Abstract
Preeclampsia (PE) is a prevalent life-threatening hypertensive disorder of pregnancy associated with increased complement activation. However, the causative factors and pathogenic role of increased complement activation in PE are largely unidentified. Here we report that a circulating maternal autoantibody, the angiotensin II type 1 receptor (AT1R) agonistic autoantibody (AT1-AA), recently emerged as a potential pathogenic contributor to PE, stimulates deposition of complement C3 in placentas and kidneys of pregnant mice via AT1R activation. Next, we provide in vivo evidence that selectively interfering with C3a signaling by C3a receptor (C3aR) specific antagonist significantly reduces hypertension from 167±7 to 143±5 mmHg and proteinuria from 223.5±7.5 to 78.8 ± 14.0 µg albumin/mg creatinine (both P<0.05) in AT1-AA-injected pregnant mice. Additionally, we demonstrated that C3aR antagonist significantly inhibited autoantibody-induced circulating soluble fms-like tyrosine kinase-1 (sFlt-1), a known antiangiogenic protein associated with PE, and reduced small placental size with impaired angiogenesis and intrauterine growth restriction. Similarly, in humans, we demonstrate that C3 deposition is significantly elevated in the placentas of preeclamptic patients compared to normotensive controls. Lastly, we show that C3aR activation is a key mechanism underlying autoantibody-induced sFlt-1 secretion and decreased angiogenesis in cultured human villous explants. Overall, we provide mouse and human evidence that AT1-AA-mediated AT1R activation contributes to elevated C3 and that C3aR signaling is a key mechanism underlying the pathogenesis of the disease. These studies are the first to link AT1-AA with complement activation and provide important new opportunities for therapeutic intervention in PE.
Keywords: hypertension, preeclampsia, autoantibody, complement C3a, C3a receptor, AT1 receptor, placenta
Introduction
Preeclampsia (PE) is a serious and common complication of pregnancy and is a leading cause of maternal and neonatal morbidity and mortality 1. It is a multisystem disorder generally appearing after the 20th week of gestation and characterized by hypertension, proteinuria, inflammation, and vascular defects. Despite intensive research efforts and several large clinical trials, the underlying cause of PE remains a mystery and treatment options continue to be unsatisfactory. Recently, a maternal autoantibody, the angiotensin II type I receptor agonistic autoantibody (AT1-AA), has been reported as a potential contributor to the pathogenesis of PE.2–7 Early studies showed that these autoantibodies can activate AT1 receptors on a variety of cells and provoke cellular responses relevant to PE.8–13 More recently, we and others demonstrated that AT1-AA contributes to key features of PE including hypertension, proteinuria, renal and placental injury in several animal studies using adoptive transfer and immunization experiments.14–17 Thus, these in vivo studies offered direct evidence of the pathophysiological role of AT1-AAs in PE. Extending animal studies to human, we and others recently demonstrated that AT1-AAs are highly prevalent in PE, and antibody titers correlate with disease severity.18–19 Thus, both human and mouse studies support a novel view that PE contains an autoimmune component associated with AT1-AA and excessive AT1 receptor activation by these autoantibodies is detrimental and underlies pathophysiology of PE.
The complement system consists of a series of more than 30 proteins that provide one of the first lines of defense against pathogens.20–22 The activation of complement component C3 is a point of convergence for all three major complement activation pathways including classical, alternative and lectin-activated21. The complement activation pathways result in the production of C3 convertases that cleave C3 into two components, C3a and C3b.23 C3a is a small molecular weight split product that functions as an anaphylatoxin that provokes a strong inflammatory response by activation of C3a receptors (C3aR) on multiple target cells including inflammatory cells, endothelial cell, vascular smooth muscle cells and epithelial cells.24–25,26 Although initial function of complement activation is protective and beneficial to get rid of pathogens, a growing body of evidence supports the claims that autoimmune conditions or tissue injury can lead to inappropriate activation of the complement cascade resulting in organ damage. Of note, recent studies show that PE is characterized by a pronounced maternal inflammatory response that is coupled with activation of innate immunity1, 27, including the complement cascade.28 Some hypothesize that the activation of complement propagates a state of chronic tissue injury in PE and other pregnancy complications.28 For example, complement activation and deposition in the placenta and kidneys are associated with abnormal placental development, miscarriage and kidney injury in several animal models, while inhibiting complement activation rescues pregnancies and prevent kidney damage.29–33 Recent clinical studies show that elevated C3a is an independent predictive factor for adverse pregnancy outcomes, suggesting that complement-related inflammatory events in pregnancy contribute to the subsequent development of poor outcomes at later stages of pregnancy.32, 34–35 However, why this heightened complement activation occurs in PE is unknown and the exact contribution of complement to the disease features remains undefined.
Because excessive complement activation is associated with autoimmune diseases and tissue injuries and PE is associated with the presence of pathogenic autoantibodies that activate the AT1 angiotensin receptor, it is possible that AT1-AA is a causative factor responsible for the increased complement activation in PE. Here, we use both human and mouse studies to explore whether AT1-AA promotes elevated complement activation in PE. Moreover, we investigate the contributory role of the increased complement activation in the pathogenesis of PE and identify potential therapeutic strategies.
Materials and Methods
An expanded Methods section is available in the Supplementary Methods Patients
Patients admitted to Memorial Hermann Hospital were identified by the obstetrics faculty of the University of Texas Medical School at Houston. Preeclamptic patients (n=20) were diagnosed with severe disease on the basis of the definition set by the National High Blood Pressure Education Program Working Group Report.36 The criteria of inclusion, including no previous history of hypertension, are reported previously.14, 18, 37 Control pregnant women were selected on the basis of having an uncomplicated, normotensive pregnancy with a normal term delivery (n=16). The research protocol was approved of by the Institutional Committee for the Protection of Human Subjects. The detailed information of human subjects is summarized in Table 1.
Table 1.
Clinical characteristic features of human subject
| variable | NT | PE | |
|---|---|---|---|
| N | 20 | 16 | |
| Age (y) | 28.4 ± 7.4 | 29.4 ± 6.6 | |
| Race (%) | African American | 45 | 50 |
| White | 26 | 20 | |
| Hispanic | 28 | 27 | |
| Other | 1 | 3 | |
| Gravity | 2.4 ± 1.2 | 2.7 ± 1.3 | |
| BMI | 32.1 ± 5.4 | 34.87 ± 7.6 | |
| Weeks gestational age | 36.6 ± 3.8 | 32.57 ± 5.6 | |
| Systolic BP (mmHg) | 116 ± 8 | 171 ± 6* | |
| Diastolic BP (mmHg) | 75 ± 6 | 96 ± 12* | |
| Proteinuria (mg/24h) | < 300 | 1769 ± 438* |
This table illustrates that the blood pressure and proteinuria are elevated in severe preeclamptic (PE) women, as compared to normotensive (NT) pregnant women. The category mean or median is indicated (± SEM, where applicable).
P<0.001 vs normotensive pregnant women.
