Abstract
Rats orient to and approach localizable visual cues paired with food delivery. Previous studies from this laboratory show that the acquisition and expression of these learned cue-directed responses depend on integrity of a system including the central nucleus of the amygdala (CeA), the substantia nigra pars compacta (SNc), and the dorsolateral striatum (DLS). Other investigators have suggested that cue-directed behaviors may also depend on interaction between CeA and the ventral striatum, perhaps via CeA projections to the ventral tegmentral area (VTA). In Experiment 1 we examined the effects of unilateral lesions of CeA and/or VTA on rats’ acquisition of conditioned responses to visual cues paired with food. Contrary to the results of previous studies that examined interactions of CeA with either SNc or DLS, rats with contralateral “disconnection” lesions of CeA and VTA were unimpaired in their acquisition of cue-directed responses. By contrast, rats with lesions of both structures in the same hemisphere failed to learn cue-directed responses, but were normal in their acquisition of conditioned responses directed to the food cup. In Experiment 2, we attempted to characterize VTA’s influence on CeA by examining FOS induction in CeA by a visual cue for food in rats with unilateral lesions of VTA. The results suggested an excitatory influence of VTA on CeA in the presence of food cues. Implications of these results for brain circuits involved in learned orienting and incentive motivation are discussed.
Keywords: amygdala central nucleus, ventral tegmental area, attention, incentive learning, orienting, rat
Circuitry that includes the amygdala central nucleus (CeA) and components of the nigrostriatal and/or mesolimbic dopamine systems is critical for learning and expression of conditioned responses (CRs) directed toward cues that predict food delivery. When visual stimuli are repeatedly paired with food, rats acquire conditioned orienting (ORs) and/or cue-approach responses. Gallagher et al. (1990) and Parkinson et al. (2000) found that rats with bilateral lesions of CeA failed to acquire these cue-directed behaviors. Nevertheless, in Gallagher et al.’s (1990) study, lesioned rats showed no deficits in the acquisition of conditioned food cup approach responses (CRs) to these cues, indicating that their failure to acquire cue-directed behaviors was not symptomatic of general deficits in learning, motivation or arousal.
Noting that CeA lesions also disrupt performance in other tasks used to assess attention, Holland and Gallagher (1999) asserted that the emergence of cue-directed CRs reflects broader changes in attention. Within their account, these CRs result from learning-dependent enhancement of initially unconditioned ORs elicited by novel stimuli (Holland, 1977). Han et al. (1997) proposed that this enhancement is mediated by CeA’s influence on nigrostriatal systems. Specifically, CeA neurons project to dopaminergic neurons in the substantia nigra pars compacta (SNc; Gonzalez & Chesselet, 1990; Lee et al., 2005), which in turn innervate the dorsolateral striatum (DLS). Elevated activity in this system is related to enhanced responsiveness to sensory stimuli (Chevalier & Deniau, 1990; Schultz, 1992), and lesion studies indicate that DLS is important for the integration of sensory and motor systems involved in unconditioned ORs (Carli et al., 1985). Consistent with this proposal, deficits in cue-directed CRs were found in rats in which CeA was functionally disconnected from SNc (Lee et al., 2005) or DLS (Han et al., 1997).
Other investigators have argued that cue-directed responding reflects the conditioning of incentive motivation to food cues (Cardinal et al., 2002), generating approach to those cues. Robbins and Everitt (2002) suggested that cue-directed CRs depend on CeA’s modulation of a structure implicated in incentive processes, the nucleus accumbens (ACB) core, via its projections to dopaminergic neurons in the ventral tegmental area (VTA). Although the lack of significant direct projections of CeA to VTA in rat undermines the particular model suggested by Robbins and Everitt (2002), CeA might modulate VTA activity indirectly, for example, via the peduncular pontine tegmental nuclei or the lateral hypothalamus, both of which have substantial projections to VTA (Geisler et al., 2007). Consistent with this view, Parkinson et al. (2002) found that 6-OHDA lesions of ACB produced deficits in cue-directed responding comparable to those produced by CeA lesions. Furthermore, lesions or transient inactivation of ACB, CeA, or VTA each disrupt another conditioning phenomenon thought to indicate incentive learning, Pavlovian-instrumental transfer (Hall et al., 2001; Murschall & Hauber, 2006).
Here, we examined the effects of CeA-VTA disconnection on the acquisition of cue-directed CRs to visual cues paired with food. If the acquisition of conditioned ORs to visual cues requires serial connectivity between CeA and VTA, as suggested by Parkinson et al. (2002), then it should be impaired by CeA-VTA disconnection.
