–5 after randomization), participants visited the clinic daily; during phase 2 (weeks 6–12), visits were reduced to thrice weekly (Monday, Wednesday, and Friday). Withdrawal was assessed and adjuvant therapy with nonopioid medications (ie, clonidine hydrochloride, hydroxyzine hydrochloride, loperamide hydrochloride, and promethazine hydrochloride) was available at each visit. Participants provided staff-observed urine specimens thrice weekly.
Figure 1.
A Schematic of the Experimental Design, Including Stabilization, Taper, and Naltrexone Maintenance Phases
Arrows represent participant randomization following stabilization into 1 of 3 experimental conditions prior to initiation of the buprenorphine (BUP) taper. MWF indicates Monday, Wednesday, and Friday.
Measures
The intake assessment included completion of a drug history, the substance dependence section of the DSM-IV,44 Addiction Severity Index,45 Beck Anxiety and Depression Inventories,46,47 Brief Pain Inventory,42 Michigan Alcoholism Screening Test,48 and Fagerstrom Test for Nicotine Dependence49; medical screening; and a timeline follow-back assessment of past-month use of opioids and other drugs.50 Additional measures administered at each visit prior to dosing included timeline follow-back of opioid and other drug use since the last visit, completion of the Clinical Institute Narcotic Assessment of opioid withdrawal,51 and visual analog ratings of withdrawal and opioid effects.
Drugs
Medications were administered in a double-blind, double-dummy manner, with participants receiving sublingual tablets and capsules at each visit. Buprenorphine/naloxone and color-matched placebo were manufactured by Reckitt Benckiser Pharmaceuticals Inc and provided by the National Institute on Drug Abuse. Doses were prepared by the hospital’s investigational pharmacy and administered under research nurse observation. Participants received 5.5 sublingual tablets at each visit, containing a combination of active and/or placebo buprenorphine. Naltrexone (12.5, 25, 50, 100, and 150 mg) and placebo capsules (size 0, opaque hard gelatin) were prepared using naltrexone hydrochloride and powdered lactose. Participants ingested 3 capsules at each visit, containing a combination of active and/or placebo naltrexone. Participants and staff remained blinded to dose, taper duration, and the point at which naltrexone began.
Procedure
Administration of Study Medications
During stabilization, participants attended the clinic daily and withdrawal was assessed before study medication was administered. Individualized dose adjustments were determined using a Clinical Institute Narcotic Assessment–based protocol aimed at stabilizing participants on a dose sufficient to achieve withdrawal suppression without intoxication or sedation. Reductions during taper occurred in a graded fashion, with the stabilization dose reduced by 2-mg steps to 2 mg, followed by a 1-mg dose and placebo. Treatment in participants who underwent tapering without resuming opioid use was transitioned to naltrexone, with induction individually tailored to prevent withdrawal precipitation using minimum criteria of at least 1 opioid-negative urine sample and no self-reported opioid use in the prior 24 hours. Naltrexone treatment began with 12.5 mg on day 1, 25 mg on days 2 and 3, and 50 mg on day 4. Daily administration of 50 mg continued through phase 1, followed by thrice-weekly doses of 100, 100, and 150 mg during phase 2. Failure to transition to naltrexone by the end of phase 1 resulted in discharge from the study and referral to a local treatment program.
Biochemical Testing
Urine specimens were analyzed on site via enzyme-multiplied immunoassay (MGC 240; Microgenics) for opioids, methadone, buprenorphine, oxycodone, and propoxyphene. Randomly selected samples were analyzed once weekly for cocaine, amphetamines, benzodiazepines, marijuana, and barbiturates. Three consecutive missed urine specimens resulted in discharge. Breath alcohol levels were assessed at each visit (Alco-Sensor III; Intoximeters Inc).
Behavioral Therapy
The platform behavioral therapy was based on the Community Reinforcement Approach but was adapted for PO-dependent patients undergoing detoxification. Participants were offered twice-weekly 1- to 1.5-hour sessions with a master’s-level therapist. Topics included coping with withdrawal, human immunodeficiency virus and hepatitis education, relapse prevention, and developing recreational activities and social networks, with additional components tailored to an individual’s needs (eg, employment, education, health insurance, insomnia, depression, and anger management). Sessions were guided by a manual-based protocol; however, the therapist maintained a flexible approach toward appointment scheduling and goal setting and, although participants were encouraged to attend all scheduled therapy sessions, no punitive consequences were administered for missed sessions. Therapists received clinical supervision weekly.