Introduction of human autoantibody into pregnant mice
Purified IgGs were isolated from preeclamptic or normotensive patient sera (PE-IgG and NT-IgG, respectively) 14–17, 38, and introduced into pregnant mice as described previously.14, 18, 37 Briefly, pregnant C57Bl/6J mice (Harlan) were anesthetized with isofluorane, and concentrated IgG purified from 200 µL of patient serum (approximately 800µg) was introduced into pregnant mice by retro-orbital sinus injection twice, on gestational day (GD) 13 and GD14 (PE: n=4; NT: n=4). For neutralization experiments, the autoantibody was simultaneously coinjected twice, with either losartan (8 mg/kg IV; n=9) or the 7-aa epitope peptide (7-aa epitope peptide is premixed with PE-IgG, 50 mg/kg IV; sequence AFHYESQ; n=9). Some of the mice (n=5) injected with the IgG from PE patients were infused with SB290157 (30mg/kg body weight/day on E13 which was dissolved in 50% DMSO) for 5 days using Alzet osmotic minipumps implanted subcutaneously (model 1007D, Alzet, Cupertino, CA).24–26 All of the animal protocols were reviewed and approved of by the institutional animal welfare committee at the University of Texas at Houston Health Science Center.
Statistical analysis
Results are expressed as mean ± s.e.m. All the data were subjected to statistical analysis using one-way analysis of variance followed by the Newman Keuls post hoc test or student’s t-test to determine the significance between groups. Statistical programs were run by GraphPad Prism 5, statistical software (GraphPad, San Diego, CA). Statistical significance was set at P < 0.05.
Results
PE-IgG induces C3 deposition in the placentas and kidneys of pregnant mice via AT1R activation
C3 is reported to be elevated in the circulation of preeclamptic women.32, 34–35 However, the causative factors for its elevation remain unknown. Our preeclamptic animal model with adoptive transfer of autoantibody14 is a valuable investigative tool to determine whether AT1-AA contributes to elevated C3. Specifically, we injected IgG from normotensive pregnant women (NT-IgG) or IgG from women with PE (PE-IgG) into pregnant mice on gestation days (GD) 13 and 14. Injected mice were sacrificed on GD18 and the C3 deposition in placentas and kidneys was examined. I Immunohistochemical analysis revealed that the C3 was predominantly deposited in the labyrinthine zone of the placentas and the glomeruli of the kidneys in PE-IgG injected pregnant mice (Fig. 1A – B).
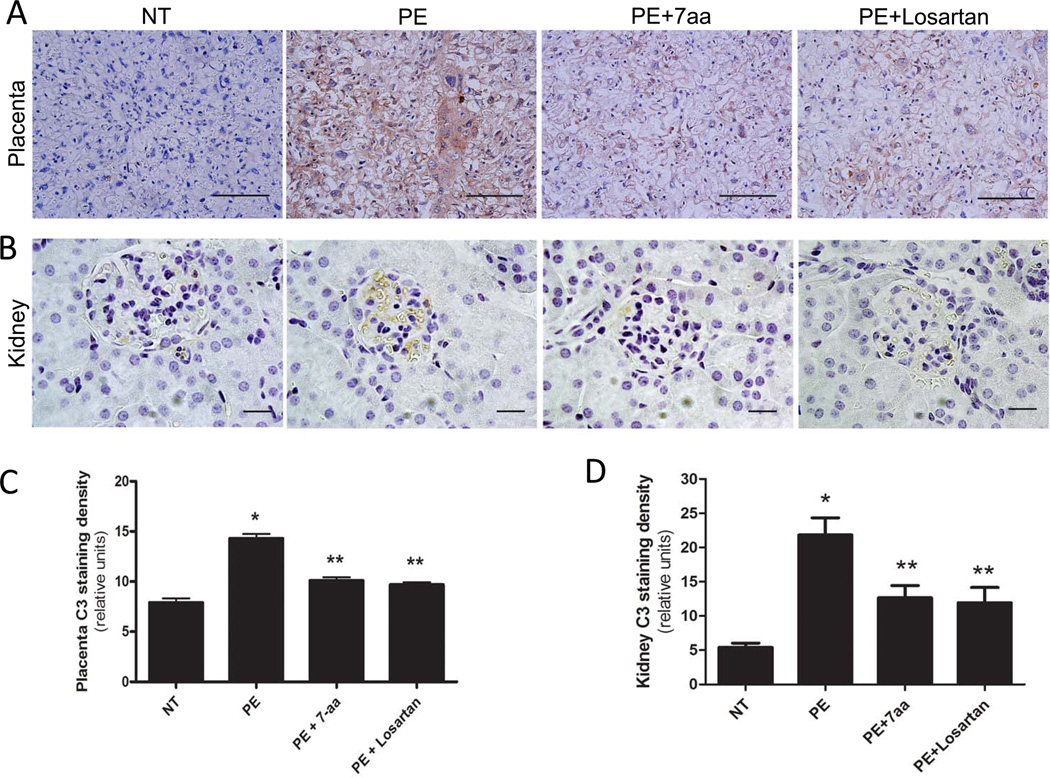
Figure 1. Maternal circulating IgG in preeclamptic women is responsible for increased C3 deposition in placentas and kidneys of PE-IgG-injected pregnant mice via AT1R activation.
Deposition of C3 was examined by immunohistochemistry. C3 expression levels are significantly elevated in (A) placenta (trophoblast cells and endothelial cells; Scale bar = 100µM) and (B) kidney (podocytes of glomeruli; Scale bar = 20µM) of PE-IgG-injected pregnant mice (n=4). Co-injection with losartan (n=9) or the 7-aa epitope peptide (n=9) resulted in substantial reduction of C3 staining. Image quantification shows that C3 staining in both placentas (C) and kidneys (D) is significantly elevated in PE-IgG-injected pregnant mice compared to NT-IgG-injected pregnant mice (n=4). * P < 0.05 compared to NT-IgG injected mice; ** P < 0.05 compared to PE-IgG injected mice.
Image quantification analysis indicated that C3 deposition was significantly enhanced in both placentas and kidneys of PE-IgG-injected pregnant mice as compared with tissues derived from NT-IgG injected pregnant mice (Fig. 1C–D). Next, to determine whether PE-IgG-induced C3 deposition is via AT1R activation, we co-injected PE-IgG with either losartan (an AT1R blocker) or an autoantibody-neutralizing 7 amino acid epitope peptide (7aa). We found that both losartan and 7aa peptide significantly reduced C3 deposition in the placentas and kidneys of pregnant mice injected with PE-IgG (Fig. 1A–D). These results are the first to show that IgG from women with PE, in contrast to IgG from normotensive pregnant women, stimulates C3 deposition in placentas and kidneys of pregnant mice via AT1R activation.