MATERIALS AND METHODS
Subjects
Sixty experimentally naive, male Long-Evans rats (Charles River Laboratories, Raleigh, NC), initially weighing 275-325 g, were individually housed in a climate-controlled vivarium on a 12:12-hr light/dark cycle (lights on at 07:00) with free access to water. They were fed ad libitum during acclimation and postoperative recovery periods, but starting 7 days before the beginning of behavioral training, they were given limited access to food to maintain their weights at 85% of free-feeding weights.
Surgery
Rats were anesthetized with isoflurane gas (Abbott Laboratories, North Chicago, IL), and stereotaxic surgery was conducted under aseptic conditions. The rats received either unilateral ibotenic acid lesions of CeA alone (n= 4), unilateral 6-OHDA lesions of VTA alone (Group VTA, n=5) or unilateral lesions of both CeA and VTA in either the same (Group Ipsi, n = 25) or opposite (Group Contra, n= 26) hemisphere. According to the logic of the disconnection lesion procedure (Everitt et al., 1991; Han et al., 1997), because CeA-VTA and VTA-CeA projections are ipsilateral, contralateral lesions of CeA and VTA should prevent communication between those two regions. Notably, such lesions should spare functions subserved by each region unilaterally, except for those that require CeA-VTA communication. By contrast, ipsilateral lesions destroy the same amount of tissue in each region as the contralateral lesions, but should leave communication between CeA and VTA intact in the unlesioned hemisphere. Thus, ipsilaterally-lesioned rats typically serve as appropriate controls for the assessment of effects of functional disconnection of two regions in contralaterally-lesioned rats.
The CeA lesions were made using stereotaxic coordinates 2.4 mm posterior to bregma and 4.35 mm from the midline, with infusions at a depth of 7.9 mm from the skull surface. Each CeA lesion was made using 0.25 μl of 10 μg/μl ibotenic acid (Sigma, St. Louis, MO) in PBS solution, infused with a Hamilton 2.0 μl syringe over a 3-min period. Each VTA lesion was made using 1.0 μl of 6 μg/μl 6-OHDA (Sigma) in a PBS/0.1% (w/v) ascorbic acid vehicle, infused over a 10-min period at the same stereotaxic coordinates 5.0 mm posterior to bregma and 0.8 mm from the midline, with infusions at a depth of 8.2 mm from the skull surface. Across all rats, there were approximately equal numbers of each lesion type in each hemisphere. After surgery, each rat received a single 0.015-ml subcutaneous injection of buprenorphine hydrochloride for amelioration of pain, and was allowed to recover from surgery for 7-10 days before behavioral testing.
Apparatus
The behavioral training apparatus consisted of eight individual chambers (22.9 × 20.3 × 20.3 cm). Each chamber had aluminum front and back walls, clear acrylic sides and top, and a floor made of stainless steel rods (0.48 cm in diameter spaced 1.9 cm apart). A food cup, fitted with phototransistors for detecting head entries, was recessed in the center of the front wall 2 cm above the floor. A jeweled 6-w lamp, mounted on the front panel of the chamber, 15 cm above the food cup (panel light), served as the source of one of the visual conditioned stimuli (CSs.) Each chamber was enclosed in a sound-attenuated box where constant dim illumination was provided by a 6-w red light and ventilation fans provided masking noise (70 dB). Another 6-w lamp, located on the inside wall of this box, 10 cm from the front wall of the experimental chamber, served as a second visual CS (house light). A television camera was mounted within each box and images were recorded during behavioral training and testing.
Behavioral procedures
All rats were first trained to eat from the recessed food cup by delivering two 45 mg pellets (Research Diets, New Brunswick, NJ) with intertrial intervals ranging from 2-6 min over a 64-min session. Next, they received a 32-min session to pretest the two visual CSs, the panel light and house light, to examine unconditioned ORs. This session included 4 10-s presentations of each CS, randomly intermixed. Finally, all rats received 16 32-min sessions of discrimination training, in which one visual CS was reinforced with food delivery (CS+), and the other was nonreinforced (CS−). In each of these sessions, there were four 10-s presentations of CS+ and four 10-s nonreinforced presentations of CS−, randomly intermixed. The identity of CS+ (panel light or house light) was counterbalanced within each lesion condition.
Behavioral observation procedures
All observation were made from videotapes and paced by auditory signals (1.25-s intervals) recorded on the tapes. Observations were made during the 5-sec period immediately prior to light presentation and during the 10-sec period of light presentation. The OR to the light, rearing, was defined as standing on the hind legs with both front legs off the floor, but not grooming. To assess the objectivity of behavioral scoring, many of the video tapes were scored by multiple observers, who agreed on 95% of over 10,000 joint observations. The number of observations scored as rearing was divided by the total number of observations in a scoring period to form the measure “% rearing”. Because the number of observations in each CS interval (scoring period) was constant, this measure is an absolute frequency measure, which does not depend on overall levels of behavior. The measure of food cup responding used was the percentage of time during each recording period that the food cup photocells reported head entry.
