Abstract
We have hypothesized that estradiol enhances basal forebrain cholinergic function and cognitive performance, at least in part, via activation of the novel estrogen receptor GPR30. Here we evaluated the effects of estradiol, G-1 (a selective GPR30 agonist), and tamoxifen (TAM; an ERα/ERβ antagonist that also acts as a GPR30 agonist), on acetylcholine (ACh) release in the hippocampus, as well as the ability to block the effects of 17β-estradiol (E) or TAM with the GPR30 antagonist G-15. Note that G-1 was included to evaluate the effects of selectively activating GPR30, whereas TAM was included to differentiate effects of E associated with activation of GPR30 vs. ERα or ERβ. The study was designed to test effects on potassium-stimulated release, as well as on ACh release stimulated by feeding. Effects of feeding were including because the tasks we used previously to demonstrate beneficial effects of E on cognitive performance were motivated by food reward, and we hypothesized that E may enhance performance by increasing ACh release in association with that reward. Ovariectomized rats were treated for 1 week, and ACh release was evaluated using in vivo microdialysis. In addition, rats were fed at the same time daily for several days and were fasted overnight prior to microdialysis. For each rat, ACh release was evaluated under basal conditions, in response to feeding, and in response to elevated potassium. Both feeding and elevated potassium increased ACh release in the hippocampus. In response to feeding, E, G-1, and TAM all significantly increased the percent change in release. The effects of E and TAM were blocked by G-15, and the effects of combining E+TAM did not differ significantly from the effects of E or TAM alone. In response to elevated potassium, E, and TAM significantly increased the percent change in ACh release. G-1 produced a slightly lesser effect. The effect of TAM was reduced by G-15, but the effect of E was not. These findings suggest that activation of GPR30 is both necessary and sufficient to account for the effects of E on ACh release associated with feeding. In contrast, activation of GPR30 appears to be sufficient, but may not be necessary for increased release associated with elevated potassium. The changes associated with feeding are consistent with the effects of E, G-1 and G-15 on acquisition of a spatial learning task previously described. These data confirm and extend previous reports, and support a hypothesis wherein E treatment can improve learning on specific tasks by activating GPR30 and enhancing ACh release in association with food reward.
Keywords: Estradiol, G-1, G-15, Tamoxifen, Basal Forebrain Cholinergic Neurons, Hippocampus, Feeding-Induced ACh Release, Potassium-Stimulated ACh Release
INTRODUCTION
Studies show that, in rodents, ovariectomy impairs and estradiol enhances performance on a variety of learning, memory, and attention tasks (Bimonte-Nelson et al., 2010; Daniel and Bohacek, 2010; Gibbs, 2010). We hypothesize that these effects are mediated, at least in part, by effects on cholinergic inputs to the hippocampus and cortex (Gibbs, 2010). This is supported by studies showing that 17β-estradiol (E) enhances basal forebrain cholinergic function as evidenced by increases in choline acetyltransferase (ChAT) (Gibbs, 1996; Gibbs, 1997; Gibbs, 2000; Gibbs and Pfaff, 1992; Gibbs et al., 1994; Luine, 1985; Singh et al., 1994), high affinity choline uptake in the hippocampus and frontal cortex (Gibbs, 2000; O'Malley et al., 1987), and increases in potassium-stimulated acetylcholine (ACh) release (Gabor et al., 2003; Gibbs et al., 1997b). In addition, selective destruction of cholinergic neurons in the medial septum blocked the effects of estradiol on learning in a delayed matching-to-position (DMP) T-maze task (Gibbs, 2007), as well as on synaptic spine density in the hippocampus (Lam and Leranth, 2003). Intrahippocampal infusion of muscarinic cholinergic receptor inhibitors likewise blocked E effects on acquisition of an 8-arm radial maze task (Daniel et al., 2005). Conversely, treating with cholinesterase inhibitors partially restored estradiol effects on learning in aged rats (Gibbs et al., 2011a; Gibbs et al., 2009), and in young rats with partial cholinergic lesions (Gibbs et al., 2011b). These findings indicate that effects of E on cholinergic function are an important mediator of effects on performance, at least within specific cognitive domains.
The mechanisms by which E mediates the effects on basal forebrain cholinergic neurons are not well understood. A subset of the cells (~10–30%) contain ERα (not ERβ) (Miettinen et al., 2002; Shughrue et al., 2000) and there is some evidence for direct effects mediated via this receptor (Pongrac et al., 2004; Szego et al., 2006). Recently, however, we found that the majority of the cholinergic neurons contain GPR30, a novel G protein-coupled estrogen receptor (Hammond et al., 2011). Hence current studies are focusing on the role of GPR30 in mediating estrogen effects on cholinergic function and cognitive performance. To that end we have shown that G-1, a selective GPR30 agonist, increases potassium-stimulated ACh release in the hippocampus and enhances acquisition of the DMP task similar to E (Hammond et al., 2009; Hammond et al., 2011). Furthermore G-15, a selective GPR30 antagonist, impaired the performance of gonadally intact rats comparable to the effect of ovariectomy, and blocked the ability of E to enhance performance in ovariectomized rats (Hammond et al., 2012). This suggests that GPR30 plays an essential role in mediating the effects of E on acquisition of this task.