Blocking C3aR activation attenuates autoantibody-induced hypertension, and proteinuria in pregnant mice
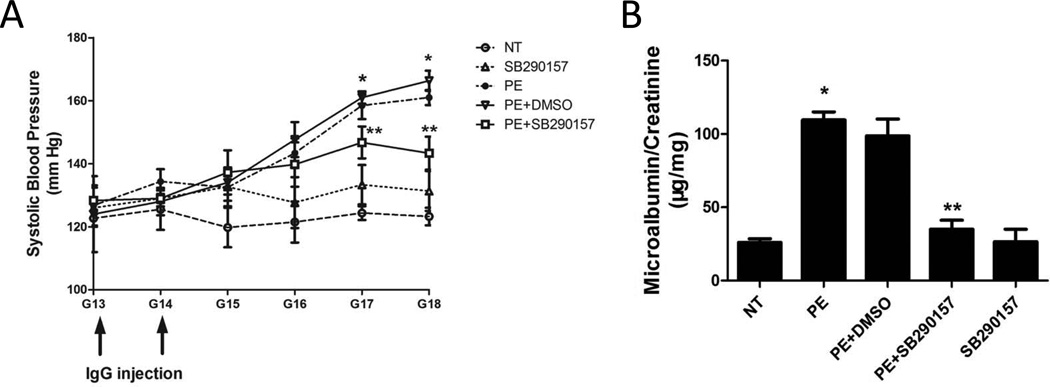
C3a receptors (C3aRs) are expressed on multiple cell types and are known to play an important role in C3a-mediated tissue injury.21, 23 To determine whether C3aRs are expressed in mouse placenta, we examined C3aR expression by immunohistochemistry. We found that C3aRs were highly expressed in trophoblast cells of mouse placenta (Supplementary data Fig. 1). To determine whether elevated C3 activation signaling via C3aR contributes to the pathophysiology of PE, we treated PE-IgG injected pregnant mice with a C3aR antagonist, SB290157. We found that systolic blood pressure increased significantly in mice injected with IgG isolated from women with PE (PE-IgG, 167±7 mmHg) relative to that of mice injected with IgG from NT pregnant women (NT-IgG, 126±1 mmHg, P < 0.05) (Fig. 2A). Additionally, we found that the increased systolic blood pressure seen in PE-IgG-injected pregnant mice was significantly attenuated by infusion of SB290157 (143±5 mmHg, P < 0.05) (Fig. 2A). DMSO, the solvent for SB290157, had no effect on PE-IgG induced hypertension. These findings demonstrate that autoantibody-induced hypertension was significantly inhibited by the infusion of a C3aR antagonist.
Figure 2. Antagonism of C3aR activation attenuates PE-IgG-induced hypertension and proteinuria in pregnant mice.
Pregnant mice were injected with NT-IgG or PE-IgG with or without infusion of SB290157, a C3aR antagonist. (A) PE-IgG injection resulted in hypertension in pregnant mice and SB290157 significantly reduced hypertension seen in these mice. (B) PE-IgG induced proteinuria in pregnant mice and SB290157 significantly attenuated proteinuria (n=3–5). * P < 0.05 compared to NT-IgG injected mice; ** P < 0.05 compared to PE-IgG injected mice.
Next, we examined injected mice for proteinuria, another key diagnostic feature of PE. The ratio of urinary albumin:creatinine was significantly increased in pregnant mice injected with PE-IgG (223.5±7.5 µg/mg) compared to mice injected with NT-IgG (58.3±5.9 µg/mg, P < 0.05) (Fig. 2B). Infusion of the C3aR antagonist significantly decreased urinary protein excretion (78.8 ± 14.0 µg/mg, P < 0.05) in mice injected with PE-IgG (Fig. 2B). DMSO, the SB290157 solvent, had no effect on urinary protein. These studies indicate that blocking C3aR activation significantly inhibited autoantibody-induced proteinuria in pregnant mice. Taken together, we have revealed that C3aR is a key molecule downstream of elevated C3 and contributes to hypertension, and proteinuria induced by detrimental maternal circulating autoantibodies in preeclamptic women.
Autoantibody-induced placental damage and fetal intrauterine growth restriction are prevented by C3aR antagonist treatment
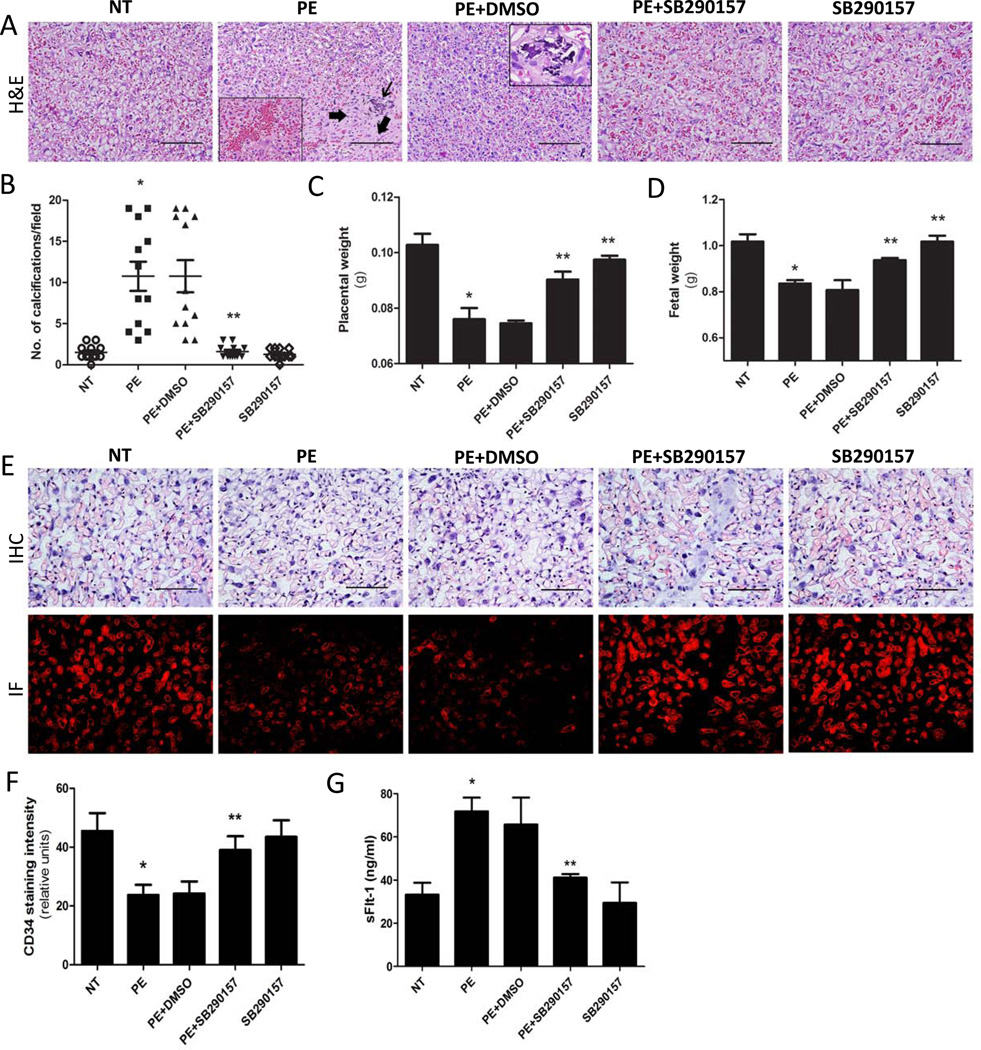
Impaired placental development is often associated with PE and has been observed in autoantibody-injected pregnant mice37, 39 Placentas were harvested for histological analysis on gestation day 18 from PE-IgG and NT-IgG injected pregnant mice. We found that placentas of PE-IgG-injected pregnant mice displayed placental calcifications, a hallmark of placental distress, and centers of fibrinoid necrosis similar to that of acute atherosis, a feature observed in human placentas from women with PE.27 These histological changes were not seen in pregnant mice injected with NT-IgG (Fig. 3A). Histomorphometric quantification analysis demonstrated that the extent of placental damage was significantly increased in PE-IgG-injected pregnant mice compared to that in NT-IgG-injected pregnant mice (Fig. 3B). Continuous infusion of the C3aR antagonist, SB290157, attenuated the placental pathological changes induced by PE-IgG (Fig. 3A & B). Placental weights (Fig. 3C) of PE-IgG injected pregnant mice were smaller (0.08±0.01 g) than placentas from NT-IgG–injected mice (0.10±0.01 g; P < 0.05). Infusion of the C3aR antagonist, but not DMSO, inhibited the autoantibody-induced reduction in placental weight (0.09±0.01 g) (Fig. 3C).