Statistical Analysis
Primary analyses included all participants randomized consistent with an intent-to-treat approach to clinical trials.52 The study was designed to have sufficient power to detect an increasing trend in retention across the 3 taper conditions, corresponding to a 30% difference between 1- and 4-week conditions. The experimental groups were compared for differences in baseline demographics and other characteristics using analyses of variance for continuous variables and χ2 tests for categorical variables. In addition, χ2 tests were used to compare treatment conditions on the percentage of participants abstinent at the end of phases 1 (weeks 1–5) and 2 (weeks 6–12), as well as conditions on treatment retention and naltrexone ingestion. Abstinence was defined as a urine specimen biochemically verified to be opioid negative. Buprenorphine-positive results during phase 1 were not considered illicit opioid positive, but any positive specimen during phase 2 was considered positive for illicit opioid use. A favorable treatment response was defined as being retained in treatment, being opioid abstinent, and receiving naltrexone at the end of the study. Stepwise logistic regression was used to examine predictors of treatment response considering treatment condition and participant characteristics as potential covariates. Statistical analyses were performed using commercial software (SAS, version 9.3; SAS Institute Inc). Statistical significance was determined using α = .05.
Results
Participants
Ninety-one individuals were screened, with 70 participants randomly assigned to the 1-week (n = 24), 2-week (n = 24), or 4-week (n = 22) tapering regimens (Figure 2). There were no significant differences between treatment conditions in baseline characteristics (Supplement [eTable]). Mean (range) duration of the stabilization phase and dose were 14.2 (8–20) days and 11.5 mg (2–20 mg), respectively; these did not differ significantly between experimental conditions.
Figure 2.
Consolidated Standards for Reporting of Trials Flow Diagram of Participants
A schematic of participant enrollment, randomization, and study completion.
Opioid Abstinence
There was a significant effect of taper duration on opioid abstinence in phase 1 (P = .02). At the final visit of phase 1, 63% (n = 14), 29% (n = 7), and 29% (n = 7) of participants were abstinent in the 4-, 2-, and 1-week conditions, respectively (Figure 3). A significantly greater percentage of participants were abstinent in the 4-week compared with the 1- and 2-week conditions. A similar effect was seen in phase 2 (P = .03),with 50% (n = 11), 16%(n = 4), and 20% (n = 5) of participants in the 4-, 2-, and 1-week conditions abstinent at the final visit and significantly better outcomes in the 4- vs 1- or 2-week conditions. When percentage of opioid-negative specimens was collapsed across all study visits, the percentage of negative specimens observed in the 4-, 2-, and 1-week conditions was 58% (n= 13), 35% (n= 8), and 38% (n= 9), respectively (P = .07).
Figure 3.
Effects of Buprenorphine Taper Duration on Illicit Opioid Abstinence Achieved
Data points represent the percentage of opioid-negative urine samples submitted at each consecutive urine-sample visit before and after randomization. Data are presented for the entire group of participants at intake and stabilization and for each experimental group after randomization: 1-week, 2-week, and 4-week taper conditions.
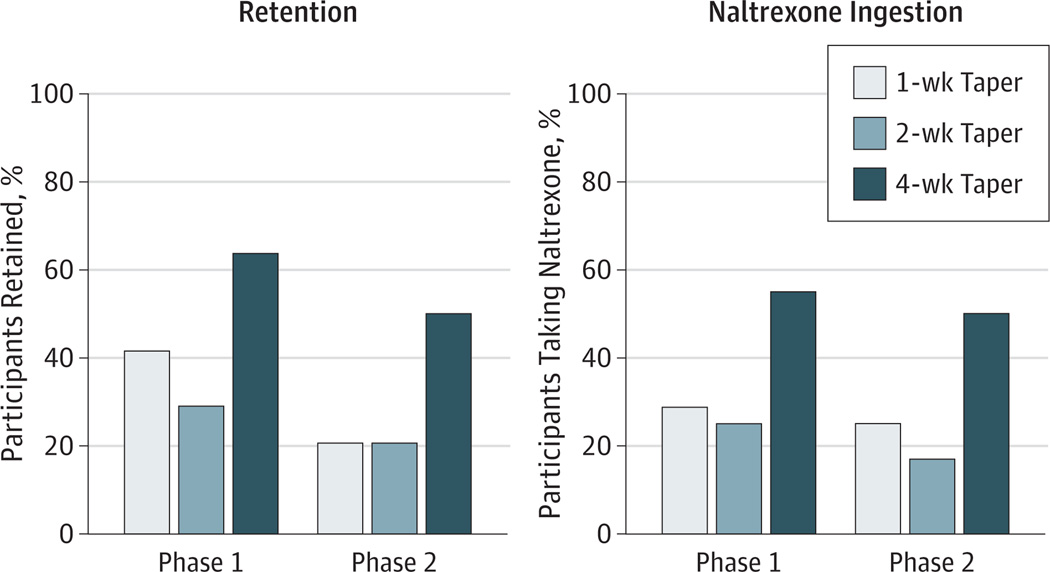
Retention
When retention was examined in phase 1, 64% (n=14), 29% (n=7), and 42% (n=10) of patients in the 4-, 2-, and 1-week conditions were retained through week 5, respectively (P=.06) (Figure 4). A significant effect of taper duration was seen in phase 2 (P = .04), with 50% (n =11), 21% (n =4), and 21% (n = 6) of participants assigned to the 4-, 2-, and 1-week conditions retained at their final study visit. The 4-week taper condition produced significantly better treatment retention than did the 1- and 2-week tapers.