The current report extends our recent findings by further evaluating the role of GPR30 in mediating the effects of E on ACh release. Here we evaluated not only potassium-stimulated release, but also release associated with presentation of food following an overnight fast. This modeled, in part, the food reward associated with acquisition of the DMP task. In vivo microdialysis was used to compare the effects of E, G-1 and tamoxifen (an ERα antagonist/GPR30 agonist) on ACh release. We also tested the ability to block effects of E and tamoxifen (TAM) with G-15. Results show that E, G-1, and TAM all increased ACh release, both potassium-stimulated release as well as release associated with feeding. The effects of E and TAM on release associated with feeding were completely blocked by G-15. In contrast, the effects of E on potassium-stimulated release were not blocked by G-15. This suggests that activation of GPR30 is both necessary and sufficient to mediate the effect of E on ACh release associated with food reward, and suggests that this effect may underlie the effects of E, G-1 and G-15 on DMP acquisition previously described.
METHODS
Animals
A total of 52 young adult female Sprague-Dawley rats (~3 months old) were purchased from Hilltop Laboratories. All rats were ovariectomized (Ovx) by the supplier prior to delivery. Rats were individually housed on a 12-hour day/night cycle (7 am to 7 pm) with food and water freely available. Rats were housed for at least 2 weeks prior to treatments. All procedures were carried out in accordance with PHS policies on the use of animals in research, and with the approval of the University of Pittsburgh’s Institutional Animal Care and Use Committee.
Drug Treatments
E was delivered by silastic capsule (6 mm length, 1.98 mm I.D., 3.18 mm O.D.) packed with 3 mm of powdered 17β-estradiol (Sigma-Aldrich, Inc.) implanted s.c. in the dorsal neck region. G-1, and G-15 were dissolved first in DMSO and then in 20% β-hydroxypropyl cyclodextrin (Aldrich, Inc.) and then administered by miniosmotic pump (Alzet model 2002; Durect, Corp.) implanted s.c.in the dorsal neck region. Tamoxifen (TAM; Tocris, Inc.) was dissolved in saline and administered daily by injection s.c. Controls received pumps containing vehicle as well as daily injections of saline.
Treatments were as follows
Controls (n=9); E (n=9); G-1 − 5 µg/day (n=5); Tam − 2 mg/Kg/day (n=5); E + TAM (n=5); G-15 − 40 µg/day (n=6); E + G-15 (n=8); TAM + G-15 (n=5)
Doses of E and G-1 were selected based on previous studies showing significant effects on DMP acquisition (Gibbs, 2007; Hammond et al., 2009) or effects on potassium-stimulated release (Gabor et al., 2003; Hammond et al., 2011). In addition, the dose of E was estimated to produce circulating levels of E in the normal physiological range (~50 pg/mL). An identical dose of G-1 was used based on similarities to E in molecular weight and structure. At the time of pump implantation rats also received a microdialysis guide cannula (CMA 12 Elite Guides, CMA Microdialysis, Inc.) lowered into the right hippocampus (−3.4 mm bregma, 1.18 mm lateral, −3.4 mm ventral) and fixed to the skull using dental cement as previously described (Gibbs et al., 2004). Microdialysis was performed 7 days later.
Feeding
Rats were fed at the same time in the morning of each day for several days prior to microdialysis. This was done so that rats would learn to anticipate food at a particular time each day. This is similar to the restricted feeding paradigm that was used when rats were trained to perform the DMP task described in prior reports.
In Vivo Microdialysis
Rats were fasted overnight prior to microdialysis. Concentric, 3 mm microdialysis probes were used (CMA 12 Elite Probes, CMA Microdialysis, Inc.). On each day of microdialysis, probes were first dialyzed for 30 minutes against a solution of ACSF containing 0.1 pmol ACh/20 µL. This sample was used to calculate and correct for probe efficiency. The probe was then rinsed and the infusate changed to ACSF containing 0.2 µM neostigmine. The neostigmine was added based on previous studies showing that modest inhibition of acetylcholinesterase activity is necessary in order to reliably detect basal levels of acetylcholine in the extracellular fluid (Gabor et al., 2003; Gibbs et al., 1997a; Rhodes et al., 1997). The probe was then inserted through the cannula into the hippocampus and the rat was placed into a large plastic container for the duration of the experiment. The probe was perfused at a rate of 1 µL/min and dialysate was collected continuously. Samples were collected and frozen every 30 min. After waiting 90 minutes for basal ACh release to stabilize, two samples were collected over one hour and averaged to represent basal release. Rats were then given food pellets every 5 minutes for 15 minutes and dialysate was collected for 30 minutes. This sample was used to assess ACh release in response to feeding. Next, probes were perfused with ACSF containing elevated levels of potassium (60 mM) and samples were collected after 30 and 60 min to measure potassium-stimulated release. Following microdialysis rats were given an overdose of ketamine (40 mg/kg) and xylazine (28 mg/kg) injected i.p. and euthanized by decapitation.
Quantification of 17β-Estradiol
Trunk blood was collected for the determination of serum estradiol levels using a sensitive LC-MS-MS method recently described (Hammond et al., 2012). Briefly, Samples were spiked with internal standard (2,4,16,16,17-d3-17β-estradiol) and then extracted with n-butyl chloride. After centrifugation and evaporation, the residue was derivatized in 0.1 mL buffered dansyl chloride (pH 10.5). E2 was eluted from a Waters Acquity UPLC BEH C18, 1.7 um, 2.1 × 150 mm reversed-phase column, with an acetonitrile:water (0.1% formic acid) gradient. Detection and quantification were achieved in the positive mode. Transitions used for analysis were 506 → 171 for E2, and 511 → 171 for the deuterated internal standard. Area under the peak was quantified and used to determine absolute levels of E2/mL of sample by comparison with a series of standards. The limit of detectability for this assay was 2.5 pg/mL.