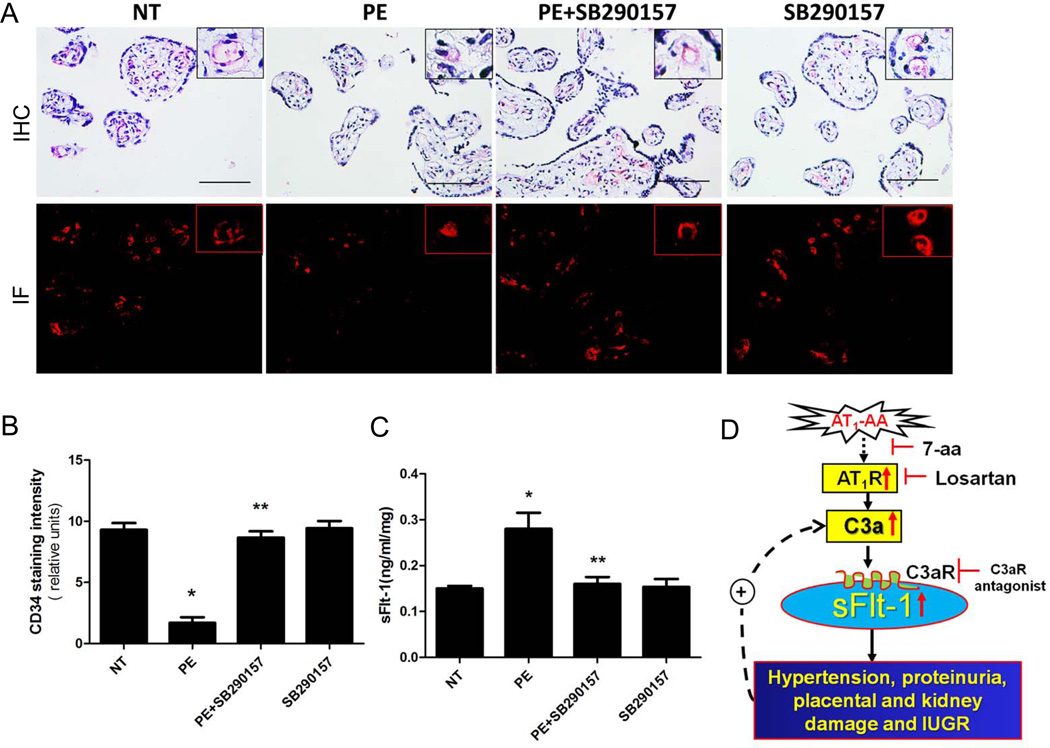
Figure 3. C3aR antagonist treatment reduces PE-IgG-induced renal and placenta damage and intrauterine growth restriction (IUGR) in pregnant mice.
(A) Placentas assessed by H&E staining indicate that PE-IgG injected pregnant mice had damaged placentas: calcifications (thin arrow) and fibrotic areas (thick arrows). The labyrinth zones appear heterogeneous and sometimes has abnormal pools of blood (inset box). Blocking C3aR activation by SB290157 significantly attenuated placental damage (scale bar=100µm). (B) An arbitrary histological quantification of the number of calcifications obtained per field under ×10 magnification (12 placentas for each group). Placental weight (C) and fetal weight (D) are remarkably reduced in PE-IgG-injected pregnant mice. SB290157 treatment significantly inhibited the reduction in placental and fetal weights in PE-IgG-injected pregnant mice (n=3–5). * P < 0.05 compared to NT-IgG injected mice; ** P < 0.05 compared to PE-IgG injected mice. (E) Mouse placental angiogenesis was assessed by CD34 dual immunostaining scale bar=100µm). CD34 staining in the placenta of PE-IgG injected pregnant mice was significantly decreased. SB290157 treatment significantly improved CD34 staining in the placenta of PE-IgG-injected pregnant mice. (F) Quantification of CD34 staining in the mouse placentas. (G) sFlt-1 levels in the maternal circulation were significantly elevated in PE-IgG-injected pregnant mice. SB290157 treatment significantly inhibited the induction of serum sFlt-1 level in these mice (n=3–5). * P < 0.05 compared to NT-IgG injected mice; ** P < 0.05 compared to PE-IgG injected mice.
Abnormal placentas are often associated with IUGR. Thus, we measured fetal weights in litter sizes of 6–8 (Fig. 3D). PE-IgG-injected mice bore fetuses of less weight (0.84±0.01 g) as compared with dams injected with NT-IgG (1.02±0.03 g; P < 0.05). Infusion of the C3aR antagonist significantly inhibited PE-IgG mediated reduction in fetal weight (0.94±0.01 g). When compared with the NT-IgG–injected animals, injection of the C3aR antagonist alone had no statistically significant effect on fetal weight (1.02±0.02 g). DMSO, the solvent for SB290157, had no effect on placental or fetal weight (Figure 3D). Overall, these studies indicate that the autoantibody-induced placenta histological changes and reductions in placental and fetal weight were inhibited by infusion of a C3aR antagonist, implying an important role for C3aR signaling in autoantibody-induced abnormal placentas and IUGR associated with PE.
C3aR antagonist treatment improves autoantibody-mediated impaired placental angiogenesis and attenuates sFlt-1 induction in pregnant mice
PE is often associated with small placentas and impaired placental angiogenesis, features also observed in the placentas of autoantibody-injected pregnant mice.13–14 To determine whether C3aR activation plays an important role in impaired placental angiogenesis, we analyzed the vasculature of isolated mouse placentas by immunostaining with antibody to CD34, an endothelial cell marker. The results show that CD34 staining was decreased in the labyrinth zone of the placentas of mice injected with PE-IgG compared to those injected with NT-IgG (Fig. 3E & F). Continuous infusion with the C3aR antagonist attenuated autoantibody induced reduction in angiogenesis as evidenced by improved CD34 staining. DMSO had no effect on placental histology.