In previous studies (e.g., Holland, 1977), ORs and food-cup entries occurred primarily during the first 5-sec and the last 5-sec periods of CS presentations, respectively. Thus, in this study, ORs were reported only for the first 5-sec period and food-cup behavior for the last 5-sec period of light presentation.
Histology
After completion of behavioral testing, the rats were anaesthetized and perfused with 0.9 % saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were removed, post-fixed and cryoprotected overnight in 4% paraformaldehyde in 0.1 M PB containing 12% sucrose, frozen with powdered dry ice, and stored at −80°C. Sections (40-μm) were taken from each brain on a freezing microtome and every third section was mounted on slides to evaluate the lesions. One series was Nissl-stained, and another was evaluated for tyrosine hydroxylase (TH), as a measure of dopaminergic activity in VTA and its striatal targets. A standard protocol for assessment of TH immunoreactivity was followed (Lee et al., 2005).
Data analysis
To reduce the impact of individual differences in baseline levels of each behavior within group, we formed elevation scores by subtracting the frequency of that behavior during the 5-s pre-CS periods from responding during the appropriate 5-s period during the CS. These elevation scores were then subjected to analysis of variance (ANOVA), followed by post-hoc Tukey honest-significant difference (HSD) tests for unequal ns.
RESULTS
Histology
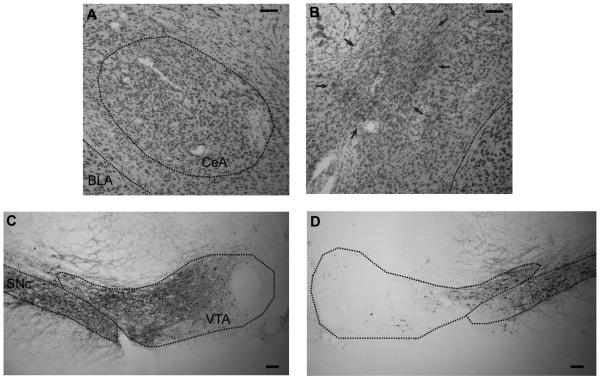
Twenty-nine rats were judged as having acceptable lesions of either CeA, VTA or both. CeA lesions were rejected (n = 24) if there was less than 40% damage to the medial portion of CeA, or if there was more than minimal damage to regions adjoining CeA. VTA lesions were rejected (n = 30) if there was less than 40% cellular damage to VTA, or if there was extensive damage to SNc. The brains with acceptable histologies averaged 77.5 +/− 7.8% and 77.0 +/− 6.0% damage to medial CeA in Groups Contra and Ipsi, respectively, and 48.6 +/− 2.0%, 52.0 +/− 7.4%, and 55.8 +/− 7.9% damage to VTA in Groups Contra, Ipsi, and VTA, respectively. Sparing of medial CeA neurons was mostly in the anterior regions and sparing of VTA adjoined SNc. There were six and eight animals with acceptable lesions to both CeA and VTA for ipsi- and contra-lateral lesions of CeA and VTA, respectively. Five animals intended for either ipsi- or contra-lateral lesions of CeA and VTA had no lesions to the CeA, but acceptable lesions to the VTA, so these animals were added to the unilateral VTA group (n = 7). Finally, There were four animals with no obvious lesions to either CeA or VTA, so these animals were grouped with the 4 unilateral CeA lesioned animals to form a single control (CTL) group (n=8). In previous studies we showed that unilateral CeA lesions alone have no effect on either OR or food cup measures of learning (e.g., Han et al., 1997, 1999). The groups did not differ in the size of either lesion, Fs < 1. Figure 1 shows typical lesions.
Figure 1.
Photomicrographs (taken with 10x objective) showing representative brain sections of CeA and VTA. (A, B) Nissl-stained sections of the intact and lesioned (arrows) CeA, respectively. (C, D) Sections stained for TH in the intact and lesioned VTA, respectively. BLA, basolateral amygdala. Scale bar = 100 μm.
Behavior
The lesions had no differential effects on unconditioned ORs in the pretest session. Across the four lesion conditions, these responses ranged from 10.6 ± 7.0 % to 21.9 ± 5.9% of behavior for the panel light and from 0.4 ±3.1% to 10.2 ± 4.0% for the houselight. A lesion X stimulus ANOVA showed that the panel light stimulus elicited more rearing than the house light, F(1, 25) = 8.06, p < .01, but there was no significant effect of lesion, nor did lesion interact with stimulus, Fs < 1.