Quantification of ACh
Levels of ACh in the dialysates were measured by HPLC with electrochemical detection as previously described (Hammond et al., 2011). Quantity of ACh was determined by measuring area under the ACh peak. This assay was able to detect as little as 30 fmol of ACh per 20 µL sample. Values from in vivo dialysates were then corrected for probe recovery and expressed as picomoles per 20 µl sample. The two samples collected prior to feeding were averaged as an estimate of basal ACh release. Subsequent samples were used to calculate percent change in ACh release relative to baseline for each rat. In this way, each rat served as its own control for estimating feeding-stimulated and potassium-stimulated release. Percent change that was calculated from the two samples collected over 60 minutes after switching to high potassium were averaged as a measure of percent change from basal release during the 60 minute period. Average basal, feeding-stimulated, and potassium-stimulated release were then calculated for each treatment group. Effects of treatment on release were analyzed using ANOVA followed by either a Tukey test or a Dunnett’s test comparing each treatment group with the controls. Effect sizes are reported as eta-squared (η2) for ANOVA and as Cohen’s d (d) for pairwise comparisons.
RESULTS
Serum estradiol levels
Mean serum levels of estradiol in the E, E+TAM and E+G-15 treated groups were 51.3 ± 3.8, 48.7 ± 5.8, 50.8 ± 10.7 pg/mL. These values did not differ significantly. Levels of E in rats that received Ovx only were below the level of detection.
Basal ACh Release
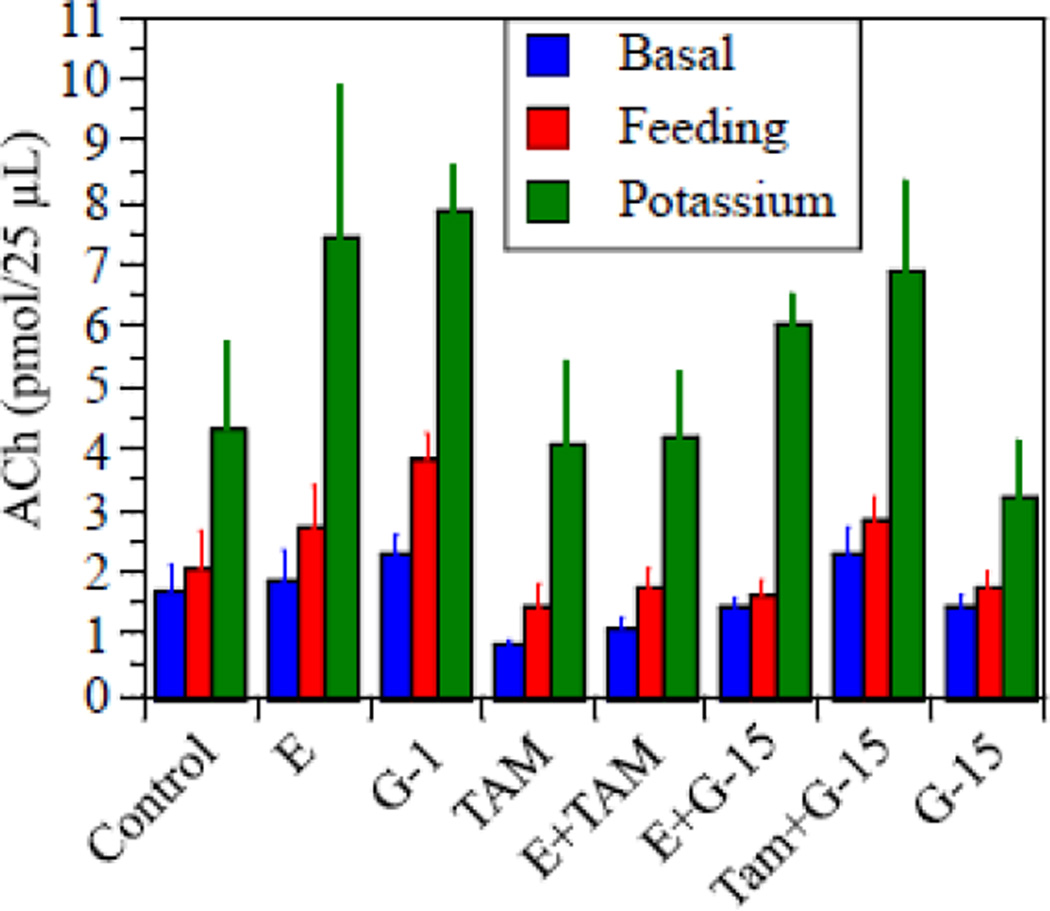
Basal levels of ACh release/20 µL sample ranged from a mean of 0.8 ± 0.10 pmol in TAM-treated rats to 2.3 ± 0.27 pmol in G-1 treated rats. Mean values for all treatment groups are summarized in Figure 1. ANOVA revealed no significant effects of treatment on basal ACh release F[7,44]=1.8, p=0.11. Comparison of the means by Dunnett’s test revealed no significant difference between any of the treatment groups vs. the controls. These findings suggest that treatments did not significantly alter basal ACh release.
Figure 1.
