Abstract
Phagosomal proteome characterization has contributed significantly to the understanding of host–pathogen interaction and the mechanism of infectious diseases caused by intracellular bacteria. The latex bead-containing phagosome has been widely used as a model system to study phagosomal proteomes at a global level. In contrast, the study of bacteria-containing phagosomes at a similar level has just begun. A number of intracellular microbial species are studied for their proteomes during the invasion of a host, providing insight into their metabolic adaptation in host cells and interaction with host-cell antimicrobial environments. In this review, we attempt to summarize the most recent advancements in the proteomic study of microbial phagosomes, especially those originating from mouse or human cells. We also briefly describe the proteomics of latex bead-containing phagosomes because they are often used as model phagosomes for study. We provide descriptions on major biological and technological components in phagosomal proteome studies. We also discuss the role of phagosomal proteome study in the broader horizon of systems biology and the technological challenges in phagosomal proteome characterization.
Keywords: Bacteria, Interaction, Microbiology, Phagosome
1 Introduction
The interaction between an intracellular bacterium and a host cell is reminiscent of the evolutionary process that eventually established the multi-organelle feature of a host cell. Some eukaryotic organelles are believed to be derived from ancestral endosymbionts. A symbiont can generally be considered an attenuated pathogen [1]. An attenuated pathogen acting as a symbiont took part in the endosymbiosis process that led to eukaryogenesis, i.e. the origin of the eukaryotic condition. Thus, successful intracellular bacteria have been the ones that are capable of co-opting the host cellular machinery for an intracellular lifestyle. The intracellular lifestyle might bear a certain symbiotic nature [2]. The opposing host and pathogen organisms may undergo a ‘détente’ in which their mutual existence is synergistic [3]. Interestingly, a pathogen established in a host niche can even learn to expel other intruders that try to invade the same host in the same niche [4], reflecting the complexity of the host–bacteria relationship that has been established over the long history of evolution. Thus, it is not surprising that the most sophisticated and successful intracellular bacteria are likely those that have co-evolved with their host, such as Mycobacterium tuberculosis [5].
Intracellular pathogens remain a major cause of infection leading to human diseases and death worldwide. Host–bacteria interaction involves a series of biological activities from both the bacterial and eukaryotic organisms. Pathogenic bacteria have acquired genetic determinants that allow them to exploit host niches as bacterial environments [6]. A number of studies have aimed at determining how bacteria can grow within a eukaryotic host and how host cells can contain and destroy intracellular bacteria [7]. These studies are providing a wealth of information on the molecular mechanisms of pathogenesis and the fundamental aspects of the host-cell immune system.
Infection by intracellular bacteria requires internalization of bacteria into a host cell by a specialized form of receptor-mediated endocytosis process called phagocytosis. Phagocytosis carries out the fundamental function of clearance of particles and particularly pathogens and thus plays a central role in host defense against bacterial invasion [8]. Phagocytosis typically results in the killing of bacteria and degradation of internalized materials, leading to antigen presentation that is important for innate and adaptive immunity.
Host-cell internalization, or phagocytosis, usually sends bacteria to lysosomes by a programmed series of membrane fusion events that occur between the bacterial vacuole and host endocytic vesicles [9]. Many pathogenic bacteria have developed strategies to avoid delivery to lysosomes. These strategies include disrupting endocytic maturation and subverting other vesicular transport pathways in the host cell [10]. Different professional intracellular bacterial pathogens use different evasion strategies to avoid the host’s antimicrobial actions in order to thrive inside a host cell [2, 7]. Among the organelles involved in the host–pathogen interaction, phagosomes play a central role in defense against invading bacteria [8].
The early advent of proteomic technologies spurred interest and facilitated studies in the area of global organellar proteomics [11]. The studies of organellar proteomes lead to a better understanding of the compositions and functions of important organelles, including the phagosome [12–14].
The application and contribution of proteomics to the understanding of phagosome maturation were reviewed by Rogers and Foster [13]. The proteomic studies of the phagosome covered in that review placed much emphasis on using phagocytosis of latex beads as a model system for phagosome maturation. By the time that review was published, the authors indicated that ‘several groups have reported procedures for biochemical enrichment of bacteria-containing vacuoles (BCVs) but no proteomic analysis of such a compartment has been reported’ [13].
In this review, we attempt to summarize the most recent advancements in the proteomic study of phagosomes with an emphasis on those originated from mouse or human cells that phagocytose pathogenic bacteria. For convenience to readers, this review will also provide some background information regarding phagocytosis, phagosome biogenesis, and phagosome purification. Because latex bead-containing phagosomes (LBPs) have been studied more extensively than BCVs, we will also summarize the most relevant advancements in large-scale proteomic studies of LBPs. We will discuss a key issue in bacterial phagosome proteomics, i.e. the preparation of bacteria-containing phagosomes for proteomic analysis that has been a more difficult task than the preparation of LBPs. We will describe the proteomic identification of proteins from phagosomal preparations, Rab proteins, and will end with a brief discussion on the application of systems biology for host–bacteria interaction studies.
2 Phagocytosis and phagosome biogenesis
2.1 Phagocytosis
Phagocytosis is a specialized form of receptor-mediated endocytosis carried out by specialized cell types, particularly professional phagocytes such as macrophages and dendritic cells [15]. It is a highly conserved process involved in the uptake of extracellular organisms or particles and the destruction of engulfed organisms within the cytoplasm. Thus, phagocytosis plays a central role in defense against bacteria [8]. Many intracellular bacterial pathogens have evolved strategies to survive in the host by replicating within the host-cell cytoplasm exploited as protected niches. These bacteria enter the host cell by subverting the cellular actin cytoskeleton to trigger their internalization in order to escape the humoral (antibody) immune defense. A common mechanism of actin assembly in the host cell is by the activation of the Rho-family GTPases Rac1 and Cdc42. Both Rac1 and Cdc42 are capable of eliciting the activation of actin-nucleating complex Arp2/3 by having proteins of the WASP and WAVE families to relay the signal from Rac1 and Cdc42 to Arp2/3 [16]. The actin-based phagocytosis process is mediated by a multitude of receptors such as Fc [17], Complement [18], PAMP [19], etc. (see, e.g. the book chapter by Hazenbos and Brown for coverage of receptor biology in phagocytosis [20]). Phagosomes are fully competent antigen-processing organelles [21] capable of presenting exogenous antigens via both MHC class II and I pathways to include cross-presentation [22].
It is generally thought that phagocytosis is initiated by the binding of particulate objects – pathogens or other particles – to receptors at the cell surface of professional phagocytes such as macrophages [23]. The particulates are internalized by an invagination step that contains the objects within a membrane-bound vacuole called the phagosome. Newly formed phagosomes display a composition similar to that of the plasma membrane from which they originate [24]. Signaling events rapidly lead to a series of complex maturation processes involving sequential fusion with other organelles in the cytoplasm including early endosomes, late endosomes, and ultimately lysosomes if the maturation process is not intervened [25]. While the plasma membrane is a major source of membrane for phagosome formation at the early stage of internalization, other endomembranes have been shown to also contribute to the phagosome maturation process [26], probably by involving a machinery distinct from receptor-mediated endocytosis [27].
2.2 Phagosome maturation
Phagocytosis is a very complex process involving specialized plasma membrane receptors. It also involves rearrangement of cytoskeleton elements and dynamic exchange of phagosomal components with several intracellular compartments.
While phagosome–lysosome fusion is the principal step in phagosome maturation, phagosomes also interact with other intracellular vesicles such as endosomes [24]. Maturation of phagosomes involves multiple fusion events with early and late endocytic compartments. The early endocytic compartment that includes early phagosomes allows for the recycling of receptors and ligands to the plasma membrane because of its closer proximity to the plasma membrane. The fusion of phagosomes with endosomes requires membrane-associated factors such as SNARE proteins responsible for vesicle docking and fusion in the secretory and endocytic pathways. When phagosomes progress to fuse with late endosomes and eventually lysosomes, they lose markers of early endocytic organelles, such as Rab5, and acquire markers of late endocytic organelles, such as Rab7, LAMP 1 and 2. Changes in the levels of Rab5/Rab7 have also been seen as early endosomes mature into late endosomes. Thus, phagosome maturation involves extensive and selective membrane trafficking events like those occurring for the endosomal compartments, leading to rapid and dynamic changes of the composition of the phagosomal membrane and contents. These observations indicate the complex interplay among the phagosome proteins that will require a more systematic study to elucidate the mechanism of the interaction between bacteria and phagosomes.
The fusion of phagosomes with late endosomes and subsequently with lysosomes leads to the formation of the acidified compartment called phagolysosome. Vacuolar-type H+-ATPases play a central role in phagosomal acidification [28].
Phagosomal acidification endows phagocytes the ability to kill internalized microorganisms so that a host can thwart bacterial invasion. It was shown that the progressive acidification of the phagosomal compartment in human monocytes occurs at levels of pH 5.0 or lower [29]. The pH within zymosan-containing phagosomes in murine bone-marrow derived macrophages also dropped to about pH 4.7 [30]. The ability of host cells to lower phagosomal pH contributes to several bactericidal mechanisms. First, an elevated proton concentration is directly lethal to most bacteria. Second, lower pH promotes the generation of hydrogen peroxide from spontaneous dismutation of superoxide. Third, acidic pH provides optimal conditions for the activity of hydrolytic enzymes such as cathepsin A [31]. In addition, progressive acidification of the phagosomal compartment, probably by fusion with late endosomes, appears to be a prerequisite for the process of phagosome–lysosome fusion [29]. Phagosome–lysosome fusion leads to the degradation of phagocytosed bacteria and facilitates antigen presentation.
2.3 Phagosome as a self-sufficient antigen-presenting organelle
Phagosomes are fully competent antigen-processing organelles [21] capable of presentation of exogenous antigens, including bacterial antigens, via both class I and II pathways [22]. While MHC class I molecules generally do not present exogenous antigens, antigens from some intracellular pathogens have been shown to elicit an MHC class I-dependent CD8+ T-cell response by the process referred to as cross-presentation [32]. Houde et al. demonstrated that phagosomes display the elements and properties necessary to be self-sufficient for the cross-presentation of exogenous antigens [22]. Proteomic analysis of phagosomes showed that all proteins needed for all steps in cross-presentation are assembled on the phagosome, explaining the fact that phagosomes are self-sufficient organelles for cross-presentation [33]. ER-mediated phagocytosis was implicated to link to the function of cross-presentation [22]. There is also evidence that ER interacts with endosomes and promotes cross-presentation [34, 35].
2.4 The role of ER in phagosome biogenesis: a controversy
Phagosomes have long been thought to originate from an invagination of the plasma membrane, thus newly formed phagosomes mainly consist of plasma membrane.
In 2001, Muller-Taubenberger et al. showed that calreticulin and calnexin in ER were important for phagocytosis in Dictyostelium [36]. In 2002, an ER-mediated phagocytosis process was proposed by Desjardins and colleagues who suggested that the ER participated in the early phagocytosis process was an important source of membrane involved in phagocytosis, and thus contributed to a significant portion of the phagosomal membrane [37]. In the following years, a controversy about the fusion of ER with phagosomes developed, peaking in the publication by Touret et al. from the Grinstein and Mellman laboratories in 2005 [38].