In an effort to identify the molecular mechanisms responsible for C3a–mediated reduction in placental angiogenesis in PE-IgG injected pregnant mice, we measured serum levels of sFlt-1, a well recognized anti-angiogenesis molecule that may contribute to impaired placental angiogenesis.40 Injection of PE-IgG increased the serum levels of sFlt-1(71.8±6.4 ng/ml) compared with NT-IgG injected pregnant mice (33.2±5.1 ng/ml, P < 0.05). Concomitant infusion with the C3aR antagonist resulted in a significant reduction in serum levels of sFlt-1 (41.1±1.7 ng/ml, P < 0.001, Fig. 3C). These findings demonstrate that C3aR signaling contributes to the induction of sFlt-1 in autoantibody-injected pregnant mice. Overall, our findings provide direct evidence that C3aR activation underlies autoantibody-induced sFlt-1 production and likely leads to impaired placenta angiogenesis.
C3 deposition is increased in the placentas of preeclampsia patients
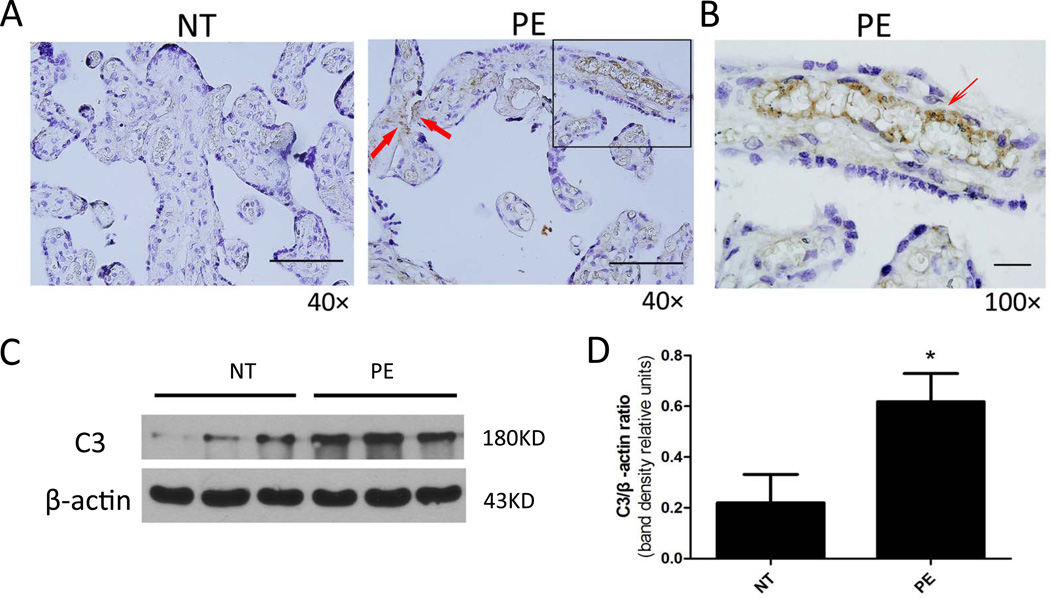
From the mouse work presented here, we have demonstrated that AT1-AAs contribute to the induction of C3 deposition in mouse placentas of autoantibody-injected pregnant mice. To extend mouse studies to humans, we determined whether C3 is also increased in the placentas of PE patients. Specifically, we examined C3 expression in placentas collected from normotensive pregnant women and PE patients. Consistent with our mouse findings, immunostaining showed that the C3 expression level was relatively low in the placentas of normotensive pregnant women. However, C3 levels were elevated in placentas of PE patients and C3 was predominantly deposited in the endothelium of placenta villi (Figure 4A–B). Similarly, western blot analysis further confirmed that C3 levels were increased in the placentas of preeclamptic women compared to normotensive pregnant women (Fig. 4C). Normalization of C3 levels to β-actin indicates that C3 expression in the placentas of PE patients was significantly elevated compared with normotensive pregnant women (Fig. 4D). These studies provide evidence that C3 deposition is significantly elevated in the placentas of preeclamptic women.
Figure 4. C3 levels are elevated in the placentas of preeclamptic women.
(A&B) Immunostaining revealed that C3 was deposited on endothelial cells (thin arrow) and trophoblast cells of human chorionic villi (A, Scale bar = 20µM; B, Scale bar = 20µM). (C) Representative western blotting analysis of expression levels of C3 and β-actin in the placentas isolated from normotensive pregnant women (NT) and preeclamptic patients (PE). (D) The ratio of C3 to β-actin protein levels was significantly increased in the placentas of preeclamptic patients (PE) as compared to normotensive pregnant women (NT). n=6 for each group. *P < 0.05 versus normotensive pregnant women.
C3aR activation contributes to autoantibody-induced impaired placental angiogenesis and increased sFlt-1 secretion from human placental villous explants
Because we found that the C3aR is a key player downstream of elevated C3 underlying autoantibody-induced PE features including impaired placental angiogenesis and increased sFlt-1 secretion in pregnant mice, it is possible that C3aR signaling also contributes to pathophysiology of PE in humans. Similar to mouse placenta (Supplementary Fig. 1), immunohistochemistry demonstrated that C3aR was expressed in the trophoblast cells of human placenta (Supplementary data Fig 2). Next, to elucidate the pathophysiologic consequences of elevated C3-mediated C3aR activation, we took advantage of human placental villous explants to assess the direct role of C3aR in impaired placental angiogenesis and increased sFlt-1secretion in human placentas. Briefly placenta explants isolated from normotensive individuals were incubated with NT-IgG or PE-IgG in the presence or absence of SB290157, a C3aR antagonist. First, CD34 immunostaining and image quantification were used to determine the effects of C3aR signaling on angiogenesis of cultured human villous explants. Similar to our mouse findings, we found that expression levels of CD34 were decreased in placental tissue incubated with PE-IgG compared to NT-IgG and that C3aR antagonism reduced the anti-angiogenic effects of PE-IgG treatment (Fig. 5A). Quantitative image analysis indicated that PE-IgG treatment significantly reduced CD34 staining and C3aR antagonism significantly prevented the PE-IgG-induced decrease in CD34 staining (Fig. 5B). Thus, this finding provides direct evidence that C3aR plays an important role in autoantiobody-induced impaired angiogenesis in the human placenta.
Figure 5. The PE-IgG–mediated decrease in angiogenesis and increase in sFlt-1 secretion from human placental villous explants are inhibited by C3aR blockade.
(A) Human placenta explants treated with NT-IgG, PE-IgG ±SB290157 or SB290157 alone. . Angiogenesis of human placental villous explants assessed by CD34 dual immunostaining (scale bar=100µm). Human villous explants incubated with PE-IgG presented decreased CD34 staining. SB290157 inhibited the antiangiogenic effect of PE-IgG. (B) Quantification of CD34 staining (n=12 for each group). (C) sFlt-1 secretion from cultured human villous explants was significantly increased after 24 hour-incubation with PE-IgG compared to NT-IgG-treated human villous explants. SB290157 significantly reduced sFlt-1 secretion induced by PE-IgG (n=6 for each group). *P < 0.05 compared to NT-IgG incubated group; **P <0.05 compared to PE-IgG incubated group. (D) Working Model: AT1-AA mediated AT1R activation is responsible for C3 elevation. Elevated C3 functions via C3aR activation and contributes to key features of PE seen in the antibody-injection model of PE in pregnant mice. C3aR-mediated sFlt-1 secretion is a key mechanism underlying impaired placenta angiogenesis and disease development. Without interference, C3aR activation-induced sFlt-1 elevation, cell damage and inflammation create a detrimental cycle, facilitating further cell damage and inflammation. However, in the presence of C3aR antagonist, this damage is decreased, slowing the malicious cycle.