Levels of ACh detected for basal release, release during feeding, and release induced by elevated potassium. Bars indicate mean ± s.e.m for each treatment group and condition. See text for a description of the statistical analyses and results.
ACh Release Induced by Feeding
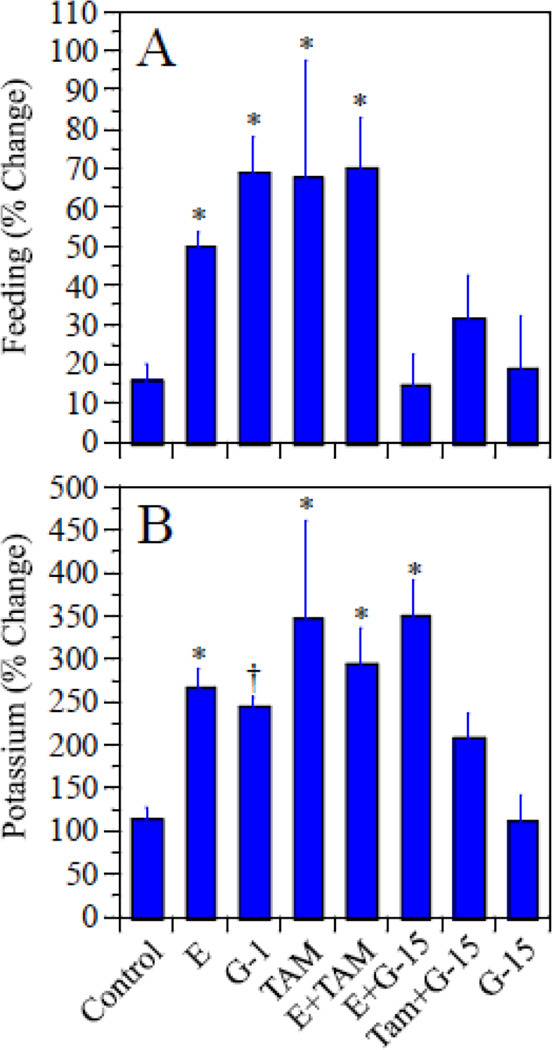
Our analysis suggests that E, G-1, and TAM all increased ACh release associated with feeding, and that these effects were blocked by G-15. Levels of ACh release during the 30 minutes when food was provided ranged from a mean of 1.4 ± 0.55 pmol in TAM-treated rats to 3.8 ± 0.55 pmol in G-1-treated rats (Fig 1). One-way ANOVA revealed a significant effect of treatment (F[7,44]=2.5, p=0.03, η2=0.28); however, neither Tukey nor Dunnett’s tests conducted on the raw data revealed significant differences between any of the drug treatments vs. the controls. A repeated measures ANOVA utilizing basal release and release during feeding as the repeated measures and treatment as the between measure, revealed a significant increase in release associated with feeding (F[1,44]=96.7, p<0.0001, η2=0.97). The overall treatment effect was nearly significant (F[7,44]=2.1, p=0.06, η2=0.23), and there was a significant treatment × feeding interaction (F[7,44]=5.1, p=0.0003, η2=0.03). This suggests that the change in release associated with feeding was differentially affected by treatment. To explore this further we calculated the percent change in release associated with feeding for each rat and analyzed this by one-way ANOVA with treatment as the between factor. This has the added advantage of enabling each rat to serve as it’s own control, which reduced inter-animal variation. Results are summarized in Figure 2A. The analysis revealed a significant effect of treatment (F[7,44]=4.6, p<0.001, η2=0.42) on percent change in ACh release associated with feeding. Post-hoc analysis confirmed significant increases in rats treated with E (d=2.7), G-1 (d=3.3), TAM (d=1.3), and E+TAM (d=2.8) relative to controls (p<0.05 in each case by Dunnett’s test). In contrast, rats treated with G-15, E+G-15, or TAM+G-15 did not differ significantly from controls. This demonstrates that treatment with E, G-1 or TAM increased ACh release associated with feeding relative to controls, and that these effects were blocked by treatment with G-15.
Figure 2.
Percent change in ACh release associated with feeding (A) and with elevated potassium(B). In this case, each rat served as its own control in calculating percent change in release. *p<0.05 relative to controls. †p<0.03 relative to controls only after removing the TAM group from the analysis. See text for discussion.