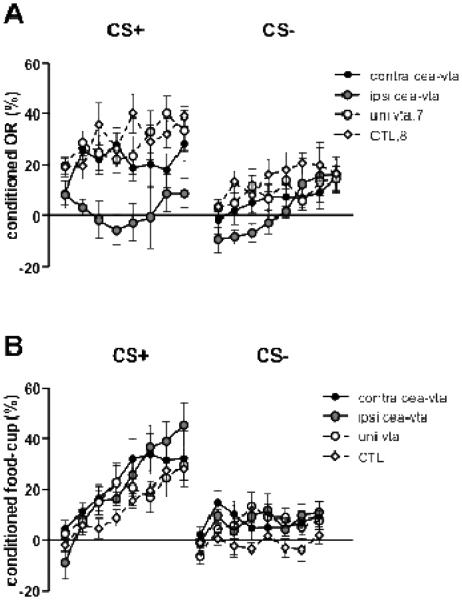
Figure 2 shows the primary data, the acquisition of conditioned ORs and food cup responses during discrimination training. In addition to the rats in the control group, rats with unilateral lesions of VTA alone or contralateral CeA-VTA lesions, which presumably prevented communication between CeA and VTA, all maintained conditioned ORs (Figure 2A) to the reinforced visual CS+, but showed declining levels of ORs during the nonreinforced CS−. By contrast, rats with ipsilateral CeA-VTA lesions, which putatively left communication between those two regions intact in one hemisphere, showed no evidence for acquisition of those conditioned ORs. Nevertheless, neither these ipsilateral lesions nor any other lesion affected the acquisition of food cup behavior to CS+ (Figure 2B), which was rapid in all groups. Thus, the learning deficit observed in the acquisition of conditioned ORs in the ipsilaterally-lesioned rats was confined to that response, and did not reflect more general deficits in learning ability, motor skills, motivation or arousal.
Figure 2.
Mean ± SEM orienting responses (A) and food-cup responses (B) during conditioning. Contra cea-vta refers to rats that received lesions of CeA in one hemisphere and of VTA in the other hemisphere. Ipsi cea-vta refers to rats that received lesions of CeA and VTA in the same hemisphere. Uni vta refers to rats that received lesions of VTA in one hemisphere only. CTL refers to rats that either received sham lesions or lesions of CeA in one hemisphere. CS+ refers to the reinforced visual conditioned stimulus and CS− refers to the nonreinforced visual conditioned stimulus. The values shown are elevation scores, calculated by subtracting pre-CS baseline responding from responding during the CS.
These descriptions of the data were supported statistically. A lesion X contingency (reinforced or nonreinforced cue) X 2-session block ANOVA of ORs showed significant main effects of lesion, F(1, 25) = 6.68, p < .002, contingency, F(1, 25) = 51.13, p < .001, and session blocks, F(7, 175) = 5.41, p < .001. Most important, the lesion X contingency, F(3, 25) = 5.25, p < .006, interaction was significant, showing that the difference between responding to the reinforced and nonreinforced cues depended on the lesion condition. Post-hoc analyses of those differences, using Tukey’s honest significant difference (HSD) tests for unequal ns, showed them to be smaller (ps < .049) in Group Ipsi than in each of the other groups, which did not differ. Comparable analyses of ORs to the reinforced cue alone likewise showed less orienting in Group Ipsi than in each of the other groups (ps <.044), whereas for ORs to the nonreinforced cue, only Groups Ipsi and CTL differed significantly (p <.047, other ps > .40).
A similar three-way ANOVA of food cup responding showed significant main effects of contingency, F(1, 25) = 67.88, p < .001, and session blocks, F(7, 175) = 19.28, p < .001. However, unlike with ORs, neither the effect of lesion nor any of its interactions was significant, Fs<1.87, ps >.161.
Lesion X blocks ANOVAs of pre-CS responding showed no significant lesion or lesion X blocks effects for either behavior, Fs < 1.48, ps > .245. Thus, the elevation scores are not confounded by between-groups differences in pre-CS responding.
DISCUSSION
Our observation that disconnection of CeA from VTA by contralateral lesions of those structures did not interfere with the acquisition of conditioned ORs is inconsistent with Parkinson et al’s (2000) suggestion that learning of CS-directed responses may depend on CeA’s modulation of the action of dopaminergic projections from VTA to ACB core. Furthermore, this finding contrasts with Lee et al’s (2005, Exp. 2) observation, using training procedures identical to those used in the present study, that lesions that disconnected CeA and SNc impaired the acquisition of conditioned ORs. Nevertheless, both findings are consistent with anatomical evidence that whereas CeA’s projections to SNc are substantial, those to VTA are relatively sparse (Kaufling et al., 2009; Zahm et al., 1999) and with evidence from studies that show that FOS expression of CeA neurons that project to SNc is sensitive to cue-reward contingencies (Lee et al., 2005, Exp. 1; Lee et al., 2010), whereas that of VTA-projecting neurons is not (Lee et al., 2010). Thus, SNc, but not VTA, is part of a serial circuit for conditioned ORs that includes the CeA.