Touret et al. used a combination of biochemical, fluorescence imaging, and electron microscopy techniques to quantitatively and dynamically assess the contribution of the plasmalemma and of the ER to phagosome formation and maturation. No evidence was found to verify even a transient physical continuity between the ER and the plasma membrane. ER was not detected as a significant contribution to the formation or maturation of phagosomes in either macrophages or dendritic cells. The data obtained by Touret et al. indicated that the plasma membrane was the main constituent of nascent and newly formed phagosomes. The newly formed phagosomes were observed to be progressively remodeled by fusion with endosomal and eventually lysosomal compartments as phagosomes matured into acidic, degradative organelles. Rogers and Foster used SILAC-based quantitative proteomics to profile 382 proteins in phagosomes with temporal resolution and argued strongly against the ER-mediated phagocytosis model for phagosome biogenesis [39].
In an attempt to reconcile the controversy between the ER-mediated and the phagosome maturation model for phagosome biogenesis, Touret et al. suggested that a disposal of the conventional phagosome maturation model might have been premature and that additional experimental analysis would be required to ascertain the applicability of the ER model [40].
On the other hand, Houde et al. [22], Guermonprez et al. [41], and Ackerman et al. [42] independently showed that fusion of the ER with the phagosome enables cells to cross-present MHC class I molecules, overthrowing a long-standing dogma that MHC class I-restricted antigen processing requires the action of the multimolecular peptide-loading complex within the ER organelle.
Recently, there is additional functional evidence for the presence of ER markers in phagosomes for several pathogens. In addition, Cresswell and colleagues showed that a peptide immobilized on latex beads gets glycosylated upon phagocytosis [43]. Because glycosylation occurs in ER, the result implied that the LBP coated with the peptide localized with ER.
Brucella abortus was found transiently in autophagosomes before localizing to the ER in nonprofessional phagocytes, but had limited localization in ER in macrophages [44]. In nonprofessional phagocytes, B. abortus was located in structures that resembled the ER [45]. In macrophages, however, only a minor percentage of B. abortus-containing phagosomes were enriched with ER [44]. These examples showed that the involvement of ER in BCVs depends upon the type of host cells.
Gueirard et al. showed that Leishmania parasites can be internalized in a calnexin and glucose-6P-positive compartment (i.e. ER) that does not fuse with lysosomes and where the parasite is not degraded [46]. Interestingly, Kima and Dunn developed a simple scheme for the isolation of Leishmania parasite-containing vacuoles with calnexin affinity selection. They exploited the observation that Leishmania parasite-containing vacuoles display ER molecules, including the transmembrane protein calnexin [47].
Goldszmid et al. showed that Toxoplasma gondii-containing vacuoles interact with the ER [48]. T. gondii tachyzoites infect host cells leading to the formation of the parasitophorous vacuole that resists fusion with endosomes and lysosomes. With immunoelectron microscopy, Goldszmid et al. documented the transfer of host ER components into the parasitophorous vacuole, demonstrating that pathogen-driven host ER-parasitophorous vacuole interactions can serve as an important mechanism for antigen entry into the MHC class I pathway and CD8+ T cell cross-priming [48].
Grotzke et al. showed the presence of ER on mycobacterial phagosomes and its functional link to cross-presentation of mycobacteria-derived antigens [49]. M. tuberculosis-containing phagosomes are relatively long-lived and resist maturation. Grotzke et al. recently showed that the M. tuberculosis-containing phagosomes derived from human patients acquire ER-localized machinery, act as a site of HLA-I loading, and play a vital role in the M. tuberculosis-derived antigen presentation similar to that described for the presentation of latex bead-associated antigens [49].
In a recent systems study of phagosome proteomes modulated by mycobacterial infections [50], we observed that ER proteins were present but did not constitute a major fraction in the 321 proteins detected in three different phagosome preparations. The three phagosome preparations were purified from bone marrow derived murine macrophages infected with three different mycobacterial strains including BCG, wild-type H37Rv, and a ΔfbpA-knockout mutant of H37Rv strain. Using an antibody to calnexin, we confirmed that our phagosome preparations were relatively free from ER membrane. The small number of lysosomal proteins detected in this study was also consistent with the results from other studies in that the maturation of the phagosomes containing the three different mycobacterial strains was either inhibited or limited [51].
Rough ER (RER) can participate in phagosome remodeling at a late stage of infection by L. pneumophila and L. longbeachaea [52]. The L. pneumophila-containing phagosome is allowed to associate sequentially with smooth vesicles, mitochondria, and RER to form a compartment called the replicative phagosome [53]. L. pneumophila secretes effectors into host cells to modulate multiple host cell processes and in particular, redirects trafficking of the L. pneumophila phagosome and mediates its conversion into an ER-derived organelle competent for intracellular bacterial replication [54]. L. pneumophila replicates in such a phagosome that excludes early and late endocytic markers and is surrounded by the RER. In contrast, the L. longbeachaea phagosome colocalizes with the early endosomal marker EEA1 and the late endosomal markers LAMP-2 and M6PR, and is surrounded by the RER. Thus, intracellular proliferation of L. longbeachaea occurs in LAMP-2-positive phagosomes that are remodeled by the RER [52].
Using fluorescence microscopy and GFP protein markers, Lu and Clarke showed that the ER membrane was not incorporated into a phagosome during the uptake of L. pneumophila by Dictyostelium [55]. They demonstrated that the entry of L. pneumophila was an actin-mediated process involving rapid transport of the newly formed phagosome within the cell. ER membrane lay close to the newly formed phagosome but was left behind by the phagosome that traversed across the cytoplasm. ER markers were acquired on phagosomes at a later stage when the movement of the newly formed phagosome diminished.
Therefore, there has been little doubt that ER proteins are found in phagosome preparations. While the controversy of the nature of phagosomal membrane [40] may continue between the ER-mediated phagocytosis model [56] and the conventional phagosome maturation model of phagosome biogenesis [39], the debate is more on the extent and timing of ER involvement in phagosome biogenesis and maturation rather than the presence or absence of ER in phagosomes and phagolysosomes. The early models of the Desjardins group that suggested that the ER membrane provides a very large proportion of the phagosomal membrane were probably overestimated. However, many other research groups have identified ER markers on the phagosome as mentioned above and even first mechanistic insights have been published [57, 58].
In addition, bacteria–host cell interactions involve a variety of bacteria and host cells that may differ significantly in bacterial pathogenesis and host immune response with unique niches to each pathogen–host system [7]. Thus, it may be overly simplistic by trying to settle on one model over another model to describe phagosome biogenesis and maturation. It remains a question whether multiple models co-exist that are suitable to describe different types of pathogen–host cell or particulate–phagocyte interactions.
3 Phagosome preparation and biomarkers
3.1 Preparation of phagosomes
Purification of phagosomes is the first crucial step in a phagosomal proteome study. Acquiring and characterizing bacteria-containing phagosomes was considered a ‘holy grail’ because few studies were reported on that important subject at the time [13]. Initial proteomic analyses of phagosomes were performed using 2-D gel analyses with few proteins identified [59]. With advances in the sensitivity of MS techniques, many more new proteins have been detected in phagosome preparations [33]. The higher sensitivity of the approach for phagosome characterization has to be accompanied by advanced techniques to isolate phagosomes with greater purity. In reality, however, biochemical purification of phagosomes can never be perfect because complete separation from other organelles is never achieved due to the complex interaction of phagosomes with other organelles in the cytoplasm [39].
Although bacteria-containing phagosomes are more difficult to purify to high homogeneity, LBPs can be prepared with a much higher purity by taking advantage of the significant density difference between LBPs and other host cell organelles. Thus, LBP fractions can be purified with a purity that exceeds that of any other organelles isolated from cells [60]. Actually, much progress and success in the field of phagosome proteomics has been made due to the fact that LBPs can be isolated at very high degrees of purity, more than any other organelle at least. Thus, LBPs have widely been used as a model system to study phagosome biology. The isolation of LBPs was originally demonstrated by Korn’s group in the 1970s [60] and further developed by Desjardins et al. in 1994 [26]. Because LBPs have a lower density than most other organelles, LBPs can be isolated on a one-step sucrose gradient while phagosomes with bacteria require multiple steps.
Because bacteria-containing phagosomes have densities closer to some other organelles, multiple passes of purification by differential density centrifugation are typically required. The general approaches used today to purify bacteria-containing phagosomes reflect variations and improvements of those procedures described earlier, e.g. by Zeichner [61], Lührmann and Haas [62], Lutz et al. [63], and Sturgill-Koszycki et al. [30].
The vast majority of phagosome purifications are performed with sucrose gradients. Host cells such as macrophages are typically infected with bacteria for a period ranging from a few hours to a few days depending on the protocol. Cells are then scraped into a buffer generally containing HEPES, EGTA, and EDTA. Infected cells are homogenized in the presence of anti-protease mixes and passed through syringes with needle gauges ranging from 25 to 28. The homogenate is usually overlaid onto a sucrose gradient consisting of 12 and 50% sucrose and centrifuged. Phagosomes are collected from the interphase between the 12 and 50% sucrose, washed, and loaded onto a second sucrose gradient for further purification [50, 64, 65].
While sucrose gradients are the predominant method for phagosome isolation, other techniques are also available. One such includes magnetically labeling mycobacteria [66], lysing infected cells and passing the homogenate through a column surrounded by a magnet. Phagosomes containing labeled mycobacteria remain in the column while other cellular debris passes through. Upon removal of the magnet, the phagosomes can be collected [49]. Another technique is very similar to the sucrose gradient; however, after completing a sucrose gradient purification, samples are run on an iodixanol gradient [67].
3.2 Purity of phagosome preparations
Electron microscopy has been used as an important tool to examine the purity, morphology, membrane structure, or protein localization in phagosomes [37, 38, 68–70]. Electron microscopy provides higher resolution than light microscopy, thus is well suited for examining the homogeneity of a phagosome preparation. It provides structure-level details of a phagosome preparation that can reveal entrapment of contaminating membrane fragments or organelles in phagosome preparations. It is also used to examine the purity of an intracellular bacteria preparation, for example, as shown in the preparation of intracellular Salmonella samples [71].
Bacteria-containing phagosomes have densities closer to some other organelles than LBPs [39] (Fig. 1). Contamination from those organelles may still be present even after several times of sucrose gradient centrifugation. In one of our works, we compared the proteome profiles of three phagosome preparations derived from macrophages infected by three different mycobacterial strains [50]. In that work, the proteomic analysis indicated that a large number of proteins were identified as from mitochondria to represent about a third of the detected proteins.
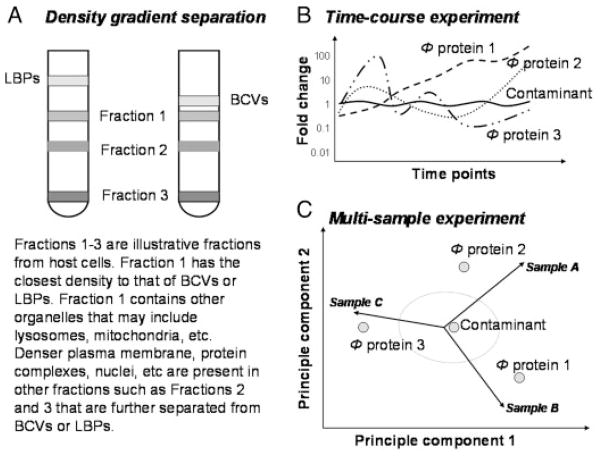
Figure 1.