ACh Release Induced by Elevated Potassium
Effects of treatments on potassium-stimulated release also were observed; however, the effects were more complex than those associated with feeding. Levels of ACh release during the 60 minutes of elevated potassium ranged from a mean of 3.2 ± 0.92 pmol in G-15-treated rats to 7.4 ± 2.4 pmol in E-treated rats (Fig 1). One-way ANOVA of the raw data revealed no significant effect of treatment (F[7,43]=1.7, p=0.14). A repeated measures ANOVA utilizing basal release and release during elevated potassium as the repeated measures and treatment as the between measure, revealed a significant increase in release associated with elevated potassium (F[1,43]=72.6, p<0.0001, η2=0.83); but no significant effect of treatment (F[7,43]=1.7, p=0.13), and no significant treatment × potassium interaction (F[7,43]=1.7, p=0.13). However, when we evaluated the percent change in release associated with elevated potassium using each rat as its own control, the one-way analysis with treatment as the between factor revealed a highly significant effect of treatment (F[7,43]=5.0, p<0.001, η2=0.45). Post-hoc analysis reveled that rats treated with E (d=2.9), Tam (d=1.6), E+Tam (d=3.3), or E+G-15 (d=2.8) all differed significantly from the controls (Fig 2B). G-1 appeared to produce an effect similar to E, however the effect of G-1 was not statistically significant. Inspection of the data suggested that this might be due to the relatively small sample size for a study of this many treatment groups, and the high degree of variance in the TAM-treated group, which added significantly to the overall error term in the analysis. Indeed, variance in the TAM-treated group was over four times the variance in any other group. This may be due to the fact that TAM is an ERα/ERβ antagonist, while at the same time also acts as a GPR30 agonist. Therefore, the effects of TAM on ACh release in a given animal will depend in part on the relative expression of ERα and GPR30 by the cholinergic cells in that animal. This could easily add to the overall inter-animal variance in the effects of TAM on ACh release. Indeed, when the TAM group was excluded, the statistical significance of the E, E+TAM, and E+G-15 treatment effects was greatly increased and the G-1-treated group now differed significantly from the controls (p<0.03, d=7.0) comparable to prior studies (Hammond et al., 2011). This suggests that E, G-1, and TAM each can increase potassium-stimulated ACh release in the hippocampus. In addition, the effect of TAM on potassium-stimulated release was reduced by G-15 as evidenced by the fact that the TAM-treated group differed significantly from controls whereas the TAM+G-15-treated group did not. In contrast, the effects of E were not reduced by G-15.
DISCUSSION
Effects on ACh Release
The results extend our previous findings demonstrating significant effects of E and G-1 on ACh release in the hippocampus. Of particular note are the increases in ACh release associated with feeding. In vehicle-treated controls feeding was associated with an increase of approximately 15%. This is consistent with a prior report showing increased ACh release in the hippocampus during anticipation and consumption of a meal (Inglis et al., 1994), although the increases observed here are much less than the 2–3 fold increases reported previously. The current data show that release was further increased approximately 3–4 fold in E, G-1 and TAM-treated groups. To our knowledge, this is the first report showing that ER agonists and antagonists can influence ACh release associated with feeding. TAM is a mixed ER agonist/antagonist that, at sufficiently high concentrations inhibits ERα (MacGregor and Jordan, 1998; Quaedackers et al., 2001; Shiau et al., 1998), but that also acts as an agonist at the GPR30 receptor (Thomas et al., 2005). The fact that TAM alone increased ACh release similar to E, and that the effect of E was not reduced by TAM suggest that the effects of E and TAM on ACh release associated with feeding are due to activation of GPR30. This is further supported by the fact that a similar effect was produced by G-1, and that the effects of E and TAM were blocked completely by G-15. Note that G-15 alone had no effect in the absence of ER agonists. The data also suggest that ERα has little role since the effect of E was not altered by TAM. Collectively, these data strongly suggest that activation of GPR30 is both necessary and sufficient to mediate the increase in ACh release induced by E in association with feeding.
The results also confirm and extend our previous reports showing that E significantly increases potassium-stimulated ACh release in the hippocampus (Gabor et al., 2003; Gibbs et al., 2004; Gibbs et al., 1997b; Hammond et al., 2011). Here we show that a similar increase also is produced by TAM. Specifically, the average percent change in release was approximately 2.5–3.5 fold greater in E and TAM-treated rats than in vehicle-treated controls. G-1 had slightly less of an effect, but even so the mean percent change in release was more than 2 fold greater than in the controls, which agrees nicely with prior results (Hammond et al., 2011). The percent change in release produced by E+TAM was similar to that produced by E alone (E+TAM: 292.8% ±41.1; E: 264.3% ± 24.0). Since TAM acts as an ERα antagonist, this suggests that the effect of E was not due to activation of ERα. Although measures for TAM vs. TAM+G-15 were not significantly different, indirect evidence suggests that the effect of TAM was reduced by G-15. This is demonstrated by the fact that the TAM-treated group differed significantly from controls whereas the TAM+G-15-treated group did not. This supports the idea that the effect of TAM was mediated, at least in part, by activation of GPR30. However, unlike the effects associated with feeding, the effects of E on potassium-stimulated release were not blocked by G-15, suggesting that receptors other than GPR30 also can support E-mediated effects on potassium-stimulated ACh release. Hence in this case we conclude that activation of GPR30 is sufficient, but may not be necessary, to produce the increase in potassium-stimulated ACh release elicited by E.
The effect of E on potassium-stimulated release may be related to a recent report by Damborsky et al. (Damborsky et al., 2013) showing that E reduces the frequency of spontaneous inhibitory postsynaptic synaptic currents (sIPSCs) and increases the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) on septal neurons (Damborsky et al., 2013). These effects were action potential-dependent. Notably, decreases in sIPSCs also were produced by G-1 and by DPN (a selective ERβ agonist), but not by PPT (a selective ERα agonist). In contrast, increases in sEPSCs were produced by G1 and also by PPT, but not by DPN. This suggests that E-treatment alters the ratio of inhibitory:excitatory inputs onto septal cholinergic neurons, and that these effects are mediated by multiple estrogen receptor subtypes. Further study will be needed to determine how selective activation of specific estrogen receptor subtypes relate to the observed effects on potassium-stimulated ACh release.