Considerably more puzzling is the additional observation of a deficit in ORs in rats with ipsilateral lesions of CeA and VTA, despite the absence of an effect of contralateral lesions. One account for this observation is that the normal relation between CeA and VTA is inhibitory. Although direct CeA-VTA projections are sparse, ipsilateral projections from VTA to CeA are more substantial (Swanson, 1982). Thus, activation of VTA might normally suppress activity in the ipsilateral CeA. Ipsilateral lesions of CeA and VTA would eliminate CeA output in the lesioned hemisphere and leave CeA inhibited by VTA in the unlesioned side. By contrast, a VTA lesion contralateral to a CeA lesion would release the surviving CeA from normal VTA inhibition, yielding greater overall CeA activity after contralateral than after ipsilateral lesions. Experiment 2 used an immediate early gene procedure to determine VTA’s influence on CeA activity in a discrimination task identical to that of Experiment 1.
Experiment 2
In Experiment 2, rats with unilateral lesions of VTA were trained with procedures identical to those of Experiment 1. On completion of that training, the rats received test presentations of the previously reinforced visual cue prior to sacrifice and preparation of brain tissue for immunochemistry for FOS-like protein. If VTA’s influence on CeA during conditioned orienting is primarily inhibitory, then we would expect to see greater FOS in the CeA ipsilateral to the VTA lesion than in the contralateral CeA. Importantly, we evaluated FOS separately in medial (m) and lateral (l) divisions of CeA. CeA neurons associated with conditioned responding in this preparation are found primarily in mCeA (Lee et al., 2005; 2010), but VTA neurons project primarily to lCeA (Lee & Wheeler, unpublished observations). Because many connections between lCeA and mCeA are GABAergic, it is possible that VTA lesions would reduce the activity of ipsilateral lCeA neurons, but consequently enhance the activity of ipsilateral mCeA activity, which is correlated with conditioned responding.
Method
Twelve rats with characteristics identical to those of Experiment 1 were given unilateral lesions of VTA, using the surgical procedures of Experiment 1. After 10-14 days recovery, the rats were trained with procedures identical to those of Experiment 1, using four identical conditioning chambers. The day after the last discrimination training session, each rat received a 15-min test session in which 4 10-s presentations of either the previously reinforced CS (8 rats) or the previously nonreinforced CS (4 rats) were given. Seventy-five min after the end of that session, rats were sacrificed by exsanguination under deep pentobarbital (100 mg/kg) anesthesia, and perfused with 0.9 % saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were removed, post-fixed and cryoprotected overnight in 4% paraformaldehyde in 0.1 M PB containing 12% sucrose, and stored at −80°C. Brains were then sliced on a freezing microtome and 30-μm coronal sections through CeA and VTA were collected in 4 series.
Immunohistochemistry
The first series of sections was used for FOS immunohistochemical staining. The second series of sections was stained for Nissl to verify anatomical locations of adjacent sections of CeA immunoreacted for FOS, and to evaluate VTA lesions. The third series was used for TH staining for evaluation of VTA lesions, as in Experiment 1. Immunohistochemical staining for FOS followed a protocol similar to that used by Lee et al. (2005). The primary antibody was rabbit FOS antibody (1:5000 dilution, Santa Cruz Biotechnology #sc-52). After the primary antibody incubation (48-72 hrs at 4 °C), sections were rinsed in 0.1 M PB containing 0.9% saline (PBS), incubated in goat anti-rabbit IgG biotinylated secondary antibody (1:250 dilution, Vector Laboratories) for 1-1.5 hrs, rinsed in PBS, and then incubated in avidin-biotin peroxidase conjugate (Vector laboratories) for 1-2 hrs. After several rinses in PBS, sections were reacted using a Vector DAB substrate kit for peroxidase (Vector Laboratories) to visualize FOS. Sections were mounted on slides, dehydrated in ascending concentrations of alcohol, and coverslipped with Permount.
Analysis of FOS expression
Analyses were conducted blind with respect to the lesion site. Medial and lateral subnuclei of CeA were defined according to Swanson’s Rat Brain Atlas (Swanson, 1992). Three sections from different rostral-caudal levels (levels 25 to 27 according to Swanson, 1992) and two sections from levels 27 to 28 were used to analyze bilateral medial and lateral CeA, respectively. Images of the FOS-stained sections and the adjacent thionin-stained sections were acquired using a MicroPublisher RTV camera (QImaging, Burnaby, B.C., Canada). Borders of the left and right medial and lateral CeA were then drawn on the images of FOS sections, guided by the thionin-stained sections. Using an image analysis system (NIH Image 1.63), a threshold for background density was set for each medial and lateral CeA regions on the FOS section, and FOS-positive cells with a density that was at least 2 standard deviations above the background threshold were counted using the software.