Next, the levels of sFlt-1 in the medium of normal human placental explant cultures were determined by ELISA. Consistent with our mouse findings, human placental villous explants incubated with PE-IgG showed an increase in secreted sFlt-1 relative to explants incubated with NT-IgG (0.28±0.04 vs 0.15±0.01 ng/ml/mg, P < 0.05; Fig. 5C). Co-incubation of PE-IgG with SB290157 markedly attenuated the induction of sFlt-1 levels (0.16±0.03, P < 0.05, versus PE-IgG; Fig. 5C). These results show that C3aR signaling contributes to autoantibody-induced sFlt-1 secretion from human placenta villous explants. Thus, these findings are consistent with those observed in the mouse model and suggest that AT1-AA-induced C3aR activation contributes to sFlt-1 induction, results in impaired placental angiogenesis.
Discussion
In this study, we have provided in vivo mouse evidence and in vitro human evidence that AT1-AA is a novel candidate directly inducing C3 deposition in placentas or kidneys via AT1R activation. Blocking C3aR activation significantly ameliorates key features associated with PE seen in autoantibody-injected pregnant mice and impaired placental angiogenesis in cultured human villous explants. Overall, these studies have identified a previously unrecognized role of AT1-AA-induced C3 elevation coupled with C3aR signaling in PE and demonstrated the importance of this complement cascade in the pathogenesis of the disorder (Fig. 5D). These findings suggest a novel therapeutic option for the complicated management of this serious condition.
While complement, in particular C3a, is reportedly increased in the circulation of preeclamptic women32, 34, the exact cause of increased C3a is unknown and its pathogenic role remains unclear. Several animal studies implicated that increased C3 expression is associated with hypertensive and renal disorders. For example, Pratt et al. showed that complement component C3 is elevated in animal models of acute renal transplant rejection.41 Subsequent studies by Lin et al. showed that complement protein C3 is elevated in the vascular smooth cells from the spontaneously hypertensive rats.42 There are few animal models of PE available and none of them have delineated the cause of increased C3. Here using a novel autoantibody-induced model of PE in pregnant mice14, we demonstrate that autoantibody-mediated AT1 receptor activation induces C3 deposition in multiple tissue including placentas and kidneys. Since IgG purified from normotensive pregnant women did not elicit the same increase, the effect can be attributed to the autoantibody itself and not a non-specific immunologic response. Furthermore, the effects are blocked by co-injection with losartan or a 7aa epitope peptide, thereby providing additional evidence for specificity. Our findings that maternal circulating autoantibody activating AT1R contributes to elevation of C3 is strongly supported by elegant studies reported by Dr. Muller and colleagues.43 For their experiments they used transgenic rats harboring human renin and angiotensinogen genes that are characterized by a progressive increase in blood pressure and proteinuria that results from elevated levels of Ang II. They provided evidence showing Ang II-mediated AT1R activation leads to increased complement activation in these rats. However, Ang II level does not increase in PE compared to normal pregnancy. Thus, our studies provide novel and compelling in vivo evidence that maternal circulating AT1-AA is a detrimental factor causing elevation of C3 by excessively activating AT1R in preeclampsia.
Multiple lines of evidence demonstrate that C3a is a critical complement component contributing to tissue injury by activating C3aRs on target cells.22–23, 25, 44 Thus, blocking C3aRs effectively attenuates inflammatory response and tissue damage in several animal models including experimental lupus26, arthritis24, myocardial ischemia and reperfusion injury45, renal tubulointerstitial damage46 and brain injury after intracerebral hemorrhage47. Here we provide strong evidence that interfering with C3aR activation by its specific antagonist significantly attenuated almost all of the features of PE seen in autoantibody-induced preeclamptic model including hypertension, proteinuria, and small placenta featured with impaired angiogenesis and IUGR. Thus, we have revealed that AT1-AA is a key mediator responsible for inducing increased C3 levels in PE and that blockade of C3aR activation can attenuate disease features. Similarly, Dr. Salmon and her colleagues have nicely showed that elevated complement activation, in particular the C3-C5 complement cascade, plays a causative role in pregnancy loss and IUGR in pregnant mice infused with human antiphospholipid antibodies.29–30, 33, 48–49 More recent studies from Dr. Salmon’s group point out that C3 activation resulted in oxidative stress and placental dysfunction, as well as proteinuria and renal pathologic features in a mouse model of spontaneous miscarriage50. In contrast, recent studies demonstrated that genetically deficient mice lacking C1q display preeclamptic like features due to abnormal placental development characterized with shallow trophoblast invasion51. These latter results suggest a role for complement component C1q in trophoblast invasion and normal placental development. Nevertheless, our current studies and others demonstrate that excessive activation of downstream signaling cascades of the complement pathways is detrimental and contributes to adverse pregnancy outcome including PE and pregnancy loss.
In an effort to determine what are the key downstream factors underlying C3aR activation and contributing to autoantibody-induced PE, we used our adoptive transfer PE animal model as an available experimental tool. We demonstrate that sFlt-1, an antagonist of VEGF and placental growth factor (PlGF)24, is elevated in PE-IgG-injected pregnant mice and that C3aR antagonist treatment inhibits PE-IgG-induced sFlt-1 elevation in vivo. As such, we demonstrate that C3aR treatment also completely prevents PE-IgG-induced small placentas featured with impaired angiogenesis and prevents IUGR in autoantibody-injected pregnant mice. Because excess circulating sFlt-1 contributes to endothelial dysfunction, hypertension, and proteinuria in animal models of preeclampsia40 and in view of our early studies showing that antagonizing elevated sFlt-1 by VEGF121 infusion significantly decreases autoantibody-induced hypertension and proteinuria in autoantibody-injected pregnant mice52, it is likely that the C3aR-mediated sFlt-1 elevation is a key underlying mechanisms for autoantibody-induced PE features in pregnant mice in vivo. Similar to our antibody-injection model of PE in pregnant mice, we found that 1) C3 deposition is significantly elevated in the human placentas from preeclamptic women and 2) blocking C3aR activation significantly attenuates AT1-AA-induced sFlt-1 secretion and subsequent impaired angiogenesis in cultured human placental villous explants. Thus, our human findings not only strongly support our mouse finding, but also demonstrate the direct role of C3aR in autoantibody-induced sFlt-1 induction and impaired placental angiogenesis. Our findings are strongly supported by early studies by Girardi et al. who showed that complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction29. Of note, inhibition of C3aR activation does not completely reduce BP to normal, suggesting that other downstream factors besides C3aR activation-mediated sFlt-1 elevation is likely involved in PE-IgG-induced hypertension. Although our present study highlights the role of C3a/C3aR signaling in AT1-AA-induced PE in pregnant mice, other studies have illustrated a potential pathogenic role for C5a/C5aR signaling in PE by stimulating increased production of sFlt-1 29. In other studies we have shown that antiangiogenic factors 14, 53–54 and inflammatory cytokines39, 55 contribute to AT1-AA-induced pathophysiology of PE. Thus, the complement cascade is one of several pathways mediating autoantibody induced features of PE in pregnant mice. Moreover, it will be very interesting to compare AT1-AA-injected PE model with Ang II-infused pregnant mice. This approach will be critical to determine if Ang II-infused pregnant mice-mediated PE features also can exactly mimic the actions of autoantibodies.