Relevance of effects on ACh Release to Effects on Cognitive Performance
Although cognitive performance was not evaluated in this study, we have conducted many studies showing that ovariectomy impairs, and E-treatment improves, the rate at which rats learn a delayed matching-to-position (DMP) T-maze task, and that cholinergic inputs to the hippocampus are critically involved (reviewed in Gibbs, 2010). For example, selective destruction of cholinergic inputs to the hippocampus completely abolishes the effect of E on DMP acquisition (Gibbs, 2007; Gibbs et al., 2011b). Effects on DMP acquisition correlate with the degree of cholinergic cells loss (Gibbs et al., 2011b), and cholinesterase inhibitors can restore effects of E in aged rats (Gibbs et al., 2011a; Gibbs et al., 2009) and in young rats with a partial loss of the cholinergic projections (Gibbs et al., 2011b). These findings are consistent with additional reports showing that effects of E on other tasks such as place learning and radial maze learning are related to acetylcholine release (Marriott et al., 1999; Marriott and Korol, 2003; McIntyre et al., 2003) or can be blocked by selective cholinergic inhibitors (Daniel et al., 2005). This has led us to hypothesize that beneficial effects of E on cognitive performance are due, at least in part, to effects on basal forebrain cholinergic function. More recently we showed that G-1 enhances DMP acquisition similar to E (Hammond et al., 2009), and that the effects of both G-1 and E on DMP acquisition are blocked by G-15 (Hammond et al., 2012). This suggests that activation of GPR30 is both necessary and sufficient to mediate the effects of E on DMP acquisition.
These behavioral results agree nicely with the current results showing that E, G-1, and TAM increase ACh release in association with feeding, and that the effects of E and TAM are blocked by G-15. The DMP task is motivated by food reward. Rats are food restricted. They receive food reward during performance of the task as well as their daily feeding shortly after completion of the task. Testing is performed at approximately the same time each day. Hence rats learn to anticipate food in association with the task as well as with the time of day. Here we show that E increases ACh release in the hippocampus in association with feeding, in rats that have experienced an overnight fast and that have been fed daily so as to anticipate feeding around a specific time of day. This suggests that one mechanism by which E improves DMP acquisition is by increasing ACh release in the hippocampus specifically in association with the anticipation and receipt of food reward.
These findings make sense when one considers that ACh release in the hippocampus and cortex is an important regulator of wakefulness and attention (Baxter and Chiba, 1999; Everitt and Robbins, 1997; Parikh and Sarter, 2008; Sarter et al., 2006). These effects are mediated in part via direct inputs onto the cholinergic neurons coming from orexin-containing cells located in the lateral hypothalamus (Eggermann et al., 2001; Espana et al., 2001; Fadel et al., 2005; Thakkar et al., 2001). At the cellular level, cholinergic inputs have been shown to modulate cellular excitability that lead to increased sensory discrimination abilities. For example, studies by Gullege and co-workers show that ACh activity modulates the excitability of neocortical and hippocampal pyramidal neurons. Increases in excitability are accomplished, in part, by the production of calcium-dependent after-depolarizing potentials following short trains of action potentials (Gulledge et al., 2009). In the prefrontal cortex these effects are mediated by M1 type ACh receptors. In the hippocampus, cholinergic modulation of the Schaffer-collateral circuit is mediated predominantly by M1 and M4 receptors (Dasari and Gulledge, 2011).
Using a different approach, Ma et al. (2012) showed that in the olfactory bulb, cholinergic activation sharpens the olfactory tuning curves of mitral and tufted cells and potentiates odor-evoked responses of periglomerular cells and granule cells. This is consistent with a cholinergic-mediated increase in olfactory discrimination capability. Likewise, in auditory cortex, cholinergic inputs have been shown to play a critical role in enabling the re-tuning of auditory cells to a stimulus used in an associative learning paradigm (Weinberger, 2003).
These findings provide the cellular basis for ACh effects on attention, with corresponding effects on learning and memory. For example, the effects of phasic ACh activity in the mouse prefrontal cortex mentioned above is predictive of successful cue detection (Gulledge et al., 2009), and the M1 receptors that mediate these effects are necessary to facilitate orienting responses and the establishment of cue-reward associations (Gulledge et al., 2009). Parikh et al. (Parikh et al., 2007) likewise showed significant correlations between ACh release in the prefrontal cortex and cue detection. One way of interpreting these data is that activation of the basal forebrain cholinergic system during reward is a mechanism for biasing the allocation of attentional resources toward sensory cues related to success or failure. With respect to spatial navigation tasks, like the DMP T-maze task and traditional radial maze tasks, we postulate that E treatment enhances learning by increasing ACh release during reward, thereby increasing attention to visual cues and the ability to incorporate those cues into an effective learning strategy. This has not been tested directly; however, the hypothesis is consistent with all of our studies to date showing effects of estrogen receptor agonists and antagonists on basal forebrain cholinergic function and cognitive performance, as well as with evidence that effects of E are abolished when cholinergic inputs are lost or compromised. This also could explain the reduced benefits of E treatment on cognitive performance in advanced age and in association with Alzheimer’s disease where basal forebrain cholinergic neurons are significantly compromised. The possibility that some of these effects may be mediated indirectly via orexin is currently being examined.