Schematic illustration of phagosome preparation and a strategy to discern phagosome proteins from contaminating proteins during quantitative proteomic analyses of phagosomes. (A) In a sucrose density gradient separation of LBPs or BCVs from a host cell lysate, LBPs are better separated from other organelles due to their lighter density. (B) In a time-course experiment to monitor the changes in protein concentrations in a phagosome biogenesis, the concentration of a contaminant protein would not change over time because it does not participate in the biogenesis or maturation process of a phagosome. The proteins showing time-dependant fluctuation in concentration are most likely phagosome proteins. The figure was drawn based on [37, 39]. Four proteins are depicted. The proteins with dashed time-course curves are phagosome proteins and the one with a solid time-course curve represents a contaminating protein. Φ-phagosome. (C) In a multi-sample experiment where multiple phagosome preparations are compared under the same condition, the concentration of a contaminant protein would not differ among the several sample preparations. Proteins that are differentially regulated among the several phagosome preparations are most likely phagosome proteins. The figure was drawn based on [50], illustrating the biplot display of a principal component analysis of three phagosome preparations. Four proteins are depicted. The eclipse represents a confidence threshold.
It has been suggested that mitochondria have a similar density with bacteria-containing phagosomes [39]. By using a centrifugation method, the mitochondrion might not be separated from the bacteria-containing phagosome. In addition, autophagy of mitochondria might result in some autophagosomes that share similar density with the bacteria-containing phagosomes. In our preliminary studies, we observed that the ΔfbpA M. tuberculosis strain colocalized into autophagosomes that are thought to include ER [72] or mitochondrial [73] membranes for biogenesis. During formation, autophagosomes sequester cytoplamic contents that might include other organelles such as mitochondria [74]. It is also likely that autophagosomes fuse with phagosomes or phagolysosomes to lead to the entrapment of other organelles within the phagosome [75].
It also cannot be excluded that some mitochondria could be trapped in the same phagosomes with the bacteria, although the entrapment of mitochondria and bacteria in the same phagosomes would be a smaller contribution of the mitochondrial proteins to the phagosome sample composition. Similarly, the co-entrapment of mitochondria with latex beads could also occur. In fact, mitochondrial proteins have been consistently detected in many phagosome and phagolysosome proteomic studies, including the studies of LBPs [13]. LBPs are presumably purer than any other cell organelle preparations because the lighter buoyancy of latex beads allows a more complete separation of the phagosome from the mitochondrion.
Mitochrondria exhibit the closest density to that of bacteria-containing phagosomes in a typical sucrose gradient. Thus, mitochondria could become one of the major sources of contaminating organelles in a phagosome preparation. In a recent study of Legionella-containing phagosomes [76], Shevchuk et al. went through a greater length of separation process to purify the phagosomes. The methods employed include elimination of lysosomes with colloidal iron-loading and magnetic separation, elimination of mitochondria by iodophenylnitrophenyltetrazolium (INT) heavy labeling, and degradation of nucleic acid by Benzonase. The percentage of mitochondrial proteins was reduced to 6.4%. Those results suggest that mitochondria contamination exists during phagosome separation and could be reduced, although not eliminated, with more elaborate treatments.
Owing to the complex interaction of phagosomes with other organelles in the cytoplasm, biochemical purification of phagosomes can never be perfect because complete separation from other organelles is never achieved as stated by Rogers et al. [39]. Because most, if not all, of the proteins in phagosome preparations (except those from bacteria) come from the plasma membrane, other endocytic organelles, and probably ER as well, it is not an easy task to distinguish phagosome proteins from those in contaminating organelles, cytoskeletons, and other membrane fragments.
3.3 Experimental design, contaminant handling, and result interpretation
With proper experimental design, the existence of some contaminating proteins from mitochondria and other organelles might not prevent the determination of phagosome protein relative abundances if the content of contaminants is consistent in phagosome preparations.
Rogers et al. [39] as well as Gagnon et al. [37] made a valid point to state that as the organelle preparations of early and late phagosomes are identical, contaminants should not fluctuate over time (Fig. 1). If a marker of another contaminating organelle is enriched with a phagosome preparation, such a marker would not participate in a phagosome biogenesis or maturation process. Thus, authentic phagosome proteins and contaminating proteins can be distinguished based on their time-dependent concentration fluctuation (Fig. 1).
Similarly, if multiple phagosome preparations are performed under identical conditions such as the preparation of three different mycobacteria-containing phagosome samples shown in the work by Rao et al. [50], proteins from contaminating organelles should not differ among different phagosome preparations. Thus, the proteins found differentially abundant among different phagosome preparations represent the effect of modulation by different bacterial strains that infected the host cells (Fig. 1). Those regulated proteins are most likely to be phagosome proteins. For example, several proteins found enriched on the wild-type M. tuberculosis-phagosome are indeed phagosome proteins, such as Nicastrin, Cdc42, and Rab11 [50].
In the experiments by Rogers et al. [39], calnexin, an ER marker, did not fluctuate more than two-fold at seven time points over 2 h, suggesting that ER could be a contaminant or it was not regulated during the LBP biogenesis process.
On the other hand, in the experiment by Gagnon et al. to monitor the degradation of calnexin over the time course of LBP formation and maturation [37], calnexin was found to be degraded dramatically during the phagolysosome biogenesis process with its highest concentrations at the early time points but nearly zero concentration several hours later [37]. Based on the assumption that the amount of markers from contaminating organelles should not fluctuate over time, calnexin should not be considered as being from contaminating ER. In other words, ER was demonstrated to be a component of the newly formed LBPs [37].
Interestingly, these results from Rogers et al. [39] and Gagnon et al. [37] add additional complexity to the ongoing controversy over the role of ER in phagosome biogenesis, which probably will require further studies to resolve [40].
3.4 Some known phagosome protein markers
In Table 1, we summarize some known phagosome proteins that have been studied by methods other than MS-based proteomics, i.e. non-MS methods, in mouse and human macrophages. Some of these proteins indicate the stage of phagosome maturation and vesicular fusion. Therefore, they could potentially serve as markers for monitoring the progression of phagosome biogenesis and maturation. They can also be used to assess the pathways of phagosome biogenesis, maturation, and antigen presentation. Because the behavior of many of these proteins is predictable, they could be useful for validation purposes in phagosomal proteome experiments as well. The functions of many of these proteins are known, but some remain for further characterization.
Table 1.
Representative phagosomal proteins detected by non-MS methods
| Protein | Host cell | Detection method | Remark/protein function | Reference |
|---|---|---|---|---|
| Cathepsin D | Balb/C BM-MΦ | Western | Hydrolase | [114] |
| Rab5 | Balb/C BM-MΦ | Confocal Microscopy | Early endosome marker | [93] |
| Rab7 | J774 | Western | Late endosome marker | [70] |
| Transferrin | Balb/C BM-MΦ | Confocal Microscopy | Recycling endosome marker | [93] |
| PI3P | C57Bl/6 BM MΦ | Fluorescence microscopy | Signaling | [115] |
| EEA1 | RAW 264.7 | Microscopy | Early endosome marker | [116] |
| Rab10 | RAW 264.7 | Microscopy | Membrane transport | [116] |
| Rab14 | RAW 264.7 | Fluorescent microscopy | Phagosome maturation arrest | [100] |
| Rab21 | RAW 264.7 | Fluorescent microscopy | Early endosome | [117] |
| Rab22 | RAW 264.7 | Fluorescent microscopy | Endosomal trafficking | [117] |
| Cellubrevin | J774 | Western | Recycling endosomes | [118] |
| NSF | J774 | Western | Membrane fusion | [118] |
| SNAP | J774 | Western | Membrane fusion | [118] |
| SNAP23 | J774 | Western | Plasma membrane | [118] |
| GDIs | J774 | Western | Maintains Rab5 | [119] |
| P38 MapK | J774 | Western | Cell signaling | [119] |
| Rab11 | J774 | Western | Recylcing endosomes marker | [120] |
| Syntaxin 3 | J774 | Western | Plasma membrane | [120] |
| Syntaxin 8 | J774 | Western | Early endosome | [120] |
| Syntaxin 13 | J774 | Western | Early endosome | [120] |
| Syntaxin 4 | J774 | Microscopy | Plasma membrane | [91] |
| TACO | J774A.1 | Western | Actin binding | [121] |
| CIP50 | J774A.1 | Western | Aids coronin | [121] |
| Annexin | THP-1 | Western | Transport/endocytosis | [122] |
| PI3K | THP-1 | Western | PI3P synthesis | [123] |
| Lamp | Human monocyte | Electron microscopy | Lysosome/phagosome marker | [81] |
| MHC class I | Human monocyte | Electron microscopy | Antigen presentation | [81] |
| MHC class II | Human monocyte | Electron microscopy | Antigen presentation | [81] |
| Calnexin | Human DC | Microscopy | ER chaperone | [124] |
| CD1 | Human DC | Fluorescence microscopy | Antigen presentation | [125] |
4 Phagosome proteins identified by proteomics
4.1 Number of potential phagosome proteins and localization evidence
It remains an open question how many proteins can eventually be extracted and characterized from a phagosome.
It was previously estimated that 1000 proteins could be present on a phagosome [14] and a recent in-depth proteomic analysis of LBPs has identified 2415 phagosome proteins with a high level of sensitivity [33]. LBPs are exceedingly pure phagosome preparations that one can obtain [60]. LBPs are probably more homogenous than any other bacteria-containing phagosome preparations as revealed by electron microscopy examinations [37, 38, 68–70].
While electron microscopy provides structure-level details about a phagosome preparation, a proteomic analysis provides greater molecular level details of a phagosome preparation. It has been shown that 3000–7000 proteins could be identified from a Hela cell sample including membrane proteins [77, 78]. If one applies such a high-sensitivity proteomic analysis for a phagosome preparation, proteins present at a very low level in a phagosome preparation, including those from trace contaminants, could also be identified.
Thus, it should be cautioned that the identification of a protein in a phagosome preparation by highly sensitive proteomic analyses does not necessarily represent the definitive and ultimate evidence for the true localization of the protein on a phagosome. This caution is particularly applicable to BCVs and is also applicable to LBPs even though they are typically purer. With many proteins identified from a phagosome preparation, there have not been secondary methods available to validate the true localization of each protein identified by proteomics at a comparably large scale. Conventional methods such as fluorescent fusion proteins or immunostaining could be used to validate a small number of identified proteins, especially abundant ones.
While LBPs contain only host proteins, bacterial phagosomes are obviously a mixture of both host and pathogen-derived proteins. Earlier studies were carried out to examine the proteome profiles of BCVs or LBPs [59, 79, 80], to decipher the endosomal–lysosomal pathway [81], and to identify the determinants of phagosome–lysosome fusion [82]. Several more recent studies have been carried out at a larger scale with more sensitive proteomic techniques to enable a systems level investigation of phagosomal proteomes.