Results
Lesions and behavior
Nine rats were judged as having acceptable VTA lesions. These lesions were slightly larger (62 ± 6% damage) than those of Experiment 1, although not significantly so, p > .20. Conditioning of food cup and OR behaviors proceeded as in Group Unilateral VTA of Experiment 1; over the final two sessions, food cup behavior was 37.9±6.0%, 20.1±6.0%, and 3.0±1.1% during CS+, CS− and pre-CS periods, and ORs were 29.2±3.6%, 16.5±1.8%, and 4.4±1.6%, respectively. In the test session, food cup and rear behaviors comprised 34.9±3.8% and 35.6±3.3% during CS+ and 4.8±2.6% and 8.6±2.1% during pre-CS periods, in the 7 rats tested with CS+. In the 2 rats tested with CS−, food cup was 3.4% and ORs 15.9%.
FOS immunochemistry
Consistent with previous observations (Lee et al., 2005), among the 7 rats with acceptable lesions tested with CS+, there were more FOS counts/area in mCeA than in lCeA. Furthermore, contrary to our prediction, FOS counts were greater contralateral to the lesion than ipsilateral, in both lCeA (196±17 vs 155±16 counts/mm2) and mCeA (340±32 vs 293±25 counts/mm2). ANOVA yielded significant main effects of both region, F(1,6)=26.26, p=.002, and hemisphere, F(1,6)=7.71, p=.032, but no interaction, F<1, p=.879. Comparisons of FOS ipsilateral vs contralateral to the lesion in the CeA subregions taken individually did not reach conventional levels of significance, 0.15 > ps > 0.05.
Discussion
These results suggest that VTA has an excitatory effect on CeA neuron activity when cues for food are presented. In turn, Lee et al. (2005) showed that CeA activity is positively correlated with conditioned responding; rats that received more pairings of CS and food showed more CeA FOS and larger CRs than those that had received fewer or no such pairings. Alternately, the influence of VTA on CeA might indeed be inhibitory, but is exerted contralaterally rather than ipsilaterally. However, this latter alternative is not straightforward anatomically because there is no evidence for direct contralateral VTA-CeA projections; nevertheless, indirect multisynaptic contralateral connections cannot be ruled out.
General Discussion
In Experiment 1, we found that ipislateral lesions of CeA and VTA, but not contralateral lesions of those structures, impaired acquisition of conditioned ORs. This outcome contrasts with previous observations that contralateral but not ipsilateral lesions of CeA and SNc or of CeA and DLS impair OR learning. Thus, VTA does not appear to contribute to the acquisition of conditioned ORs in the same manner as structures within the serial CeA-SNc-DLS circuit identified previously. At the same time, the observation of effects of ipsilateral CeA-VTA lesions shows that VTA must play some role in circuitry responsible for the conditioned visual ORs we observe. Other findings within Experiments 1 and 2 place some limitations on what that role might be. One possibility is that whereas (as suggested by the results of Experiment 2) ipsilateral VTA-CeA projections upregulate SNc-projecting neurons in CeA, VTA may exert an inhibitory influence on SNc and its projections to DLS. Notably, some VTA neuron groups have substantial projections to other VTA cell groups in the opposite hemisphere, and many of these target VTA neurons project to SNc (Ferreira et al., 2008). The further assumption that these contralateral VTA-VTA projections disinhibit VTA projections that otherwise downregulate SNc/DLS would readily account for our observations, including greater impairment in ORs after ipsilateral CeA-VTA lesions than after contralateral lesions of those structures (Figure 3).
Figure 3.
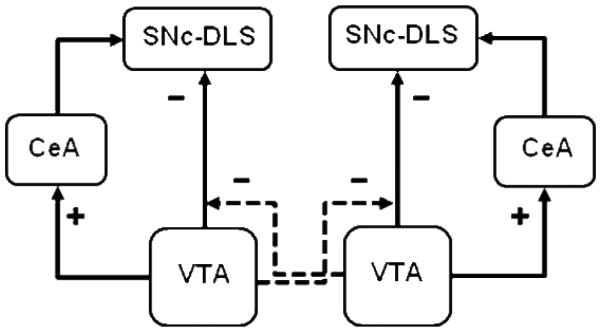
A schematic diagram of postulated influence of VTA on the neural circuitry (i.e. CeA-SNc/DLS) important for conditioned orienting behavior. Solid arrowed lines represent ipsilateral connections and dotted arrowed lines represent contralateral connections. + represents excitatory connections and − represents inhibitory connections.