The idea that E treatment can improve cognitive performance by altering ACh release in response to food and hence biasing attentional resources toward specific sensory cues agrees nicely with results showing positive effects of E on tasks that are motivated by food reward and that have high attentional requirements. However, what about tasks that are not motivated by food reward or tasks for which attentional demands are low. For example, Bimonte-Nelson and co-workers have shown that E-treatment significantly improves working memory performance on a water radial maze task and on Morris water maze tasks, and that the effects are most apparent as working memory demands increase (Acosta et al., 2009; Bimonte and Denenberg, 1999; Talboom et al., 2008). In these cases, attentional requirements are high, but there is no food reward. Reward consists of escape to a hidden platform. Whether ACh release in hippocampus and cortex is associated with escape to a hidden platform is not known. If it is, then effects of E on ACh release could underlie the effects of E on this task, similar to the effects of E on the DMP task described above. If not, then other cellular mechanisms must be involved. Recently we evaluated the effects of E on acquisition of a simple operant discrimination/reversal learning task (Hammond et al., 2012). This task is motivated by the same food reward that is used in the DMP task and tests the ability to alter behavior in response to a change in reinforcement contingency; however, unlike the DMP task, sensory cues are extremely easy to discriminate and attentional demands are relatively low. In this case, E had no significant effect on task performance. Based on our hypothesis, we would argue this is because there is no significant advantage afforded by biasing attentional resources, since the task is relatively easy to solve.
E treatment also has been shown to affect performance on tasks that do not involve reward at all. For example, several labs have reported that ovariectomy impairs and E-treatment improves novel object recognition memory in rats and mice (Fernandez et al., 2008; Luine et al., 2003; Walf and Frye, 2008; Walf et al., 2008; Walf et al., 2006). This task relies on the animals’ innate predisposition to investigate novel objects in their environment. Animals are presented with two objects in an arena. At a later time the animal is returned to the same arena where one of the two objects has been replaced with a novel object. The investigator measures the percentage of time that an animal spends interacting with the novel vs. the familiar object. Studies show that E-treated rats and mice spend more time interacting with the novel vs. the familiar object, suggesting better recognition memory for the familiar object. Our own studies show that basal forebrain cholinergic lesions do not appear to affect novel object recognition memory in rats (Cai et al., 2012), which would indicate that the effects of E on this task are not due to effects on basal forebrain cholinergic function. Studies by Frick and co-workers suggest that the effects of E on novel object recognition involve direct effects on hippocampal neurons resulting in the activation of ERK (Fernandez et al., 2008; Harburger et al., 2009). These findings demonstrate that there are likely multiple mechanisms by which E can affect cognitive performance, and that mechanisms will depend in large part on the cognitive demands associated with each task. With respect to the DMP task and to other food-motivated tasks with significant attentional demands, we propose that enhancing hippocampal and cortical ACh release in response to food reward, and hence biasing attentional resources toward specific sensory cues, is an important mechanism by which E improves performance on these tasks.
SUMMARY
Results replicate and extend our recent findings showing effects of E and G-1 on ACh release in the hippocampus. We show for the first time that E, G-1, and TAM can increase ACh release in association with food reward and that these effects are blocked by G-15, suggesting that activation of GPR30 is both necessary and sufficient to induce the effects. In addition, we replicate previous findings showing that E and G-1 increase potassium-stimulated release and show that TAM produces a similar effect. Unlike the effects associated with feeding, however, the effect of E on potassium-stimulated release was not blocked by G-15, suggesting that GPR30 is not solely responsible for mediating this effect. The findings are consistent with the effects of E on food-motivated spatial learning tasks that we and others have described, and suggest a model in which E enhances learning on these tasks by increasing ACh release during food reward, thereby increasing attention to visual cues and the ability to incorporate those cues into an effective learning strategy.
Highlights.
Estradiol increases hippocampal acetylcholine release
Activation of GPR30 is necessary to enhance release associated with feeding
This may underlie estrogen’s ability to enhance learning on specific tasks.
ACKNOWLEDGEMENTS
This work was supported by NSF grant 0948796 to RBG and NIH grants F31 AG034035 and R36 AG039381 to RH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors have nothing to disclose.