4.2 Proteomic studies of latex-bead phagosomes
Garin et al. employed 2-D gel, 1-D SDS-PAGE, MALDI-TOF, and electrospray ionization-MS to characterize LBPs with an identification of about 140 proteins [83]. The most important finding of that study was that it identified proteins involved in cell signaling and membrane fusion events. It also revealed contamination from mitochondrial proteins and the inability of 2-D gels to detect some membrane proteins. The ability to identify membrane proteins was enhanced by Triton X-114 partitioning of phagosomal membrane proteins followed by 1-D SDS-PAGE separation and MS identification.
Stuart et al. used a 2-D gel to extensively fractionate LBP proteins into 96 fractions for subsequent LC/MS protein identification using a QTOF mass spectrometer [84]. Six hundred and seventeen proteins were identified to potentially associate with Drosophila LBPs. Of these 617 LBP proteins, 20% were predicted to have transmembrane domains and 70% were identified to have mammalian orthologues. Of the 140 mammalian phagosome proteins identified by that time [56, 83, 85], 100 had orthologues in the Drosophila LBP. In that study, a control experiment was carried out to confirm the purity of LBPs. Specifically, LBPs were generated from unlabeled Drosophila S2 cells and mixed with [35S]-labeled S2 cells that had not seen latex beads. LBPs were re-isolated and contaminating proteins were detected by 2-D-gel electrophoresis and autoradiography. It was confirmed that <30 proteins could be identified as potential contaminants resulting from the LBP purification process.
Using 2-D-gel electrophoresis, Gotthardt et al. generated 6-time point profiles for 1388 protein-spot intensities over the course of LBP maturation in Dictyostelium [86]. Clustering analyses of the time profiles revealed 24 functional groups that were mapped to discrete steps during LBP maturation. Hundred and seventy-nine phagosome proteins were identified by MALDI-TOF or electrospray ionization-QTOF MS. Among those 179 identified proteins, two G proteins appeared at the early stage of phagocytosis. Mutation of those two G proteins was found to generate a phagocytic defect in latex bead uptake in Dictyostelium.
In the study by Rogers and Foster [39], 505 phagosome proteins were identified for the LBP proteome from RAW 267.4 macrophages. In that study, protein samples were also fractionated with 1-D SDS-PAGE. Peptides resulting from in-gel digestion of gel bands were analyzed with nano-LC/LTQ-Orbitrap for protein identification and quantitation. Of the 505 identified LBP proteins, 382 could be reliably quantified across various time points. The authors stressed that biochemical purification of phagosomes could never be perfect because complete separation from other organelles was never achieved. Markers for other organelles were indeed also measured in the LBP preparations.
Rogers and Foster used SILAC as the quantitative proteomics method to model the LBP maturation process. Three SILAC formulations were used to label seven time points of LBP maturation in a combination of three experiments. Samples labeled at the same time point in different experiments with the same SILAC formulation were used to scale the different experiments. By doing so, the triplet time-point measurements in separate experiments could be combined to generate the seven time point abundance profile of a protein during a 2-h LBP maturation process. The more detailed description of the experimental design is available in [39].
In general, those early studies of phagosomal proteomes were limited in the ability to extensively identify proteins from LBPs.
Most recently, Trost et al. identified 2415 LBP proteins from RAW 267.4 macrophages by employing an LTQ-Orbitrap mass spectrometer, the state-of-the-art proteomic instrumentation [33]. The LTQ-Orbitrap mass spectrometer is several times more sensitive than other mass spectrometers. In that work, as little as 20 μg of LBP protein lysate was fractionated into 17 fractions by 1-D SDS-PAGE for subsequent protein identification with nanoLC/LTQ-Orbitrap analyses. Two thousand four hundred and fifteen LBP proteins were identified at a false discovery rate of <1%. A total of 29 941 unique peptides were identified to result in an average of 24% sequence coverage for the identified proteins. The high coverage of the protein sequences suggests that the LBP proteins were probably exhaustively identified from the LBP preparation. The reported 2415 LBP proteins identified in that study represents the most comprehensive coverage of a phagosomal proteome reported to date.
The study by Trost et al. [33] identified proteins differentially expressed in LBPs harvested from IFN-γ activated macrophages compared with those from naïve ones. By examining the differential expression and modification of the proteins, Trost et al. demonstrated the role played by signaling molecules in assembly and maturation of the LBP with proteomic analysis. The activation of macrophages by IFN-γ leads to complex antimicrobial activities that allow macrophages to sequester and kill pathogens for efficient antigen presentation. Many signaling molecules such as Ras-like proteins and kinases were identified. IFN-γ activation was found to increase the phosphorylation of many proteins due to an increase in the activity of various kinases.
4.3 Proteomic studies of bacterial phagosomes
For bacteria-containing phagosomes, the number of phagosome proteins identified by proteomics is generally lower, probably due to the fact that bacteria-containing phagosome preparations in large quantities are more difficult to obtain with purity as high as that of LBP preparations. Similarly, the proteomic analysis of intracellular bacteria from a variety of bacteria–host cell interactions [7] also revealed that the numbers of proteins identified for intracellular bacteria samples were significantly lower than those obtained for pure bacterial cultures. Li and Lostumbo ascribed the discrepancy to the use of older proteomic techniques and the lack of purity and amount in the intracellular bacterial sample preparation in many of those studies [7].
Using the 2-D-gel/MALDI-TOF technique, Shevchuk et al. identified 157 host proteins from Legionella-containing phagosomes isolated from Dictyostelium [76]. In that work, mitochondrial contaminations were reduced by INT ‘heavy labeling’. Succinate dehydrogenase activity of mitochondria reduced INT and the resulting product, formazan, was deposited on mitochondrial membranes. This step increased the density of mitochondria and enhanced their separation from bacteria-containing phagosomes during a continuous density gradient centrifugation.
Urwyler et al. purified Legionella-containing phagosomes from Dictyostelium by magnetic immunoseparation followed by density gradient centrifugation [67]. Five hundred and sixty-six host proteins were identified from the Legionella-containing phagosome by microLC/MS with an LTQ mass spectrometer. Of those 566 proteins, 44 were found among the 176 proteins identified from LBPs in Dictyostelium [86]. Twenty-three of those 566 proteins were found among the 116 proteins identified from LBPs in the J774 mouse macrophage-like cell line [83]. In that study, proteins were resolved by 2-D-gel electrophoresis and proteins were identified by MALDI-TOF or LC/MS with a QTOF mass spectrometer [83]. The underlying reasons for the low overlap among those three different data sets are difficult to deconvolute because two different hosts and three different proteomic platforms were used [67, 83, 86]. Nevertheless, the identification of the 566 host proteins suggested that both known and novel components were found in the Legionella-containing phagosome and that the Legionella-containing phagosome communicated with late secretory and endosomal pathways [67].
In a proteomic study of BCG-containing phagosomes in PMA-differentiated THP-1 human macrophages, Lee et al. identified 447 and 289 host proteins in the BCG-containing and the latex bead-containing phagosomes, respectively [87]. Phagosome preparations were fractionated on 1-D SDS-PAGE and proteins were identified by LC/MS with a QTOF mass spectrometer. In total, 475 host proteins were identified from either of the two phagosome compartments, i.e. either the BCV or the LBP. 260 proteins were identified in both of the BCV and LBP preparations, indicating a high degree of overlap in protein composition between the two phagosome compartments.
Despite the high degree of overlap in protein compositions, Lee et al. indicated that the BCG-containing phagosome had a number of proteins that consistently were not detected from the LBP [87]. It is noteworthy that 178 proteins were identified in three out of five BCG-containing phagosome preparations, and all of those 178 but 32 proteins were identified in at least one of the three LBP preparations. The authors further suggested that those 32 proteins present in the BCG-containing phagosomes but not detected in the LBP might reflect an important difference in cell biology between the two phagosome compartments.
But, the additional proteins detected in the BCG-containing phagosome preparation might be due to technical variation in proteomic analysis more than due to biological differences between the two phagosome compartments. This concern is because a higher number of host proteins are usually detected from an LBP preparation, ranging from ~500 [39] to ~2000 [33]. Therefore, further studies await to verify whether the missing 32 proteins in the LBP preparation were indeed due to biological rather than technical variation during analyses [87]. On the other hand, many of those 32 proteins were actually identified on LBPs in a most recent work by Trost et al. [33]. Nevertheless, Lee et al. identified the most host proteins from a mycobacterial phagosome reported to date (447) [87].
We employed a nanoLC/LTQ-FTMS-based proteomics system to analyze the bacillus-containing phagosomes purified from bone marrow derived BMA3.A3 macrophages infected with three different mycobacterial strains [50]. The results allowed us to gain insight into the modulation of the phagosomal proteome by the M. tuberculosis H37Rv wild-type strain, a ΔfbpA attenuated H37Rv mutant strain, and the BCG vaccine strain, respectively. At a false discovery rate of 2%, we identified a total of 322 proteins from the three phagosome preparations containing the three different mycobacterial strains, respectively. Using a label-free proteomic method [88], we cross-referenced the identified peptides among the three samples so that we were able to quantify those identified proteins in all of the three phagosome samples. All but one of the 322 proteins were host proteins. Comparison of our list of proteins identified with the proteins identified by Trost et al. [33] indicates that about 71 proteins were likely unique to mycobacterial infections. An analysis of certain phagosomal maturation markers suggests that BCG- and H37Rv-infected phagosomes were able to inhibit phagosome maturation while ΔfbpA-infected phagosomes proceeded further in maturation.
5 Rab markers in phagosome maturation
5.1 The Rab5/Rab7 switch
Phagosome maturation involves multiple fusion events with early and late endocytic compartments and probably some other organelles such as ER. The involvement of extensive and selective membrane trafficking events like those occurring for the endosomal compartments leads to rapid and dynamic changes of the composition of the phagosomal membrane and contents. Thus, proper trafficking of various organelles to the phagosomal membrane is critical for phagosome maturation. Among the signaling molecules important for the trafficking process, small GTPases form the signaling cascades through which many effectors are recruited to the phagosome membrane.
Among the small GTPase molecules, Rab proteins are responsible for transport, recruitment, and fusion of vesicles [89]. For example, Rab5 is the central organizer of the protein machinery that assembles on early endosomes. It provides a unique identity to the compartment that directs cargo sorting, transport, and signaling. Thus Rab5 is essential for early endosomal fusion. Not only is Rab5 essential for early endosomal fusion, it is also essential for the recruitment of Rab7 and for the progression of phagosomes to phagolysosomes [90]. When phagosomes progress to fuse with late endosomes and eventually lysosomes, they lose Rab5 and acquire markers of late endocytic organelles such as Rab7. A change in the relative levels of Rab5 and Rab7, or the Rab5/Rab7 switch, is thus observed in the phagosome maturation process. The Rab5/Rab7 switch has also been seen as early endosomes mature into late endosomes. Thus, detection and quantitation of selective Rab markers such as the Rab5/Rab7 switch is useful to discern a ‘stalled’ phagosome from a mature one [91].