First, in intact rats, the inhibitory influence of VTA on ipsilateral SNc/DLS would be suppressed by the influence of contralateral VTA neurons activated by food cues, allowing CeA’s projections to SNc to be effective. Second, unilateral VTA lesions would have no net effect on ORs. Although these lesions would release SNc/DLS activity from inhibition in the hemisphere ipsilateral to the lesion, enhancing activity of the OR circuit in that hemisphere, they would also eliminate the normal disinhibition of such activity in the contralateral hemisphere, lowering the output of SNc in that hemisphere. Third, contralateral CeA-VTA disconnection lesions would prevent activity in the CeA-SNc-DLS circuit ipsilateral to the CeA lesion, but removal of VTA in the other hemisphere would compensate for that loss by eliminating the normal inhibition of SNc-DLS by VTA. Finally, if VTA and CeA lesions are ipsilateral, removal of CeA makes activation of SNc-DLS by CeA impossible in the hemisphere with the lesion, and destruction of VTA enables inhibition of SNc-DLS by VTA in the contralateral hemisphere by removing the normal contralateral VTA-VTA inhibition.
Another implication of this circuit not explicitly examined in this study is that lesions of both SNc and VTA in the same hemisphere would eliminate conditioned ORs, regardless of the status of CeA. SNc output would be eliminated on the side with the lesion, and the release of the contralateral VTA from inhibition would result in the inhibition of SNc in that hemisphere as well. Notably, the data from several rats in Experiments 1 and 2 were discarded from those studies because the attempted VTA lesion extended substantially into SNc as well. Likewise, in a previous unpublished study with behavioral training procedures identical to those used here, both SNc and VTA were lesioned intentionally in one hemisphere, either contralateral or ipislateral to excitotoxic or sham lesions of CeA. Consistent with the aforementioned prediction, in all of these cases, the rats failed to acquire conditioned ORs beyond the level of pre-CS periods. However well it accounts for the present data, this particular account remains speculative. Other VTA afferents that also arise bilaterally, for example, those from the pedunculopontine tegmental area (Geisler et al., 2007; Jackson & Crossman, 1983; Yeomans et al., 1993), which in turn is innervated by CeA, might play comparable roles.
Regardless of the precise circuitry that underlies it, our observation of greater disruption after ipsilateral lesions of CeA and VTA than after contralateral lesions of those structures is consistent with previous observations of the effects of CeA-VTA disconnection on the acquisition of incentive value to food-paired CSs. A common measure of the learned incentive value of a Pavlovian CS is its ability to elevate the rate of instrumental responding supported by the same reinforcer, known as Pavlovian-instrumental transfer (PIT). PIT is disrupted by bilateral lesions of CeA (Hall et al., 2001; Holland & Gallagher, 2003) or bilateral inactivation of the VTA (Murschall & Hauber, 2006). Importantly, although some aspects of their data differed from the present observations, El-Amamy and Holland (2007) found reductions in PIT after ipsilateral lesions of CeA and VTA but not after contralateral lesions of those regions. It may be reasonable to speculate that the somewhat unusual role of VTA in influencing ORs to visual cues in the present study might reflect interactions with acquisition of incentive value to those cues. That is, expression of visual ORs, which often involve both reorientation and approach to the source of the visual cue (e.g. Holland, 1980), may engage both attentional (as emphasized by Holland & Gallagher, 1999) and motivational (as emphasized by [Everitt’s group]) learning systems. In that case, the influences of VTA on visual ORs that we observed might reflect its modulation of function of contralateral ACB core, rather than of SNc-DLS circuitry. Notably, previously we found no evidence for a role of VTA in the acquisition of conditioned ORs to auditory cues, which do not involve approach-like ORs. Although the conditioning of startle ORs to auditory cues paired with food depends on the serial connectivity of CeA and SNc (Gallagher et al., 1990; Groshek et al., 2005; El-Amamy & Holland, 2007), El-Amamy & Holland (2007) found no effect of either ipsilateral or contralateral lesions of VTA and CeA on these conditioned startle responses.
Our present observation is also reminiscent of previous findings in the study of reward from electrical stimulation of the brain (ESB) in various sites along the medial forebrain bundle (e.g., Waraczynski, 2006). ESB reward effects have been found to survive massive lesions in locations ipsilateral to the stimulation site (e.g., Colle & Wise, 1987), and Miguelez et al. (2004) found that amygdala lesions contralateral to the site of ESB produced larger changes in ESB reward thresholds than ipsilateral lesions. These observations also suggest substantial contralateral influence of dopaminergic circuits in reward functions.