REFERENCES
- Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav. 2009;55:454–464. doi: 10.1016/j.yhbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–183. doi: 10.1016/s0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Acosta JI, Talboom JS. Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models. Molecules. 2010;15:6050–6105. doi: 10.3390/molecules15096050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Gibbs RB, Johnson DA. Recognition of novel objects and their location in rats with selective cholinergic lesion of the medial septum. Neuroscience letters. 2012;506:261–265. doi: 10.1016/j.neulet.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damborsky JC, DuBois DW, Fincher AS, Roth C, Griffith WH. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2013. Rapid membrane estrogen receptor activation increases the excitatory/inhibitory ratio in the rat basal forebrain. pp. Online. [Google Scholar]
- Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta. 2010;1800:1068–1076. doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience. 2005;132:57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Dasari S, Gulledge AT. M1 and M4 receptors modulate hippocampal pyramidal neurons. Journal of neurophysiology. 2011;105:779–792. doi: 10.1152/jn.00686.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu. Rev. Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fadel J, Pasumarthi R, Reznikov LR. Stimulation of cortical acetylcholine release by orexin A. Neuroscience. 2005;130:541–547. doi: 10.1016/j.neuroscience.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor R, Nagle R, Johnson DA, Gibbs RB. Estrogen enhances potassium-stimulated acetylcholine release in the rat hippocampus. Brain Research. 2003;962:244–247. doi: 10.1016/s0006-8993(02)04053-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Fluctuations in Relative Levels of Choline Acetyltransferase mRNA in Different Regions of the Rat Basal Forebrain Across the Estrous Cycle: Effects of Estrogen and Progesterone. J. Neurosci. 1996;16:1049–1055. doi: 10.1523/JNEUROSCI.16-03-01049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Research. 1997;757:10–16. doi: 10.1016/s0006-8993(96)01432-1. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52:352–359. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Chipman AM, Hammond R, Nelson D. Galanthamine plus estradiol treatment enhances cognitive performance in aged ovariectomized rats. Hormones and behavior. 2011a;60:607–616. doi: 10.1016/j.yhbeh.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Chipman AM, Nelson D. Donepezil plus estradiol treatment enhances learning and delay-dependent memory performance by young ovariectomized rats with partial loss of septal cholinergic neurons. Horm Behav. 2011b;59:503–511. doi: 10.1016/j.yhbeh.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Hashash A, Johnson DA. Effects of estrogen on potassium-evoked acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Research. 1997a;749:143–146. doi: 10.1016/s0006-8993(96)01375-3. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Hashash A, Johnson DA. Effects of estrogen on potassium-stimulated acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Res. 1997b;749:143–146. doi: 10.1016/s0006-8993(96)01375-3. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Mauk R, Nelson D, Johnson DA. Donepezil treatment restores the ability of estradiol to enhance cognitive performance in aged rats: evidence for the cholinergic basis of the critical period hypothesis. Horm Behav. 2009;56:73–83. doi: 10.1016/j.yhbeh.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Pfaff DW. Effects of estrogen and fimbria/fornix transection on p75NGFR and ChAT expression in the medial septum and diagonal band of Broca. Exp. Neurol. 1992;116:23–39. doi: 10.1016/0014-4886(92)90173-n. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Wu D-H, Hersh L, Pfaff DW. Effects of estrogen replacement on relative levels of ChAT, TrkA and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp. Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Bucci DJ, Zhang SS, Matsui M, Yeh HH. M1 receptors mediate cholinergic modulation of excitability in neocortical pyramidal neurons. J Neurosci. 2009;29:9888–9902. doi: 10.1523/JNEUROSCI.1366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology. 2011;36:182–192. doi: 10.1016/j.psyneuen.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Kline E, Gibbs RB. Chronic treatment with a GPR30 antagonist impairs acquisition of a spatial learning task in young female rats. Hormones and behavior. 2012;62:367–374. doi: 10.1016/j.yhbeh.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Saadi A, Frick KM. Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160:6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis FM, Day JC, Fibiger HC. Enhanced acetylcholine release in hippocampus and cortex during the anticipation and consumption of a palatable meal. Neuroscience. 1994;62:1049–1056. doi: 10.1016/0306-4522(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Lam TT, Leranth C. Role of the medial septum diagonal band of Broca cholinergic neurons in oestrogen-induced spine synapse formation on hippocampal CA1 pyramidal cells of female rats. Eur J Neurosci. 2003;17:1997–2005. doi: 10.1046/j.1460-9568.2003.02637.x. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp. Neurol. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Ma M, Luo M. Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:10105–10116. doi: 10.1523/JNEUROSCI.0058-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor JI, Jordan VC. Basic guide to the mechanisms of antiestrogen action. Pharmacological reviews. 1998;50:151–196. [PubMed] [Google Scholar]
- Marriott LK, Gold PE, Korol DL. Estradiol effects on acetylcholine output in the hippocampus during spatial learning in female rats. Society for Neuroscience Abstracts. 1999;25:2161. [Google Scholar]
- Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiol Learn Mem. 2003;80:315–322. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, Gold PE. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol Learn Mem. 2003;79:177–183. doi: 10.1016/s1074-7427(02)00014-x. [DOI] [PubMed] [Google Scholar]
- Miettinen RA, Kalesnykas G, Koivisto EH. Estimation of the total number of cholinergic neurons containing estrogen receptor-alpha in the rat basal forebrain. J Histochem Cytochem. 2002;50:891–902. doi: 10.1177/002215540205000703. [DOI] [PubMed] [Google Scholar]
- O'Malley CA, Hautamaki RD, Kelley M, Meyer EM. Effects of ovariectomy and estradiol benzoate on high affinity choline uptake, ACh synthesis, and release from rat cerebral cortical synaptosomes. Brain Research. 1987;403:389–392. doi: 10.1016/0006-8993(87)90082-5. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124:809–816. doi: 10.1016/j.neuroscience.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Quaedackers ME, Van Den Brink CE, Wissink S, Schreurs RH, Gustafsson JA, Van Der Saag PT, Van Der Burg BB. 4-hydroxytamoxifen trans-represses nuclear factor-kappa B activity in human osteoblastic U2-OS cells through estrogen receptor (ER)alpha, and not through ER beta. Endocrinology. 2001;142:1156–1166. doi: 10.1210/endo.142.3.8003. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Li PK, Burke AM, Johnson DA. Enhanced plasma DHEAS, brain acetylcholine and memory mediated by steroid sulfatase inhibition. Brain Res. 1997;773:28–32. doi: 10.1016/s0006-8993(97)00867-6. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERα and ERβ) in the cholinergic neurons of the rat basal forebrain. Neurosci. 2000;96:41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Research. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Szego EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, Abraham IM. Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–328. [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Conjugated equine estrogen enhances rats' cognitive, anxiety, and social behavior. Neuroreport. 2008;19:789–792. doi: 10.1097/WNR.0b013e3282fe209c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiol Learn Mem. 2003;80:268–284. doi: 10.1016/s1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]