The absence of Rab7 from stalled phagosomes is not simply because they do not mature. Bacteria such as those in the M. tuberculosis complex actually selectively exclude Rab7 from phagosomes [70]. In addition to Rab5, functional phosphatidylinositol 3-kinases (PI3K) are also required for successful phagosome maturation [90]. Inhibition of PI3K prevents phagosome maturation but not the accumulation of Rab5 or Rab7 on phagosomes. When PI3K is inhibited, however, the accumulation of Rab7 alone is not sufficient to induce phagosome maturation. It was suggested that Rab5 activates both PI3K-dependent and PI3K-independent effectors that act in parallel to promote phagosome maturation [90].
5.2 Rab proteins identified by proteomics
Rabs are also phosphorylated during the maturation of phagosomes [33]. The phagosome formation and maturation process is complex and requires Rab proteins to interact with each other, concoct signaling cascades, and recruit effector proteins. Modulation of the fusogenic events can lead to the arrest of the phagosome, making Rabs critical in host–pathogen interaction.
Table 2 lists 52 Rab proteins detected from LBPs and BCVs in several proteomic studies that provide the most comprehensive coverage of phagosomal proteomes reported to date. Of these 52 Rab proteins, only a limited number of them have known functions [89]. While the Rab5/Rab7 switch is critical in phagosome maturation, Rab5 is also known to be important in the acquisition of iron in mycobacterial studies [92, 93]. As described earlier, Rab7 does not accumulate in pathogen-inhabited phagosomes as efficiently as Rab5 because Rab7 is involved in late endosomal transport [91]. Phosphorylation of Rabs was implicated in the activation of macrophages [33] and protein–protein interactions [89]. Because Rabs may play multiple roles, they are important proteins to target for proteomic study to improve the understanding of the modulation of BCVs by a variety of intracellular bacteria [7]. Meanwhile, proteomic studies of LBPs have provided immense information to elucidate the localization and possible regulation of some Rab proteins in the phagosome biogenesis and maturation processes.
Table 2.
Rab proteins detected in LBPs or BCVs
| Rab protein |
Drosophila/ LB [84] |
RAW264.7/ LB [33] |
J774/ LB [83] |
Dictyostelium/ LB [86] |
Dictyostelium/ L. pneumophila [67] |
THP-1/ BCG [87] |
BMA/ H37Rv [50] |
BMA/ ΔfbpA [50] |
BMA/ BCG [50] |
|---|---|---|---|---|---|---|---|---|---|
| Rab1 | x | ||||||||
| Rab10 | x | x | x | x | x | x | x | ||
| Rab11 | x | ||||||||
| Rab11A | x | x | x | ||||||
| Rab11B | x | x | x | x | x | ||||
| Rab11C | x | ||||||||
| Rab12 | x | ||||||||
| Rab13 | x | x | |||||||
| Rab14 | x | x | x | x | x | x | x | x | |
| Rab18 | x | ||||||||
| Rab19 | x | ||||||||
| Rab1A | x | x | x | ||||||
| Rab1B | x | x | x | ||||||
| Rab1D | x | x | |||||||
| Rab2 | x | x | |||||||
| Rab20 | x | ||||||||
| Rab21 | x | x | |||||||
| Rab22A | x | ||||||||
| Rab23 | x | ||||||||
| Rab24 | x | ||||||||
| Rab2A | x | x | |||||||
| Rab2B | x | ||||||||
| Rab32 | x | ||||||||
| Rab32A | x | ||||||||
| Rab34 | x | ||||||||
| Rab35 | x | x | |||||||
| Rab37 | x | ||||||||
| Rab38 | x | ||||||||
| Rab39A | x | ||||||||
| Rab39B | x | ||||||||
| Rab3B | x | ||||||||
| Rab3C | x | x | |||||||
| Rab3D | x | ||||||||
| Rab4 | x | ||||||||
| Rab43 | x | ||||||||
| Rab4B | x | ||||||||
| Rab5 | x | ||||||||
| Rab5A | x | x | |||||||
| Rab5B | x | x | |||||||
| Rab5C | x | x | x | x | x | ||||
| Rab6 | x | ||||||||
| Rab6A | x | x | |||||||
| Rab6B | x | ||||||||
| Rab7 | x | x | x | x | x | x | |||
| Rab7A | x | x | x | ||||||
| Rab7B | x | ||||||||
| Rab7L1 | x | ||||||||
| Rab8 | x | ||||||||
| Rab8A | x | x | x | ||||||
| Rab8B | x | x | |||||||
| Rab9A | x | ||||||||
| RabC | x |
Using in-depth proteomic analysis, Trost et al. demonstrated the regulation of signaling molecules in the assembly and maturation of LBPs in IFN-γ activated macrophages [33]. Forty-one different Rab proteins were identified from LBPs in that study (Table 2), consistent with their importance in the regulation of membrane fusion and trafficking during phagosome maturation. Of the 41 identified Rab proteins, only four displayed substantial changes in abundance or phosphorylation, including Rab7b, Rab12, Rab20, and Rab43. With network analysis, Trost et al. observed that the IFN-γ activation of macrophages affected transport vesicles, endosomes, and lysosomes. Contrary to the results shown in some other studies that IFN-γ activation enhanced phagosome maturation [94], the IFN-γ activation of macrophages delayed the fusion of phagosomes with lysosomes and led to an overall reduction in the degradative capacity of phagosomes. On the other hand, the IFN-γ activation increased cross-presentation by MHC class I, consistent with the observation that activation of macrophages by IFN-γ leads to efficient antigen presentation [94]. Thus, the proteomic study of LBPs by Trost et al. provided a detailed molecular view of phagosome biology and antigen presentation.
5.3 Change of Rab protein levels during phagosome biogenesis and maturation
An analysis of BCVs and LBPs suggests that most Rabs are recruited to the phagosome membrane but their concentrations may fluctuate due to impaired or limited recruitment. Many intracellular pathogens that survive in BCVs are known to modulate the acquisition of Rabs on phagosomes in host cells. Pathogens such as M. tuberculosis interfere with Rab effector proteins and hence the ability of the phagosome proteins to interact with each other [95–98].
In a recent study of Legionella-containing vacuoles over a time course of 15 min to 14 h [67], it was observed that there were no qualitative changes, i.e. the presence or absence, in the different classes of identified proteins. Instead, many changes in the phagosomal proteome involved fluctuation of protein concentrations in a quantitative manner, suggesting that a small change in the concentrations of some Rab proteins might be sufficient to trigger down-stream signaling processes leading to vesicle fusion events.
That a small change in the concentration of a phagosome protein can trigger molecular events can be in part explained by the phagosome individuality described by Griffiths [99]. Griffiths indicated that phagosomes formed via the same receptors could find themselves in different chemical states even within the same macrophage. Because heterogeneity of phagosomes exists within the same macrophage as well as among different macrophages, we typically sample an average of the concentrations of a phagosome protein on different phagosomes at different states. A clearly synchronized cascade of molecular events would not be readily observed in many typical experiments. Therefore, although the change in the average concentration of a phagosome protein might appear to be moderate, it does not exclude that a subpopulation of the phagosomes already incur more qualitative changes and proceed further into next phase of molecular events.
While LBPs go on to fuse with their target vesicles and their proteomic profiles display an entire gamut of proteins associated with a maturing phagosome, BCV proteomes show more diverse changes of profiles in phagosome biogenesis and maturation processes. For intracellular pathogens such as L. pneumophilia and mycobacterial species, the phagosomal Rab protein amounts seem to be limited. Studies done on mycobacterial phagosomes also gave conflicting results depending upon which host cells were used for the study.
Studies on mycobacterial phagosomes isolated from J774 macrophages showed that mycobacteria-containing phagosomes did not acquire Rab7 even seven days after infection, whereas Rab5 accumulated in large quantities. This result shows that the bacteria-containing phagosomes were able to fuse with some early endosomal vesicles but were not able to proceed further to fuse with late endosomal vesicles necessary for eventual fusion with lysosomes [91]. Rab14 was shown to be critical to maintain M. tuberculosis phagosome maturation arrest [100].
In contrast, immunostaining analyses of phagosomes containing M. tuberculosis in Hela cells suggested that M. tuberculosis-containing phagosomes acquire Rab7 on day one of infection [92]. Despite the acquisition of Rab7, the M. tuberculosis-containing phagosome still exhibited maturation arrest. However, it should be noted that HeLa cells are not only not phagocytes but also not the natural niche of mycobacteria. Endosomal sorting processes may be different in phagocytic versus non-phagocytic cells. While M. tuberculosis-containing phagosomes were able to acquire both Rab5 and Rab7, L. pneumophilia-containing phagosomes acquired Rab5 first and then Rab7 after further maturation. Arrest of the phagosome might take place sometime after the acquisition of Rab7.
Proteomic analyses indicate that BCG- and M. tuberculosis-containing phagosomes from different host cells contain both Rab5 and Rab7, suggesting that acquisition of specific Rab proteins is dependent on the pathogen (Table 2). Western blot analysis of live versus dead Salmonella-containing phagosomes revealed that Salmonella acquires Rab5 early in the phagosome but selectively depletes Rab7 and Rab9 in order to block the phagosome–lysosome fusion [101]. In a recent proteomic analysis of phagosomes containing L. pneumophilia (virulent) and L. hackeliae (less virulent), respectively, 157 host proteins were identified with 2-D-gel/MALDI-TOF [76]. No Rab proteins were found, but a few other signaling proteins were detected. However, the absence of Rab proteins from the list of 157 detected proteins could be simply due to the limitation in methodological sensitivity rather than of biological regulatory effects. That example stresses the need to comprehensively profile the phagosomal proteome or to employ targeted analysis strategies to specifically monitor a subgroup of proteins such as the Rab proteins [102].
In studies of pathogen-containing phagosomes, Rab proteins associated with late-endosomal vesicles might not be detected. This appears to be the case for Rab9 but not the case for Rab11 (Table 2). Rab9 and Rab11 are associated with late endosomal compartments and hence with the arrest of phagosomal maturation. Both Rab9 and Rab11 have been detected in LBPs [33]. While Rab9 has not been detected in pathogen-containing phagosomes in the studies listed in Table 2, Rab11 has been detected in most of those studies.
Because proteomic analyses of pathogen-containing phagosomes have not identified as many proteins as in LBP analyses, the presence or absence of Rab proteins from specific BCVs or LBPs remains to be confirmed with more controlled experiments. It is quite likely that many of the Rab proteins and other signaling molecules are regulated in a precise manner with relatively small changes in concentrations on phagosomes. Therefore, quantitative proteomic approaches with high precision and large dynamic range are needed to obtain useful information to help decipher the roles of many signaling proteins in the phagosomal proteome.
As discussed earlier in Section 4, the numbers of identified proteins in bacteria-containing phagosomes are generally fewer than those in LBP preparations. This discrepancy in the number of identified proteins between BCVs and LBPs is similar to the scenario in intracellular bacterial proteome analyses where more proteins are typically identified from pure bacterial culture cells than from intracellular bacterial preparations [7]. Such a discrepancy will likely be reduced when more sensitive proteomics techniques are applied or when the phagosome sample preparation techniques are further improved to generate higher amounts of purer materials for in-depths proteomic analyses, which can support a systems analysis of the important roles of phagosomes in innate and adaptive immunity [33].
6 Systems biology of host-cell and microbial-pathogen interaction
Früh et al. suggested that “scientists in the area of microbial pathobiology will soon regard the integrated ‘omics’ approach as the fundamental tool of their discipline” [3].