Figure 4.
Effects of Buprenorphine Taper Duration on Treatment Retention and Naltrexone Ingestion at the End of Each Study Phase
Data are presented for 1-week, 2-week, and 4-week taper conditions.
Naltrexone Ingestion
There was a significant effect of taper duration on naltrexone ingestion at the end of phase 2 (P = .04), with 50%, 17%, and 25% of participants in the 4-, 2-, and 1-week conditions still receiving naltrexone at the final visit (Figure 4). A significantly greater percentage of participants in the 4- vs 2-week condition were still receiving naltrexone at the end of the study.
Predictors of Favorable Treatment Response
Multivariate analyses were conducted to identify baseline participant characteristics associated with favorable treatment response. When participants were dichotomized on the basis of treatment response, there were significantly more treatment responders in the 4-week condition (P=.03), with 50% (n = 11), 17% (n = 4), and 21% (n = 5) of participants assigned to the 4-, 2-, and 1-week conditions considered responders at the end of treatment, respectively. In analyses that considered experimental condition and stratification variables as potential predictors of treatment response, taper duration and stabilization dose met the entry criteria (P < .05). Participants assigned to the 4-week taper had an estimated 4-fold greater odds of a favorable treatment response compared with those in the 1-week taper (odds ratio [OR], 4.1; 95% CI, 1.1–16.0, P = .04) and nearly 6 times compared with the 2-week condition (5.9; 1.4–24.7; P = .01). The only covariate that met criteria for entry was patients’ stabilization dose, with doses of 8 mg or greater being associated with decreased odds of a favorable treatment response (OR, 0.26; 95% CI, 0.08–0.90; P = .03).
Discussion
The aim of this randomized, double-blind trial was to evaluate, following a brief stabilization period, the relative efficacy of 1-, 2-, and 4-week buprenorphine tapering regimens and subsequent naltrexone therapy for PO-dependent adults. The 4-week taper produced superior outcomes over briefer durations, with 50% of participants retained, abstinent, and receiving naltrexone at the end of the study compared with 17% and 21% for the 2- and 1-week groups, respectively. These results suggest that the duration of taper influences treatment outcome and are consistent with prior studies33,53–58 showing favorable outcomes with longer- vs briefer-duration detoxification.
The amounts of abstinence achieved in the present study are generally greater than those seen in prior studies of outpatient opioid detoxification,32 including a recently completed National Institute on Drug Abuse Clinical Trials Network trial59 that sought to evaluate the efficacy of brief and extended buprenorphine treatment for PO abusers. In that study, which is, to our knowledge, the only other to prospectively evaluate outpatient buprenorphine detoxification for PO abusers, only 6% to 9% of the participants remained abstinent following a 2-week taper. Several factors may contribute to these differences. First, our behavioral therapy was more intensive than the counseling used in the Clinical Trials Network trial and could have contributed to the favorable outcomes. It is impossible to disentangle the contribution of the counseling component to outcomes. Second, the inclusion of naltrexone therapy could have helped prevent resumption of illicit opioid use following detoxification. Third, we used a double-blind, double-dummy design wherein neither participants nor staff knew the doses received, the duration of taper, or the point at which naltrexone therapy began. Although this permitted rigorous evaluation of outpatient buprenorphine detoxification for PO dependence, it is possible that remaining blinded to one’s medication status could help facilitate outcomes. We know of no empirical data demonstrating more favorable outcomes under blinded vs non-blinded tapering conditions. Furthermore, because all participants received double-blind medication administration, this would not explain the differential treatment response across conditions. In addition, the differential outcomes are not likely the result of differences in patient characteristics. For example, participants in the present study and the Clinical Trials Network study were largely similar in age (28 vs 33 years), sex (69% vs 60% male), race (94% vs 91% white), educational level (12 vs 13 years), employment (63% vs 64% full-time), duration of opioid use (5 vs 5 years), and past-month frequency of opioid use (26 vs 28 days).