Taken together we provide both human and mouse studies showing that C3aR-mediated sFlt-1 induction is a key mechanism underlying AT1-AA-induced features of PE in pregnant mice. We believe that without interference, C3aR activation-induced cell damage and inflammation create a detrimental cycle, facilitating further cell damage and inflammation. However, in the presence of a C3aR antagonist, this damage is decreased, slowing the malicious cycle (Fig. 5D).
Perspective
In conclusion, our studies identified AT1-AA as a novel candidate causing increased C3 deposition via AT1 receptor activation in pregnant mice. Of significant importance, C3aR antagonism reduces key features of the disease in an adoptive transfer mouse model of PE. In humans, we demonstrate that C3 deposition is significantly elevated in placentas of preeclamptic women and AT1-AA-induced placental damage can be alleviated by preventing C3aR activation in human placental villous explants. Finally, both human and mouse studies indicate that increased C3 coupled with C3aR-mediated sFlt-1 elevation is a key mechanism underlying placental damage and subsequent disease symptom development. Thus, our human studies and preclinical mouse studies could be the foundation leading to future human trials and a possible therapy for PE, a life-threatening disorder of pregnancy for which the current treatment is extremely limited.
Supplementary Material
Novelty and Significance.
1) What is new?
Our study is the first to link AT1-AA with the complement system in the pathogenesis of preeclampsia.
2) What is relevant?
We demonstrated C3a can mediate AT1-AA induced features of preeclampsia pregnant mice by signaling through the C3aR.
3) Summary
Our studies indicated: a). AT1-AA can stimulate C3 activation via AT1R activation in both pregnant mice and in human placental villous explants; b). Activated C3a, signaling via C3aR, mediates AT1-AA induced placenta sFlt-1 production and other features of preeclampsia including hypertension and proteinuria. Our studies identify complement components c3a and its receptor as potential therapeutic targets for PE.
Acknowledgments
Sources of Funding
Support for this work was provided by the National Institute of Health grants HL076558, HD34130 and RC4HD067977 and American Heart Association Grant 10GRNT3760081.
Abbreviations
- Ang II
angiotensin II
- AT1R
angiotensin II type 1 receptor
- AT1-AA
angiotensin receptor agonistic autoantibody
- NT
normotensive
- PE
preeclampsia
- sFlt-1
soluble Fms-like tyrosine kinase-1
- C3aR
complement C3a receptor
- IUGR
intrauterine growth restriction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Redman CW, Sargent IL. Preeclampsia and the systemic inflammatory response. Semin Nephrol. 2004;24:565–570. doi: 10.1016/s0270-9295(04)00127-5. [DOI] [PubMed] [Google Scholar]
- 2.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin at1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia Y, Zhou CC, Ramin SM, Kellems RE. Angiotensin receptors, autoimmunity, and preeclampsia. J Immunol. 2007;179:3391–3395. doi: 10.4049/jimmunol.179.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia Y, Kellems RE. Receptor-activating autoantibodies and disease: Preeclampsia and beyond. Expert review of clinical immunology. 2011;7:659–674. doi: 10.1586/eci.11.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaMarca B. Progress toward identifying potential markers for preeclampsia: Role of agonistic autoantibody to the angiotensin ii type i receptor. Hypertension. 2011;55:236–237. doi: 10.1161/HYPERTENSIONAHA.109.141465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irani RA, Xia Y. Renin angiotensin signaling in normal pregnancy and preeclampsia. Semin Nephrol. 2010;31:47–58. doi: 10.1016/j.semnephrol.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irani RA, Xia Y. The functional role of the renin-angiotensin system in pregnancy and preeclampsia. Placenta. 2008;29:763–771. doi: 10.1016/j.placenta.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig. 2003;10:82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 9.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, 3rd, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110:1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 10.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. At(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 11.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN. Agonistic autoantibodies to the at1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 12.Bobst SM, Day MC, Gilstrap LC, 3rd, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. American journal of hypertension. 2005;18:330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Zhou CC, Irani RA, Zhang Y, Blackwell SC, Mi T, Wen J, Shelat H, Geng YJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody-mediated tumor necrosis factor-alpha induction contributes to increased soluble endoglin production in preeclampsia. Circulation. 2011;121:436–444. doi: 10.1161/CIRCULATIONAHA.109.902890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenzel K, Rajakumar A, Haase H, Geusens N, Hubner N, Schulz H, Brewer J, Roberts L, Hubel CA, Herse F, Hering L, Qadri F, Lindschau C, Wallukat G, Pijnenborg R, Heidecke H, Riemekasten G, Luft FC, Muller DN, Lamarca B, Dechend R. Angiotensin ii type 1 receptor antibodies and increased angiotensin ii sensitivity in pregnant rats. Hypertension. 2011;58:77–84. doi: 10.1161/HYPERTENSIONAHA.111.171348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parrish MR, Wallace K, Tam Tam KB, Herse F, Weimer A, Wenzel K, Wallukat G, Ray LF, Arany M, Cockrell K, Martin JN, Dechend R, LaMarca B. Hypertension in response to at1-aa: Role of reactive oxygen species in pregnancy-induced hypertension. American journal of hypertension. 2011;24:835–840. doi: 10.1038/ajh.2011.62. [DOI] [PubMed] [Google Scholar]
- 17.Parrish MR, Ryan MJ, Glover P, Brewer J, Ray L, Dechend R, Martin JN, Jr, Lamarca BB. Angiotensin ii type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gender medicine. 2011;8:184–188. doi: 10.1016/j.genm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: Correlation with disease severity. Hypertension. 2010;55:386–393. doi: 10.1161/HYPERTENSIONAHA.109.140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herse F, Verlohren S, Wenzel K, Pape J, Muller DN, Modrow S, Wallukat G, Luft FC, Redman CW, Dechend R. Prevalence of agonistic autoantibodies against the angiotensin ii type 1 receptor and soluble fms-like tyrosine kinase 1 in a gestational age-matched case study. Hypertension. 2009;53:393–398. doi: 10.1161/HYPERTENSIONAHA.108.124115. [DOI] [PubMed] [Google Scholar]
- 20.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 21.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 22.Sjoberg AP, Trouw LA, Blom AM. Complement activation and inhibition: A delicate balance. Trends Immunol. 2009;30:83–90. doi: 10.1016/j.it.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Sacks SH. Complement fragments c3a and c5a: The salt and pepper of the immune response. Eur J Immunol. 2010;40:668–670. doi: 10.1002/eji.201040355. [DOI] [PubMed] [Google Scholar]
- 24.Hutamekalin P, Takeda K, Tani M, Tsuga Y, Ogawa N, Mizutani N, Yoshino S. Effect of the c3a-receptor antagonist sb 290157 on anti-ova polyclonal antibody-induced arthritis. J Pharmacol Sci. 2010;112:56–63. doi: 10.1254/jphs.09180fp. [DOI] [PubMed] [Google Scholar]