In the last decade, there has been a considerable increase in activities aiming at genome-wide analyses of pathogenic microbes and molecular pathogenesis processes. Those activities were largely facilitated by the completion of the human genome sequence, publication of genome sequences for many human pathogens, development of microarray technology and high-throughput proteomics, and maturation of bioinformatics [103]. The advancements in those fields also revealed the tremendous needs to develop and use an integrated approach to enhance our understanding of the disease process. Systems biology is such an integrated approach.
Systems biology of infection is a particularly fascinating research area. It begins to bear fruit [104–106]. One can use a systems-level analysis to study interaction of co-evolved systems with major importance for human health [107].
Bumann indicated that the prevailing focus on the action of individual pathogen or host components could be one potential reason why it is so difficult to translate basic research into effective strategies for combating infectious diseases [107]. There is no doubt that the conventional reductionist approach has contributed tremendously to identifying and characterizing key virulence and immune factors [108, 109]. Such a conventional approach, however, lacks the ability to explain the course of complex multi-factorial infectious diseases involving many interacting pathogen and host factors. It was suggested that the vast existing knowledge and development of appropriate methodologies need to be integrated for analyzing host–pathogen interaction networks [107]. Such an integration of multi-factorial host–pathogen interaction networks will be required to facilitate rational development of new control strategies against infectious disease [110].
It should be noted that, while the reductionist approach is certainly not resulting in a comprehensive recapitulation of a complex host–pathogen interaction, the technical limitations currently still hinder real systems biology approaches that likely would gain more insights.
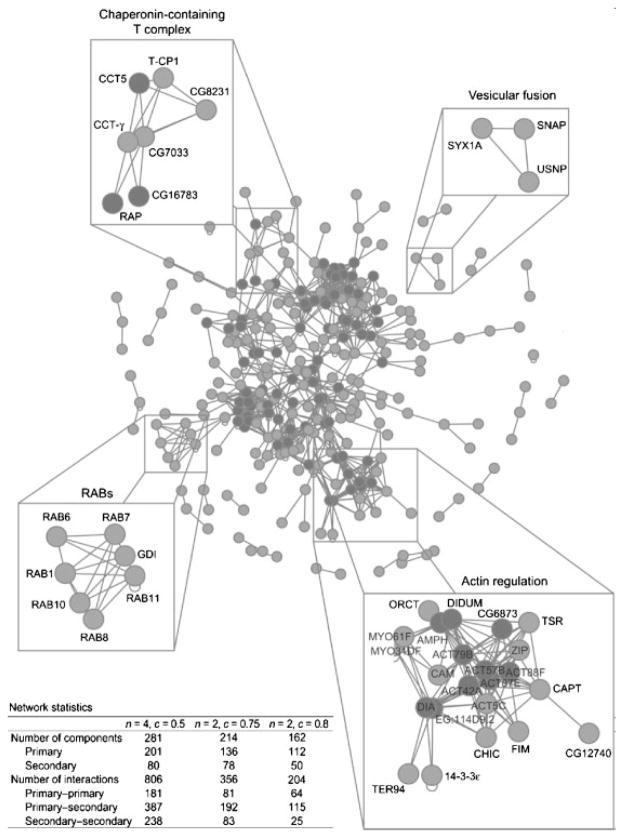
Stuart et al. applied the systems biology approach to study LBPs isolated from the model organism D. melanogaster and constructed a ‘phagosome interactome’ from the 617 proteins identified in LBP preparations (Fig. 2) [84]. Among the 617 proteins, 201 (the lighter vertices) were of high confidence with ≥4 connections at c>0.5. At the same confidence level, 80 proteins (the darker vertices) were predicted to interact with those 201 detected proteins although they were not detected in the LBP proteomic analysis. The phagosome interactome network was also optimized to reveal clusters of functional modules and protein complexes for proteins at a somewhat lower confidence level [84]. To ascribe functions to the LBP proteins in the network, Stuart et al. used RNAi and a fluorescence-activated cell-sorting assay to screen 837 genes for their roles in the phagocytosis of S. aureus and E. coli. The assay confirmed the role of genes previously implicated in phagocytosis including Rac, Cdc42, and Rabs. The analysis also identified tubulin, Rab-GDI, and chaperonin-containing T-complexes as important for the phagocytosis of bacteria, among some other new findings. Thus, the detailed interactome model derived from the proteomic analysis of LBPs provided some new insights into the functional organization of the LBP and the necessary framework to build upon to extend the understanding of phagocytosis in mammalian host–pathogen interaction [84].
Figure 2.
The protein–protein interaction network in LBPs of D. melanogaster. The LBP protein interaction network consists of the predicted high-confidence protein–protein interactions among the 201 LBP proteins identified by proteomics (‘primary components’; lighter vertices) and the 80 proteins predicted to interact with at least four primary components (‘secondary components’; darker vertices) although these 80 proteins were not directly detected in the proteomic analysis. The network corresponds to (n,c)=(4, 0.5) where n is the number of interactions and c is the confidence of the interaction. Network statistics for three values of (n,c) are tabulated. Adapted from [84] with permission.
In tuberculosis research, systems biology integrates approaches to study the host, the pathogen, and their interactions. Young et al. used M. tuberculosis as an example to illustrate how systems-biology approaches have begun to take on some challenging aspects in host–pathogen interaction, especially the basis of pathogen persistence [104]. The outcome of M. tuberculosis infection was attributed to interactions ranging from molecular and cellular to anatomical and population levels. Several mathematical models arising from systems-biology approaches were suggested to potentially establish quantitative links across multiple biological scales. These models include continuous modeling, discrete modeling, deterministic modeling, and stochastic modeling that were best fit for different scales [104]. For example, modeling at cellular and molecular levels begins to unravel the ways in which the pathogen manipulates host-cell signaling via the TLR4 agonist LPS [111] and to capture the complexity of the fate of mycobacteria in macrophages [112].
7 Concluding remarks
Proteomics has demonstrated an important utility in deciphering compositions and workings of bacteria-containing phagosomes at a global level. The phagosomal proteome represents a scenario where other genomics tools such as microarray cannot be directly applied. While high-throughput RNAi could be useful to interrogate the function of individual proteins in a phagosome, proteomics is suitable to provide an integral view of a phagosome proteome with molecular level details. The quantitative information provided by a phagosomal proteome survey is well suited for a systems analysis. On the broad horizon of systems biology, proteomic studies of phagosomal proteomes will likely continue to make substantial contributions to the progress in eliminating difficult infectious diseases such as tuberculosis.
There are several technological challenges to be met in phagosomal proteome analyses.
First, it is more difficult to obtain pure phagosome preparations from host cells being infected by bacteria than from those internalizing latex beads. In reality, proteins from other contaminating organelles will be present in many experiments. It will be critical to maintain a consistent phagosome preparation condition when one studies the modulation of a phagosomal proteome by different bacterial strains or to assess the phagosome biogenesis, maturation, and antigen presentation processes with a time course study. By doing so, the concentrations of contaminating proteins will not fluctuate over time or across multiple sample preparations. Then we will be able to correctly attribute the response of authentic phagosome proteins to bacterial infections, to the phagosome biogenesis process, or to the antigen presentation pathways.
Second, it is difficult to detect and quantify both bacterial and phagosome proteins from whole lysates of bacteria-containing phagosomes. This difficulty is especially prominent at an early stage of host–pathogen interaction due to the overwhelming presence of host proteins in a bacteria-containing phagosome where bacteria do not replicate. Thus, few bacterial proteins have been directly detected from the whole lysate of bacteria-containing phagosomes [7] with exceptions that exist for the BCVs of aggressively replicating intracellular bacteria such as Salmonella [71]. With the most recent advent in the high-sensitivity proteomic technologies that enable the identification and quantitation of thousands of proteins in a sample [33, 77, 78], many opportunities exist for a meaningful or in-depth analysis of bacteria-containing phagosomes to include both bacterial and host proteins. Although detection of a small amount of bacterial proteins from a complex host–pathogen protein mixture is challenging, an encouraging example is that a number of bacterial effector protein molecules can be detected in the cytosolic fraction of a bacterium-infected host cell [113].
Third, while the protein–protein interaction networks have been modeled for phagosomal host proteins and intracellular bacterial proteins, respectively, a bioinformatic approach is still needed to define the protein–protein interaction networks in integral bacteria-containing phagosomes. Currently, in silico approaches are used to predict and construct host–pathogen protein-interaction networks based on homologous domains that exist between host and bacterial proteins. But, in silico approaches alone are not very likely to give real interactions between proteins. Real interaction data such as those obtained with tandem-affinity-purification tagging or immunoprecipitation will be necessary to identify interacting proteins. Proteomic analyses are capable of uncovering bona fide interactions between host and bacterial proteins without relying on a prediction from homologous domains. Proteomic analysis also provides a unique and exciting opportunity to decipher the correlation of abundances or modifications between the detected bacterial and host proteins in an integral bacteria-containing phagosome. With such an opportunity, it is possible to directly deduce an interaction between bacterial and host proteins at a systems level for different bacteria–host interaction models [7].
Abbreviations
- BCV
bacteria-containing vacuole
- LBP
latex bead-containing phagosome
- INT
iodophenylnitrophenyltetrazolium
- RER
rough ER
Footnotes
The authors have declared no conflict of interest.