The finding of superior outcomes with longer vs briefer detoxification is inconsistent with results from a Clinical Trials Network trial conducted by Ling et al35 in which opioid-dependent patients were randomized to a 7- or 28-day buprenorphine tapering regimen. In that study, 44% of the 7-day group was abstinent at the end of taper compared with 30% of the 28-day group. These differences were no longer evident after the taper, with 17% and 13% of participants abstinent at 1- and 3-month follow-up, respectively. There were numerous differences between the present study and that trial. Participants in the Ling et al trial were older than our sample and were primary heroin abusers. In addition, that study was open label, with a 4-week stabilization period and no naltrexone component. Intensity of treatment was lower, with participants attending the clinic only weekly and encouraged to participate in a standard psychosocial program that varied across study sites. Finally, the 2 trials differed in how the abstinence outcome was calculated. In the present study, taper conditions were compared on the percentage of participants abstinent at a consistent time point (ie, end of weeks 5 and 12). In the Ling et al study, the percentage of participants abstinent was calculated from 2 different time points (ie, end of week 1 for the 7-day group vs end of week 4 for the 28-day group). The extent to which these differences may account for the differential outcomes is unknown, but the differences are important to consider when comparing findings.
When predictors of treatment response were examined, taper duration was the strongest predictor, followed by stabilization dose. Given that lower stabilization doses reflect lower physiologic dependence, this finding of lower baseline severity predicting favorable treatment response is consistent with the larger literature.29–31 Several other aspects of our results also are consistent with earlier studies. First, prior reports25–28,60–63 have noted that at least a subset of PO-dependent patients may be young, have less severe opioid and other drug use, have less use of the intravenous route, and have greater psychosocial stability. The mean age in our study was 27 years, with 35 participants (49%) aged 25 or younger. Although all participants were opioid dependent, most reported their primary route of administration as intranasal and their daily doses as moderate. Participants also had a relatively high rate of employment and educational level and few medical or psychiatric problems. Second, an earlier report4 suggested that PO abusers may express less interest in methadone maintenance than in other forms of treatment. Our patients were asked at intake to rate their interest in methadone maintenance, buprenorphine maintenance, and buprenorphine detoxification on a visual analog scale that ranged from 0 (not at all) to 100 (extremely). Mean ratings of these modalities were 46.5, 70.3, and 70.8, respectively, providing some evidence that at least a subset of PO-dependent individuals may be unwilling to seek methadone maintenance. Finally, access to agonist maintenance can be limited in many geographic regions, particularly in areas where PO dependence is common. For example, although rates of PO abuse in Vermont are among the highest in the country,62,64,65 there remains a lengthy waiting list for access to our state’s primary methadone service. Taken together, our data support the importance of exploring whether some PO-dependent patients may achieve favorable outcomes without extended agonist maintenance. However, although these data suggest that a meaningful subset of PO-dependent patients may do well with a carefully implemented buprenorphine detoxification regimen, ongoing antagonist therapy and other services will likely be important for longer-term success.36 To the extent that naltrexone helped prevent relapse in the present study, recently developed sustained-release naltrexone formulations could provide ongoing pharmacologic support following detoxification.66
Several potential limitations of the study relate to the generality of our sample to the larger population of PO-dependent treatment seekers. Participants were primarily white and reported oxycodone as their primary drug of abuse, both of which may limit the generality of our results to the larger population of PO abusers. However, most PO users are white,25,26,67,68 and oxycodone is one of the most commonly abused POs.64,65,69 Patients requiring opioids for pain also were excluded, although it seems reasonable to expect that PO-dependent individuals with significant or chronic pain are not likely to be candidates for detoxification and naltrexone. Our sample was not a “pure” PO-dependent population in that we did not exclude individuals with a history of heroin use. However, our definition of primary PO abusers is consistent with that of several prior studies in this population.26,70 Participants also exhibited a clear predominance of PO use, with all identifying a PO as their primary drug of abuse and only 1 of 70 identifying heroin as their secondary opioid. Furthermore, although approximately half of the participants reported ever using heroin, only 3% had used heroin on 5 or more days in the month prior to intake.