- 25.Ames RS, Lee D, Foley JJ, Jurewicz AJ, Tornetta MA, Bautsch W, Settmacher B, Klos A, Erhard KF, Cousins RD, Sulpizio AC, Hieble JP, McCafferty G, Ward KW, Adams JL, Bondinell WE, Underwood DC, Osborn RR, Badger AM, Sarau HM. Identification of a selective nonpeptide antagonist of the anaphylatoxin c3a receptor that demonstrates antiinflammatory activity in animal models. J Immunol. 2001;166:6341–6348. doi: 10.4049/jimmunol.166.10.6341. [DOI] [PubMed] [Google Scholar]
- 26.Jacob A, Bao L, Brorson J, Quigg RJ, Alexander JJ. C3ar inhibition reduces neurodegeneration in experimental lupus. Lupus. 2010;19:73–82. doi: 10.1177/0961203309348978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Molecular aspects of medicine. 2007;28:192–209. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Fang CJ, Richards A, Liszewski MK, Kavanagh D, Atkinson JP. Advances in understanding of pathogenesis of ahus and hellp. Br J Haematol. 2008;143:336–348. doi: 10.1111/j.1365-2141.2008.07324.x. [DOI] [PubMed] [Google Scholar]
- 29.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girardi G, Bulla R, Salmon JE, Tedesco F. The complement system in the pathophysiology of pregnancy. Mol Immunol. 2006;43:68–77. doi: 10.1016/j.molimm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Salmon JE, Girardi G. Antiphospholipid antibodies and pregnancy loss: A disorder of inflammation. J Reprod Immunol. 2008;77:51–56. doi: 10.1016/j.jri.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch AM, Gibbs RS, Murphy JR, Byers T, Neville MC, Giclas PC, Salmon JE, Van Hecke TM, Holers VM. Complement activation fragment bb in early pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2008;199:354, e351–e358. doi: 10.1016/j.ajog.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen D, Buurma A, Goemaere NN, Girardi G, le Cessie S, Scherjon S, Bloemenkamp KW, de Heer E, Bruijn JA, Bajema IM. Classical complement activation as a footprint for murine and human antiphospholipid antibody-induced fetal loss. The Journal of pathology. 2011;225:502–511. doi: 10.1002/path.2893. [DOI] [PubMed] [Google Scholar]
- 34.Lynch AM, Gibbs RS, Murphy JR, Giclas PC, Salmon JE, Holers VM. Early elevations of the complement activation fragment c3a and adverse pregnancy outcomes. Obstet Gynecol. 2011;117:75–83. doi: 10.1097/AOG.0b013e3181fc3afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derzsy Z, Prohaszka Z, Rigo J, Jr, Fust G, Molvarec A. Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol. 2010;47:1500–1506. doi: 10.1016/j.molimm.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the nhlbi working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 37.Irani RA, Zhang Y, Blackwell SC, Zhou CC, Ramin SM, Kellems RE, Xia Y. The detrimental role of angiotensin receptor agonistic autoantibodies in intrauterine growth restriction seen in preeclampsia. J Exp Med. 2009;206:2809–2822. doi: 10.1084/jem.20090872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. At1 receptor agonistic antibodies from preeclamptic patients stimulate nadph oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 39.Irani RA, Zhang Y, Zhou CC, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-alpha signaling. Hypertension. 2010;55:1246–1253. doi: 10.1161/HYPERTENSIONAHA.110.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component c3 regulates acute renal transplant rejection. Nat Med. 2002;8:582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 42.Lin ZH, Fukuda N, Jin XQ, Yao EH, Ueno T, Endo M, Saito S, Matsumoto K, Mugishima H. Complement 3 is involved in the synthetic phenotype and exaggerated growth of vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 2004;44:42–47. doi: 10.1161/01.HYP.0000129540.83284.ca. [DOI] [PubMed] [Google Scholar]
- 43.Shagdarsuren E, Wellner M, Braesen JH, Park JK, Fiebeler A, Henke N, Dechend R, Gratze P, Luft FC, Muller DN. Complement activation in angiotensin ii-induced organ damage. Circulation research. 2005;97:716–724. doi: 10.1161/01.RES.0000182677.89816.38. [DOI] [PubMed] [Google Scholar]
- 44.Rampersad R, Barton A, Sadovsky Y, Nelson DM. The c5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta. 2008;29:855–861. doi: 10.1016/j.placenta.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Busche MN, Pavlov V, Takahashi K, Stahl GL. Myocardial ischemia and reperfusion injury is dependent on both igm and mannose-binding lectin. American journal of physiology. 2009;297:H1853–1859. doi: 10.1152/ajpheart.00049.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bao L, Wang Y, Haas M, Quigg RJ. Distinct roles for c3a and c5a in complement-induced tubulointerstitial injury. Kidney international. 2011;80:524–534. doi: 10.1038/ki.2011.158. [DOI] [PubMed] [Google Scholar]
- 47.Rynkowski MA, Kim GH, Garrett MC, Zacharia BE, Otten ML, Sosunov SA, Komotar RJ, Hassid BG, Ducruet AF, Lambris JD, Connolly ES. C3a receptor antagonist attenuates brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2009;29:98–107. doi: 10.1038/jcbfm.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, Espinola R, Xiaowei LE, Mao D, Vialpando CG, Salmon JE. Complement c3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195:211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girardi G, Prohaszka Z, Bulla R, Tedesco F, Scherjon S. Complement activation in animal and human pregnancies as a model for immunological recognition. Mol Immunol. 2011;48:1621–1630. doi: 10.1016/j.molimm.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Qing X, Redecha PB, Burmeister MA, Tomlinson S, D'Agati VD, Davisson RL, Salmon JE. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney international. 2010;79:331–339. doi: 10.1038/ki.2010.393. [DOI] [PubMed] [Google Scholar]
- 51.Singh J, Ahmed A, Girardi G. Role of complement component c1q in the onset of preeclampsia in mice. Hypertension. 2011;58:716–724. doi: 10.1161/HYPERTENSIONAHA.111.175919. [DOI] [PubMed] [Google Scholar]
- 52.Siddiqui AH, Irani RA, Zhang Y, Dai Y, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Recombinant vascular endothelial growth factor 121 attenuates autoantibody-induced features of pre-eclampsia in pregnant mice. American journal of hypertension. 2011;24:606–612. doi: 10.1038/ajh.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou CC, Irani RA, Zhang Y, Blackwell SC, Mi T, Wen J, Shelat H, Geng YJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody-mediated tumor necrosis factor-alpha induction contributes to increased soluble endoglin production in preeclampsia. Circulation. 2010;121:436–444. doi: 10.1161/CIRCULATIONAHA.109.902890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L, Day MC, Ramin SM, Ahmed A, Kellems RE, Xia Y. utoantibody from women with preeclampsia induces soluble fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated t-cells signaling. Hypertension. 2008;51:1010–1019. doi: 10.1161/HYPERTENSIONAHA.107.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou CC, Irani RA, Dai Y, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-mediated il-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia. J Immunol. 2011;186:6024–6034. doi: 10.4049/jimmunol.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.