References
- 1.Corsaro D, Venditti D, Padula M, Valassina M. Intracellular life. Crit Rev Microbiol. 1999;25:39–79. doi: 10.1080/10408419991299167. [DOI] [PubMed] [Google Scholar]
- 2.Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 3.Fruh K, Finlay B, McFadden G. On the road to systems biology of host–pathogen interactions. Future Microbiol. 2010;5:131–133. doi: 10.2217/fmb.09.130. [DOI] [PubMed] [Google Scholar]
- 4.Fialho AM, Stevens FJ, Das Gupta TK, Chakrabarty AM. Beyond host-pathogen interactions: microbial defense strategy in the host environment. Curr Opin Biotechnol. 2007;18:279–286. doi: 10.1016/j.copbio.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Liu PT, Modlin RL. Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol. 2008;20:371–376. doi: 10.1016/j.coi.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 6.O’Callaghan D, Stebbins CE. Host-microbe interactions: bacteria. Curr Opin Microbiol. 2010;13:1–3. doi: 10.1016/j.mib.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Lostumbo G. Proteomic analyses of a variety of intracellular bacterial species infecting different host cell lines. Curr Proteomics. 2010;7 In press. [Google Scholar]
- 8.Kaufmann SH, Walker BD. Host–pathogen interactions. Curr Opin Immunol. 2006;18:371–373. doi: 10.1016/j.coi.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy C, Sansonetti P. Host–microbe interactions: bacteria: host–pathogen interactions: interpreting the dialogue. Curr Opin Microbiol. 2004;7:1–3. [Google Scholar]
- 10.Duclos S, Desjardins M. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell Microbiol. 2000;2:365–377. doi: 10.1046/j.1462-5822.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 11.Taylor SW, Fahy E, Ghosh SS. Global organellar proteomics. Trends Biotechnol. 2003;21:82–88. doi: 10.1016/S0167-7799(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 12.Au CE, Bell AW, Gilchrist A, Hiding J, et al. Organellar proteomics to create the cell map. Curr Opin Cell Biol. 2007;19:376–385. doi: 10.1016/j.ceb.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Rogers LD, Foster LJ. Contributions of proteomics to understanding phagosome maturation. Cell Microbiol. 2008;10:1405–1412. doi: 10.1111/j.1462-5822.2008.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths G, Mayorga L. Phagosome proteomes open the way to a better understanding of phagosome function. Genome Biol. 2007;8:207. doi: 10.1186/gb-2007-8-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverstein SC, Greenberg S, Di Virgilio F, Steinberg TH. Fundamental Immunology. Raven Press Ltd; New York: 1989. [Google Scholar]
- 16.Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci. 2001;114:1801–1809. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg S, Burridge K, Silverstein SC. Colocalization of F-actin and talin during Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1990;172:1853–1856. doi: 10.1084/jem.172.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helmy KY, Katschke KJ, Jr, Gorgani NN, Kljavin NM, et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K, Suda T, Naito T, Ide K, et al. Impaired toll-like receptor 9 expression in alveolar macrophages with no sensitivity to CpG DNA. Am J Respir Crit Care Med. 2005;171:707–713. doi: 10.1164/rccm.200408-1078OC. [DOI] [PubMed] [Google Scholar]
- 20.Hazenbos WLW, Brown EJ. In: Phagocytosis of Bacteria and Bacterial Pathogenicity. Ernst JD, Stendahl O, editors. Cambridge University Press; Cambrige: 2006. pp. 4–53. [Google Scholar]
- 21.Ramachandra L, Song R, Harding CV. Phagosomes are fully competent antigen-processing organelles that mediate the formation of peptide:class II MHC complexes. J Immunol. 1999;162:3263–3272. [PubMed] [Google Scholar]
- 22.Houde M, Bertholet S, Gagnon E, Brunet S, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 23.Shaw DR, Griffin FM., Jr Phagocytosis requires repeated triggering of macrophage phagocytic receptors during particle ingestion. Nature. 1981;289:409–411. doi: 10.1038/289409a0. [DOI] [PubMed] [Google Scholar]
- 24.Lang T, de Chastellier C, Ryter A, Thilo L. Endocytic membrane traffic with respect to phagosomes in macrophages infected with non-pathogenic bacteria: phagosomal membrane acquires the same composition as lysosomal membrane. Eur J Cell Biol. 1988;46:39–50. [PubMed] [Google Scholar]
- 25.Desjardins M. Biogenesis of phagolysosomes: the ‘kiss and run’ hypothesis. Trends Cell Biol. 1995;5:183–186. doi: 10.1016/s0962-8924(00)88989-8. [DOI] [PubMed] [Google Scholar]
- 26.Desjardins M, Huber LA, Parton RG, Griffiths G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol. 1994;124:677–688. doi: 10.1083/jcb.124.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinchen JM, Ravichandran KS. Phagosome maturation: going through the acid test. Nat Rev Mol Cell Biol. 2008;9:781–795. doi: 10.1038/nrm2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukacs GL, Rotstein OD, Grinstein S. Phagosomal acidification is mediated by a vacuolar-type H(+)-ATPase in murine macrophages. J Biol Chem. 1990;265:21099–21107. [PubMed] [Google Scholar]
- 29.Horwitz MA, Maxfield FR. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984;99:1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 31.Coffey JW, De Duve C. Digestive activity of lysosomes. I. The digestion of proteins by extracts of rat liver lysosomes. J Biol Chem. 1968;243:3255–3263. [PubMed] [Google Scholar]
- 32.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol. 2001;1:126–134. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 33.Trost M, English L, Lemieux S, Courcelles M, et al. The phagosomal proteome in interferon-gamma-activated macrophages. Immunity. 2009;30:143–154. doi: 10.1016/j.immuni.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Saveanu L, Carroll O, Weimershaus M, Guermonprez P, et al. IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science. 2009;325:213–217. doi: 10.1126/science.1172845. [DOI] [PubMed] [Google Scholar]
- 35.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 36.Muller-Taubenberger A, Lupas AN, Li H, Ecke M, et al. Calreticulin and calnexin in the endoplasmic reticulum are important for phagocytosis. Embo J. 2001;20:6772–6782. doi: 10.1093/emboj/20.23.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gagnon E, Duclos S, Rondeau C, Chevet E, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 38.Touret N, Paroutis P, Terebiznik M, Harrison RE, et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell. 2005;123:157–170. doi: 10.1016/j.cell.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Rogers LD, Foster LJ. The dynamic phagosomal proteome and the contribution of the endoplasmic reticulum. Proc Natl Acad Sci USA. 2007;104:18520–18525. doi: 10.1073/pnas.0705801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Touret N, Paroutis P, Grinstein S. The nature of the phagosomal membrane: endoplasmic reticulum versus plasmalemma. J Leukoc Biol. 2005;77:878–885. doi: 10.1189/jlb.1104630. [DOI] [PubMed] [Google Scholar]
- 41.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 42.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci USA. 2003;100:12889–12894. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 44.Arenas GN, Staskevich AS, Aballay A, Mayorga LS. Intracellular trafficking of Brucella abortus in J774 macrophages. Infect Immun. 2000;68:4255–4263. doi: 10.1128/iai.68.7.4255-4263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Detilleux PG, Deyoe BL, Cheville NF. Entry and intracellular localization of Brucella spp. in Vero cells: fluorescence and electron microscopy. Vet Pathol. 1990;27:317–328. doi: 10.1177/030098589002700503. [DOI] [PubMed] [Google Scholar]
- 46.Gueirard P, Laplante A, Rondeau C, Milon G, Desjardins M. Trafficking of Leishmania donovani promastigotes in non-lytic compartments in neutrophils enables the subsequent transfer of parasites to macrophages. Cell Microbiol. 2008;10:100–111. doi: 10.1111/j.1462-5822.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 47.Kima PE, Dunn W. Exploiting calnexin expression on phagosomes to isolate Leishmania parasitophorous vacuoles. Microb Pathog. 2005;38:139–145. doi: 10.1016/j.micpath.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Goldszmid RS, Coppens I, Lev A, Caspar P, et al. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med. 2009;206:399–410. doi: 10.1084/jem.20082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grotzke JE, Harriff MJ, Siler AC, Nolt D, et al. The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoS Pathog. 2009;5:e1000374. doi: 10.1371/journal.ppat.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao PK, Singh CR, Jagannath C, Li Q. A systems biology approach to study the phagosomal proteome modulated by mycobacterial infections. Int J Clin Exp Med. 2009;2:233–247. [PMC free article] [PubMed] [Google Scholar]
- 51.Katti MK, Dai G, Armitige LY, Rivera Marrero C, et al. The Delta fbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cell Microbiol. 2008;10:1286–1303. doi: 10.1111/j.1462-5822.2008.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asare R, Abu Kwaik Y. Early trafficking and intracellular replication of Legionella longbeachaea within an ER-derived late endosome-like phagosome. Cell Microbiol. 2007;9:1571–1587. doi: 10.1111/j.1462-5822.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 53.Miyake M. Intracellular survival and replication of legionella pneumophila within host cells. Yakugaku Zasshi. 2008;128:1763–1770. doi: 10.1248/yakushi.128.1763. [DOI] [PubMed] [Google Scholar]
- 54.Shin S, Roy CR. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol. 2008;10:1209–1220. doi: 10.1111/j.1462-5822.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 55.Paludan C, Schmid D, Landthaler M, Vockerodt M, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 56.Desjardins M. ER-mediated phagocytosis: a new membrane for new functions. Nat Rev Immunol. 2003;3:280–291. doi: 10.1038/nri1053. [DOI] [PubMed] [Google Scholar]
- 57.Hatsuzawa K, Hashimoto H, Hashimoto H, Arai S, et al. Sec22b is a negative regulator of phagocytosis in macrophages. Mol Biol Cell. 2009;20:4435–4443. doi: 10.1091/mbc.E09-03-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatsuzawa K, Tamura T, Hashimoto H, Hashimoto H, et al. Involvement of syntaxin 18, an endoplasmic reticulum (ER)-localized SNARE protein, in ER-mediated phagocytosis. Mol Biol Cell. 2006;17:3964–3977. doi: 10.1091/mbc.E05-12-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desjardins M, Celis JE, van Meer G, Dieplinger H, et al. Molecular characterization of phagosomes. J Biol Chem. 1994;269:32194–32200. [PubMed] [Google Scholar]
- 60.Ulsamer AG, Wright PL, Wetzel MG, Korn ED. Plasma and phagosome membranes of Acanthamoeba castellanii. J Cell Biol. 1971;51:193–215. doi: 10.1083/jcb.51.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeichner SL. Isolation and characterization of macrophage phagosomes containing infectious and heat-inactivated Chlamydia psittaci: two phagosomes with different intracellular behaviors. Infect Immun. 1983;40:956–966. doi: 10.1128/iai.40.3.956-966.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luhrmann A, Haas A. A method to purify bacteria-containing phagosomes from infected macrophages. Methods Cell Sci. 2000;22:329–341. doi: 10.1023/a:1017963401560. [DOI] [PubMed] [Google Scholar]
- 63.Lutz DA, Chen XM, McLaughlin BJ. Isolation of the phagocytic compartment from macrophages using a paramagnetic, particulate ligand. Anal Biochem. 1993;214:205–211. doi: 10.1006/abio.1993.1478. [DOI] [PubMed] [Google Scholar]
- 64.Ullrich HJ, Beatty WL, Russell DG. Interaction of Mycobacterium avium-containing phagosomes with the antigen presentation pathway. J Immunol. 2000;165:6073–6080. doi: 10.4049/jimmunol.165.11.6073. [DOI] [PubMed] [Google Scholar]
- 65.Fischer K, Chatterjee D, Torrelles J, Brennan PJ, et al. Mycobacterial lysocardiolipin is exported from phagosomes upon cleavage of cardiolipin by a macrophage-derived lysosomal phospholipase A2. J Immunol. 2001;167:2187–2192. doi: 10.4049/jimmunol.167.4.2187. [DOI] [PubMed] [Google Scholar]