Several potential limitations also relate to our study design. Although this study offers a rigorous evaluation of buprenorphine taper duration and naltrexone maintenance in PO-dependent patients, there was no effort to isolate the effects of the behavioral therapy. Thus, the contribution of the Community Reinforcement Approach to outcomes is unknown. We also did not include a comparison group receiving buprenorphine maintenance, and inclusion of such a group would have permitted a comparison of outcomes associated with brief buprenorphine taper vs continued administration. However, scientific and clinical evidence generally show that extended agonist maintenance produces favorable outcomes compared with detoxification. In this study, we simply sought to develop and evaluate a protocol that might produce good outcomes for patients for whom detoxification is potentially appropriate or for whom extended agonist maintenance is undesirable or unavailable. In addition, our ability to document abstinence was influenced by retention, which differed as a function of experimental condition. Further studies could aim to disentangle abstinence and retention, perhaps by offering financial incentives contingent on study completion (but independent of urinalysis results). Finally, stabilization was brief, with a mean duration of 14.2 days. Although its duration was individualized and sufficient to achieve an 82% rate of illicit opioid abstinence among participants prior to randomization, a longer duration might have produced greater stabilization in other areas of psychosocial functioning. To our knowledge, the contribution of the duration of stabilization preceding the taper regimen has not been empirically evaluated. Further investigation of this parameter could inform future efforts to develop more efficacious detoxification strategies.
Finally, several possible limitations relate to the generality of our findings to routine clinical practice. First, all 3 taper durations were shorter than those often used in outpatient clinical settings. Comparison of the 4-week taper with longer durations would be of scientific and clinical interest. Second, the frequency of patient visits exceeded that often used in office-based practices. The degree to which this contributed to outcomes is unknown; the clinical support and oversight needed for successful detoxification may be more compatible with a specialized outpatient treatment program than routine clinical practice in an office-based setting. Third, our taper schedule included a 1-mg dose. It may be common practice for physicians to have their patients split their doses to achieve the prescribed dose; however, there are no federal or manufacturer guidelines supporting this practice. Finally, access to agonist maintenance is more limited in Vermont than in some other geographic regions. Whether this influenced our treatment outcomes is unknown but may also be an important consideration when comparing across studies on this topic.
Taken together, this study provides a rigorous experimental investigation of the treatment needs of the growing population of PO-dependent adults. Our results suggest that a subset of PO abusers may respond favorably to a brief but carefully crafted outpatient treatment involving buprenorphine detoxification, naltrexone maintenance, and behavioral therapy. Additional controlled studies are needed to better understand the parameters of efficacious treatments for PO dependence, as well as to identify the individuals for whom brief vs longer-term treatments are warranted. Overall, this new information will inform efforts to develop efficacious treatments for the growing problem of PO dependence.
Supplementary Material
Acknowledgments
In recent years, Dr Sigmon has received consulting payments from Alkermes and, through her university, has received research support from Titan Pharmaceuticals. In the past, Dr Brooklyn was a paid mentor in the Physician Clinical Support System for training physicians in buprenorphine use.
Funding/Support: This study was supported by research grant R01-DA019989 (Dr Sigmon) and training grant T32 DA007242 (Dr Higgins) from the National Institute on Drug Abuse.
Buprenorphine/naloxone and color-matched placebo sublingual tablets were provided by Reckitt Benckiser Pharmaceuticals Inc through the National Institute on Drug Abuse.
Role of the Sponsor: The National Institute on Drug Abuse and Reckitt Benckiser Pharmaceuticals Inc had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamapsychiatry.com
Author Contributions: Dr Sigmon had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sigmon, Heil, Higgins.
Acquisition of data: Sigmon, Dunn, Saulsgiver, Patrick, Brooklyn.
Analysis and interpretation of data: Sigmon, Dunn, Saulsgiver, Patrick, Badger, Heil.
Drafting of the manuscript: Sigmon, Dunn, Saulsgiver, Patrick, Heil, Brooklyn, Higgins.
Critical revision of the manuscript for important intellectual content: Sigmon, Dunn, Saulsgiver, Badger, Higgins.
Statistical analysis: Sigmon, Badger.
Obtained funding: Sigmon, Heil, Higgins.
Administrative, technical, and material support: Sigmon, Saulsgiver, Patrick, Brooklyn.
Study supervision: Sigmon, Dunn.
Conflict of Interest Disclosures: No other disclosures were reported.