- 66.Lonnbro P, Nordenfelt P, Tapper H. Isolation of bacteria-containing phagosomes by magnetic selection. BMC Cell Biol. 2008;9:35. doi: 10.1186/1471-2121-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urwyler S, Nyfeler Y, Ragaz C, Lee H, et al. Proteome analysis of Legionella vacuoles purified by magnetic immunoseparation reveals secretory and endosomal GTPases. Traffic. 2009;10:76–87. doi: 10.1111/j.1600-0854.2008.00851.x. [DOI] [PubMed] [Google Scholar]
- 68.Diakonova M, Gerke V, Ernst J, Liautard JP, et al. Localization of five annexins in J774 macrophages and on isolated phagosomes. J Cell Sci. 1997;110:1199–1213. doi: 10.1242/jcs.110.10.1199. [DOI] [PubMed] [Google Scholar]
- 69.Stendahl O, Hazenbos WLW, Brown EJ, Huynh KK, et al. Phagocytosis of Bacteria and Bacterial Pathogenicity. Cambridge University Press; Cambridge: 2006. [Google Scholar]
- 70.Via LE, Deretic D, Ulmer RJ, Hibler NS, et al. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 1997;272:13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- 71.Shi L, Adkins JN, Coleman JR, Schepmoes AA, et al. Proteomic analysis of Salmonella enterica serovar typhimurium isolated from RAW 264.7 macrophages. J Biol Chem. 2006;281:29131–29140. doi: 10.1074/jbc.M604640200. [DOI] [PubMed] [Google Scholar]
- 72.Bernales S, Schuck S, Walter P. ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy. 2007;3:285–287. doi: 10.4161/auto.3930. [DOI] [PubMed] [Google Scholar]
- 73.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2007;141:656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. Faseb J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 75.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 76.Shevchuk O, Batzilla C, Hagele S, Kusch H, et al. Proteomic analysis of Legionella-containing phagosomes isolated from dictyostelium. Int J Med Microbiol. 2009;299:489–508. doi: 10.1016/j.ijmm.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Wisniewski JR, Zougman A, Mann M. Combination of FASP and StageTip-based fractionation allows in-depth analysis of the hippocampal membrane proteome. J Proteome Res. 2009;8:5674–5678. doi: 10.1021/pr900748n. [DOI] [PubMed] [Google Scholar]
- 78.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Meth. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 79.Sturgill-Koszycki S, Haddix PL, Russell DG. The interaction between Mycobacterium and the macrophage analyzed by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis. 1997;18:2558–2565. doi: 10.1002/elps.1150181411. [DOI] [PubMed] [Google Scholar]
- 80.Hasan Z, Schlax C, Kuhn L, Lefkovits I, et al. Isolation and characterization of the mycobacterial phagosome: segregation from the endosomal/lysosomal pathway. Mol Microbiol. 1997;24:545–553. doi: 10.1046/j.1365-2958.1997.3591731.x. [DOI] [PubMed] [Google Scholar]
- 81.Clemens DL, Horwitz MA. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kielian MC, Cohn ZA. Phagosome-lysosome fusion. Characterization of intracellular membrane fusion in mouse macrophages. J Cell Biol. 1980;85:754–765. doi: 10.1083/jcb.85.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garin J, Diez R, Kieffer S, Dermine JF, et al. The phagosome proteome: insight into phagosome functions. J Cell Biol. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stuart LM, Boulais J, Charriere GM, Hennessy EJ, et al. A systems biology analysis of the Drosophila phagosome. Nature. 2007;445:95–101. doi: 10.1038/nature05380. [DOI] [PubMed] [Google Scholar]
- 85.Desjardins M, Griffiths G. Phagocytosis: latex leads the way. Curr Opin Cell Biol. 2003;15:498–503. doi: 10.1016/s0955-0674(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 86.Gotthardt D, Blancheteau V, Bosserhoff A, Ruppert T, et al. Proteomics fingerprinting of phagosome maturation and evidence for the role of a Galpha during uptake. Mol Cell Proteomics. 2006;5:2228–2243. doi: 10.1074/mcp.M600113-MCP200. [DOI] [PubMed] [Google Scholar]
- 87.Lee BY, Jethwaney D, Schilling B, Clemens DL, et al. The Mycobacterium bovis bacille Calmette-Guerin phagosome proteome. Mol Cell Proteomics. 2010;9:32–53. doi: 10.1074/mcp.M900396-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Q, Roxas BA. An assessment of false discovery rates and statistical significance in label-free quantitative proteomics with combined filters. BMC Bioinformatics. 2009;10:43. doi: 10.1186/1471-2105-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 90.Vieira OV, Bucci C, Harrison RE, Trimble WS, et al. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol. 2003;23:2501–2514. doi: 10.1128/MCB.23.7.2501-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vergne I, Fratti RA, Hill PJ, Chua J, et al. Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol Biol Cell. 2004;15:751–760. doi: 10.1091/mbc.E03-05-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clemens DL, Lee BY, Horwitz MA. Mycobacterium tuberculosis and Legionella pneumophila phagosomes exhibit arrested maturation despite acquisition of Rab7. Infect Immun. 2000;68:5154–5166. doi: 10.1128/iai.68.9.5154-5166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kelley VA, Schorey JS. Mycobacterium’s arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron. Mol Biol Cell. 2003;14:3366–3377. doi: 10.1091/mbc.E02-12-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Via LE, Fratti RA, McFalone M, Pagan-Ramos E, et al. Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci. 1998;111:897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- 95.Vojo D, Sudha S, Sharon M, James H, et al. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cell Microbiol. 2006;8:719–727. doi: 10.1111/j.1462-5822.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- 96.Koul A, Herget T, Klebl B, Ullrich A. Interplay between mycobacteria and host signalling pathways. Nature Rev Microbiol. 2004;2:189–202. doi: 10.1038/nrmicro840. [DOI] [PubMed] [Google Scholar]
- 97.Malik ZA, Denning GM, Kusner DJ. Inhibition of Ca2+signaling by Mycobacterium tuberculosisis associated with reduced phagosome–lysosome fusion and increased survival within human macrophages. J Exp Med. 2000;191:287–302. doi: 10.1084/jem.191.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fortune SM, Solache A, Jaeger A, Hill PJ, et al. Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. J Immunol. 2004;172:6272–6280. doi: 10.4049/jimmunol.172.10.6272. [DOI] [PubMed] [Google Scholar]
- 99.Griffiths G. On phagosome individuality and membrane signalling networks. Trends Cell Biol. 2004;14:343–351. doi: 10.1016/j.tcb.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 100.Kyei GB, Vergne I, Chua J, Roberts E, et al. Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. Embo J. 2006;25:5250–5259. doi: 10.1038/sj.emboj.7601407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hashim S, Mukherjee K, Raje M, Basu SK, Mukhopadhyay A. Live Salmonella modulate expression of rab proteins to persist in a specialized compartment and escape transport to lysosomes. J Biol Chem. 2000;275:16281–16288. doi: 10.1074/jbc.275.21.16281. [DOI] [PubMed] [Google Scholar]
- 102.Anderson L, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol Cell Proteomics. 2006;5:573–588. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 103.Musser JM, DeLeo FR. Toward a genome-wide systems biology analysis of host–pathogen interactions in group A Streptococcus. Am J Pathol. 2005;167:1461–1472. doi: 10.1016/S0002-9440(10)61232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Young D, Stark J, Kirschner D. Systems biology of persistent infection: tuberculosis as a case study. Nat Rev Microbiol. 2008;6:520–528. doi: 10.1038/nrmicro1919. [DOI] [PubMed] [Google Scholar]
- 105.Dozmorov MG, Kyker KD, Saban R, Shankar N, et al. Systems biology approach for mapping the response of human urothelial cells to infection by Enterococcus faecalis. BMC Bioinformatics. 2007;8:S2. doi: 10.1186/1471-2105-8-S7-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baerenfaller K, Grossmann J, Grobei MA, Hull R, et al. Genome-scale proteomics reveals Arabidopsis thaliana gene models and proteome dynamics. Science. 2008;320:938–941. doi: 10.1126/science.1157956. [DOI] [PubMed] [Google Scholar]
- 107.Bumann D. System-level analysis of Salmonella metabolism during infection. Curr Opin Microbiol. 2009;12:559–567. doi: 10.1016/j.mib.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 108.Poltorak A, He X, Smirnova I, Liu MY, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 109.Isberg RR, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 110.Forst CV. Host–pathogen systems biology. Drug Discov Today. 2006;11:220–227. doi: 10.1016/S1359-6446(05)03735-9. [DOI] [PubMed] [Google Scholar]
- 111.Gilchrist M, Thorsson V, Li B, Rust AG, et al. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006;441:173–178. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- 112.Jordao L, Bleck CK, Mayorga L, Griffiths G, Anes E. On the killing of mycobacteria by macrophages. Cell Microbiol. 2008;10:529–548. doi: 10.1111/j.1462-5822.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 113.Samoilis G, Aivaliotis M, Vranakis I, Papadioti A, et al. Proteomic screening for possible effector molecules secreted by the obligate intracellular pathogen Coxiella burnetii. J Proteome Res. 2010;9:1619–1626. doi: 10.1021/pr900605q. [DOI] [PubMed] [Google Scholar]
- 114.Sturgill-Koszycki S, Schaible UE, Russell DG. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. Embo J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- 115.Chua J, Deretic V. Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles. J Biol Chem. 2004;279:36982–36992. doi: 10.1074/jbc.M405082200. [DOI] [PubMed] [Google Scholar]
- 116.Cardoso CM, Jordao L, Vieira OV. Rab10 regulates phagosome maturation and its overexpression rescues Mycobacterium-containing phagosomes maturation. Traffic. 2010;11:221–235. doi: 10.1111/j.1600-0854.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 117.Roberts EA, Chua J, Kyei GB, Deretic V. Higher order Rab programming in phagolysosome biogenesis. J Cell Biol. 2006;174:923–929. doi: 10.1083/jcb.200603026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fratti RA, Chua J, Deretic V. Cellubrevin alterations and Mycobacterium tuberculosis phagosome maturation arrest. J Biol Chem. 2002;277:17320–17326. doi: 10.1074/jbc.M200335200. [DOI] [PubMed] [Google Scholar]
- 119.Fratti RA, Chua J, Deretic V. Induction of p38 mitogen-activated protein kinase reduces early endosome auto-antigen 1 (EEA1) recruitment to phagosomal membranes. J Biol Chem. 2003;278:46961–46967. doi: 10.1074/jbc.M305225200. [DOI] [PubMed] [Google Scholar]
- 120.Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc Natl Acad Sci U S A. 2003;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Deghmane AE, Soualhine H, Bach H, Sendide K, et al. Lipoamide dehydrogenase mediates retention of coronin-1 on BCG vacuoles, leading to arrest in phagosome maturation. J Cell Sci. 2007;120:2796–2806. doi: 10.1242/jcs.006221. [DOI] [PubMed] [Google Scholar]
- 122.Pittis MG, Muzzolin L, Giulianini PG, Garcia RC. Mycobacteria-containing phagosomes associate less annexins I, VI, VII and XI, but not II, concomitantly with a diminished phagolysosomal fusion. Eur J Cell Biol. 2003;82:9–17. doi: 10.1078/0171-9335-00293. [DOI] [PubMed] [Google Scholar]
- 123.Cho JE, Kim YS, Park S, Cho SN, Lee H. Mycobacterium tuberculosis-induced expression of Leukotactin-1 is mediated by the PI3-K/PDK1/Akt signaling pathway. Mol Cells. 2010;29:35–39. doi: 10.1007/s10059-010-0003-5. [DOI] [PubMed] [Google Scholar]
- 124.Tailleux L, Neyrolles O, Honore-Bouakline S, Perret E, et al. Constrained intracellular survival of Mycobacterium tuberculosis in human dendritic cells. J Immunol. 2003;170:1939–1948. doi: 10.4049/jimmunol.170.4.1939. [DOI] [PubMed] [Google Scholar]
- 125.Schaible UE, Hagens K, Fischer K, Collins HL, Kaufmann SH. Intersection of group I CD1 molecules and mycobacteria in different intracellular compartments of dendritic cells. J Immunol. 2000;164:4843–4852. doi: 10.4049/jimmunol.164.9.4843. [DOI] [PubMed] [Google Scholar]