Additional Contributions: Betsy Bahrenburg, RN, Bruce Brown, BS, LICSW, LADC, and other research staff assisted in conducting this study. Leigh Ann Holterman, BS, provided statistical support. As paid staff members, these individuals received salary support but no additional financial compensation from the authors.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) CDC grand rounds: prescription drug overdose—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10–13. [PubMed] [Google Scholar]
- 2.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States. Drug Alcohol Depend. 2006;81(2):103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell JC. The prescription drug epidemic in the United States: a perfect storm. Drug Alcohol Rev. 2011;30(3):264–270. doi: 10.1111/j.1465-3362.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- 4.Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69(3):215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaum HG, White AG, Reynolds JL, et al. Estimated costs of prescription opioid analgesic abuse in the United States in 2001: a societal perspective. Clin J Pain. 2006;22(8):667–676. doi: 10.1097/01.ajp.0000210915.80417.cf. [DOI] [PubMed] [Google Scholar]
- 6.Strassels SA. Economic burden of prescription opioid misuse and abuse. J Manag Care Pharm. 2009;15(7):556–562. doi: 10.18553/jmcp.2009.15.7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA. Non-medical use, abuse and dependence on prescription opioids among U.S. adults. Drug Alcohol Depend. 2008;94(1–3):38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Bruneau J, Roy E, Arruda N, Zang G, Jutras-Aswad D. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction. 2012;107(7):1318–1327. doi: 10.1111/j.1360-0443.2012.03803.x. [DOI] [PubMed] [Google Scholar]
- 9.McLellan AT, Turner B. Prescription opioids, overdose deaths, and physician responsibility. JAMA. 2008;300(22):2672–2673. doi: 10.1001/jama.2008.793. [DOI] [PubMed] [Google Scholar]
- 10.Substance Abuse and Mental Health Services Administration (SAMHSA) The DAWN Report: Trends in Emergency Department Visits Involving Nonmedical Use of Narcotic Pain Relievers. Rockville, MD: Center for Behavioral Health Statistics and Quality; 2010. [Google Scholar]
- 11.Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305(13):1346–1347. doi: 10.1001/jama.2011.369. [DOI] [PubMed] [Google Scholar]
- 12.Ball JC, Ross A. The Effectiveness of Methadone Maintenance Treatment. New York, NY: Springer-Verlag; 1991. [Google Scholar]
- 13.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343(18):1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 14.Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10(11):1727–1740. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amato L, Davoli M, Ferri M, Gowing L, Perucci CA. Effectiveness of interventions on opiate withdrawal treatment: an overview of systematic reviews. Drug Alcohol Depend. 2004;73(3):219–226. doi: 10.1016/j.drugalcdep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci. 2007;9(4):455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appel PW, Ellison AA, Jansky HK, Oldak R. Barriers to enrollment in drug abuse treatment and suggestions for reducing them. Am J Drug Alcohol Abuse. 2004;30(1):129–153. doi: 10.1081/ada-120029870. [DOI] [PubMed] [Google Scholar]
- 18.Luty J. Treatment preferences of opiate-dependent patients. Psychiatr Bull. 2004;28:47–50. [Google Scholar]
- 19.Pinto H, Maskrey V, Swift L, Rumball D, Wagle A, Holland R. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39(4):340–352. doi: 10.1016/j.jsat.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Cicero TJ, Surratt H, Inciardi JA, Munoz A. Relationship between therapeutic use and abuse of opioid analgesics in rural, suburban, and urban locations in the United States. Pharmacoepidemiol Drug Saf. 2007;16(8):827–840. doi: 10.1002/pds.1452. [DOI] [PubMed] [Google Scholar]
- 21.Fortney J, Booth BM. Access to substance abuse services in rural areas. Recent Dev Alcohol. 2001;15:177–197. doi: 10.1007/978-0-306-47193-3_10. [DOI] [PubMed] [Google Scholar]
- 22.Lenardson J, Gale JA. Distribution of substance abuse treatment facilities across the rural-urban continuum. US Department of Health and Human Services, Federal Office of Rural Health Policy. [Accessed September 3, 2013];2007 http://muskie.usm.maine.edu/Publications/rural/wp35b.pdf. [Google Scholar]
- 23.Rosenblum A, Cleland CM, Fong C, Kayman DJ, Tempalski B, Parrino M. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rounsaville BJ, Kosten TR. Treatment for opioid dependence: quality and access. JAMA. 2000;283(10):1337–1339. doi: 10.1001/jama.283.10.1337. [DOI] [PubMed] [Google Scholar]
- 25.Moore BA, Fiellin DA, Barry DT, et al. Primary care office–based buprenorphine treatment. J Gen Intern Med. 2007;22(4):527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenblum A, Parrino M, Schnoll SH, et al. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 2007;90(1):64–71. doi: 10.1016/j.drugalcdep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Sigmon SC. Characterizing the emerging population of prescription opioid abusers. Am J Addict. 2006;15(3):208–212. doi: 10.1080/10550490600625624. [DOI] [PubMed] [Google Scholar]
- 28.Wu LT, Woody GE, Yang C, Blazer DG. How do prescription opioid users differ from users of heroin or other drugs in psychopathology: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Addict Med. 2011;5(1):28–35. doi: 10.1097/ADM.0b013e3181e0364e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franken IH, Hendriks VM. Predicting outcome of inpatient detoxification of substance abusers. Psychiatr Serv. 1999;50(6):813–817. doi: 10.1176/ps.50.6.813. [DOI] [PubMed] [Google Scholar]
- 30.Iguchi MY, Stitzer ML. Predictors of opiate drug abuse during a 90-daymethadone detoxification. Am J Drug Alcohol Abuse. 1991;17(3):279–294. doi: 10.3109/00952999109027552. [DOI] [PubMed] [Google Scholar]
- 31.Kampman KM, Pettinati HM, Volpicelli JR, et al. Cocaine dependence severity predicts outcome in outpatient detoxification from cocaine and alcohol. Am J Addict. 2004;13(1):74–82. doi: 10.1080/10550490490265389. [DOI] [PubMed] [Google Scholar]
- 32.Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: a review. Drug Alcohol Depend. 2011;119(1–2):1–9. doi: 10.1016/j.drugalcdep.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amass L, Bickel WK, Higgins ST, Hughes JR. A preliminary investigation of outcome following gradual or rapid buprenorphine detoxification. J Addict Dis. 1994;13(3):33–45. doi: 10.1300/j069v13n03_04. [DOI] [PubMed] [Google Scholar]
- 34.Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling W, Hillhouse M, Domier C, et al. Buprenorphine tapering schedule and illicit opioid use. Addiction. 2009;104(2):256–265. doi: 10.1111/j.1360-0443.2008.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial and pharmacological treatments vs pharmacological treatments for opioid detoxification. Cochrane Database Syst Rev. 2011;(9) doi: 10.1002/14651858.CD005031.pub4. [update of Cochrane Database Syst Rev. 2008;(4):CD005031]. CD005031. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez JP, Brogden RN. Naltrexone: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs. 1988;35(3):192–213. doi: 10.2165/00003495-198835030-00002. [DOI] [PubMed] [Google Scholar]
- 38.Martin WR, Jasinski DR, Mansky PA. Naltrexone, an antagonist for the treatment of heroin dependence: effects in man. Arch Gen Psychiatry. 1973;28(6):784–791. doi: 10.1001/archpsyc.1973.01750360022003. [DOI] [PubMed] [Google Scholar]
- 39.Abbott PJ. A review of the community reinforcement approach in the treatment of opioid dependence. J Psychoactive Drugs. 2009;41(4):379–385. doi: 10.1080/02791072.2009.10399776. [DOI] [PubMed] [Google Scholar]
- 40.Azrin NH, Sisson RW, Meyers R, Godley M. Alcoholism treatment by disulfiram and community reinforcement therapy. J Behav Ther Exp Psychiatry. 1982;13(2):105–112. doi: 10.1016/0005-7916(82)90050-7. [DOI] [PubMed] [Google Scholar]
- 41.Higgins ST, Sigmon SC, Wong CJ, et al. Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psychiatry. 2003;60(10):1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- 42.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 43.Weiss RD, Potter JS, Provost SE, et al. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS) Contemp Clin Trials. 2010;31(2):189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feingold A, Rounsaville B. Construct validity of the dependence syndrome as measured by DSM-IV for different psychoactive substances. Addiction. 1995;90(12):1661–1669. doi: 10.1046/j.1360-0443.1995.901216618.x. [DOI] [PubMed] [Google Scholar]
- 45.McLellan AT, Luborsky L, Cacciola J, et al. New data from the Addiction Severity Index. J Nerv Ment Dis. 1985;173(7):412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 47.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 48.Selzer ML. The Michigan Alcoholism Screening Test. Am J Psychiatry. 1971;127(12):1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 49.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 50.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 51.Peachey JE, Lei H. Assessment of opioid dependence with naloxone. Br J Addict. 1988;83(2):193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- 52.Armitage P. Exclusions, losses to follow-up, and withdrawals in clinical trials. In: Shapiro SH, Lewis TA, editors. Clinical Trials: Issues and Approaches. New York, NY: Marcel Dekker, Inc; 1983. pp. 99–113. [Google Scholar]
- 53.Fudala PJ, Jaffe JH, Dax EM, Johnson RE. Use of buprenorphine in the treatment of opioid addiction: II: physiologic and behavioral effects of daily and alternate-day administration and abrupt withdrawal. Clin Pharmacol Ther. 1990;47(4):525–534. doi: 10.1038/clpt.1990.67. [DOI] [PubMed] [Google Scholar]
- 54.Gossop M, Griffiths P, Bradley B, Strang J. Opiate withdrawal symptoms in response to 10-day and 21-day methadone withdrawal programmes. Br J Psychiatry. 1989;154:360–363. doi: 10.1192/bjp.154.3.360. [DOI] [PubMed] [Google Scholar]
- 55.Katz EC, Schwartz RP, King SD, et al. Brief vs. extended buprenorphine detoxification in a community treatment program. Am J Drug Alcohol Abuse. 2009;35(2):63–67. doi: 10.1080/00952990802585380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kosten TR, Kleber HD. Buprenorphine detoxification from opioid dependence: a pilot study. Life Sci. 1988;42(6):635–641. doi: 10.1016/0024-3205(88)90454-7. [DOI] [PubMed] [Google Scholar]
- 57.Nosyk B, Sun H, Evans E, et al. Defining dosing pattern characteristics of successful tapers following methadone maintenance treatment: results from a population-based retrospective cohort study. Addiction. 2012;107(9):1621–1629. doi: 10.1111/j.1360-0443.2012.03870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Senay EC, Dorus W, Goldberg F, Thornton W. Withdrawal from methadone maintenance: rate of withdrawal and expectation. Arch Gen Psychiatry. 1977;34(3):361–367. doi: 10.1001/archpsyc.1977.01770150119014. [DOI] [PubMed] [Google Scholar]
- 59.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brands B, Blake J, Sproule B, Gourlay D, Busto U. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug Alcohol Depend. 2004;73(2):199–207. doi: 10.1016/j.drugalcdep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Fischer B, Patra J, Cruz MF, Gittins J, Rehm J. Comparing heroin users and prescription opioid users in a Canadian multi-site population of illicit opioid users. Drug Alcohol Rev. 2008;27(6):625–632. doi: 10.1080/09595230801956124. [DOI] [PubMed] [Google Scholar]
- 62.Treatment Episode Data Set (TEDS): National Admissions to Substance Abuse Treatment Services: 1992–2002. Rockville, MD: Dept of Health and Human Services; 2004. DASIS series: S-23, DHHS Publication No. (SMA) 04-3965. [Google Scholar]
- 63.Torrington M, Domier CP, Hillhouse M, Ling W. Buprenorphine 101: treating opioid dependence with buprenorphine in an office-based setting. J Addict Dis. 2007;26(3):93–99. doi: 10.1300/J069v26n03_10. [DOI] [PubMed] [Google Scholar]
- 64.Office of National Drug Control Policy (ONDCP) Vermont drug control update. [Accessed June 29, 2011];2008 www.whitehouse.gov/sites/default/files/docs/state_profile_-_vermont.pdf.
- 65.Schneider MF, Bailey JE, Cicero TJ, et al. Integrating nine prescription opioid analgesics and/or four signal detection systems to summarize statewide prescription drug abuse in the United States in 2007. Pharmacoepidemiol Drug Saf. 2009;18(9):778–790. doi: 10.1002/pds.1780. [DOI] [PubMed] [Google Scholar]
- 66.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- 67.Havens JR, Walker R, Leukefeld CG. Prevalence of opioid analgesic injection among rural nonmedical opioid analgesic users. Drug Alcohol Depend. 2007;87(1):98–102. doi: 10.1016/j.drugalcdep.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 68.National Survey on Drug Use and Health (NSDUH) report. Nonmedical oxycodone users: a comparison with heroin users. Office of Applied Studies publication. [Accessed June 29, 2011];2005 http://www.oas.samhsa.gov.
- 69.Cicero TJ, Inciardi JA, Muñoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002–2004. J Pain. 2005;6(10):662–672. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Motamed M, Marsch LA, Solhkhah R, Bickel WK, Badger GJ. Differences in treatment outcomes between prescription opioid-dependent and heroin-dependent adolescents. J Addict Med. 2008;2(3):158–164. doi: 10.1097/ADM.0b013e31816b2f84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.