Dopaminergic systems have been widely described as important in focusing attention on significant and rewarding stimuli, and in providing reinforcement and reinforcement error signals important for learning (Schultz et al. 1997; Wise & Rompre, 1989), primarily through VTA and its projections to ACB. Similarly, Holland and Gallagher (1999) and Holland and Madddux (2010) argued that CeA was important in the modulation of attention, including, via its actions on SNc-DLS circuitry involved in sensory-motor expression, conditioning of ORs to predictors of important events. The present results, taken together with earlier findings, indicate that CeA plays important roles in both functions, and that both functions may contribute to the emergence of CS-directed behaviors. Thus, CeA plays a key role in integrating cognitive, affective and behavioral processes in learning situations, through its interactions with dopaminergic systems.
Acknowledgments
This research was supported in part by grant MH53667 from the National Institutes of Health. We thank Emily Corcoran and Bayan Adileh for assistance in behavioral and FOS data scoring, and Frank Groshek, Mary Keough and Heather El-Amamy for their work on studies preliminary to these.
Abbreviations
- ACB
nucleus accumbens
- CeA
central nucleus of amygdala
- CR
conditioned response
- CS
conditioned stimulus
- DLS
dorsolateral striatum
- OR
orienting response
- PBS
phosphate buffered saline
- PIT
Pavlovian-instrumental transfer
- SNc
substantia nigra pars compacta
- TH
typrosine hydroxylase
- VTA
ventral tegmental area
- 6-OHDA
6-hydropxydopamine
References
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carli M, Evendon JL, Robbins TW. Depletion of unilateral striatal dopamine impairs initiation of contralateral actions and not sensory attention. Nature. 1985;313:679–682. doi: 10.1038/313679a0. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal function. Trends Neurosci. 1990;13:277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Colle LM, Wise RA. Opposite effects of unilateral forebrain ablations on ipsilateral and contralateral hypothalamic self-stimulation. Brain Res. 1987;407:285–93. doi: 10.1016/0006-8993(87)91106-1. [DOI] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC. Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. Eur J Neurosci. 2007;25:1557–67. doi: 10.1111/j.1460-9568.2007.05402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neurosci. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Ferreira JGP, Del-Fava F, Hasue RH, Shammah-Lagnado J. Organization of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neurosci. 2008;153:196–213. doi: 10.1016/j.neuroscience.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J. Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RQ, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J. Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales C, Chesselet M-F. Amygdalonigral pathway: An anterograde study in the rat with phaseolus vulgaris leucoagglutinin (PHA-L) J. Comp. Neurol. 1990;297:182–200. doi: 10.1002/cne.902970203. [DOI] [PubMed] [Google Scholar]
- Groshek F, Kerfoot E, McKenna V, Polackwich AS, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning, but not expression, of conditioned auditory orienting. Behav. Neurosci. 2005;119:202–12. doi: 10.1037/0735-7044.119.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behavior. Eur. J. Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Han JS, Holland PC, Gallagher M. Disconnection of the amygdala central nucleus and substantia innominata/nucleus basalis disrupts increments in conditioned stimulus processing in rats. Behav. Neurosci. 1999;113:143–51. doi: 10.1037//0735-7044.113.1.143. [DOI] [PubMed] [Google Scholar]
- Han J-S, McMahan RW, Holland PC, Gallagher M. The role of an amygdalo-nigrostriatal pathway in associative learning. J. Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J. Exp. Psychol. Anim. Behav. Proc. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Influence of visual conditioned stimulus characteristics on the form of Pavlovian appetitive conditioned responding in rats. J. Exp. Psychol. Anim. Behav. Process. 1980;6:81–97. [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cog. Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur. J. Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Maddux J-M. Brain systems of attention in associative learning. In: Mitchell CJ, LePelley ME, editors. Attention and learning. Oxford University Press; Oxford: 2010. pp. 305–349. [Google Scholar]
- Jackson A, Crossman AR. Nucleus tegmenti pedunculopontinus: Efferent connections with special reference to the basal ganglia, studied in the rat by anterograde and retrograde transport of horseradish peroxidase. Neurosci. 1983;10:725–765. doi: 10.1016/0306-4522(83)90213-0. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J. Comp. Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Cantalini JP, Petrovich GD, Gallagher M, Holland PC. Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. J. Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Gallagher M, Holland PC. The central amygdala projection to the substantia nigra reflects prediction error information in appetitive conditioning. Learn. Mem. 2010;17:531–538. doi: 10.1101/lm.1889510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguelez M, Kentner AC, Deslauriers K, Parkinson M, Fouriezos G, Bielajew C. Interhemispheric involvement of the anterior cortical nuclei of the amygdala in rewarding brain stimulation. Brain Res. 2004;1003:138–150. doi: 10.1016/j.brainres.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Murschall A, Hauber W. Inactivation of the ventral tegmental area abolished the general excitatory influence of Pavlovian cues on instrumental performance. Learn. Mem. 2006;13:123–126. doi: 10.1101/lm.127106. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Dalley RN, Cardinal A, Bamford B, Fehnert G, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behavior: implications for mesoaccumbens dopamine function. Behav. Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur. J. Neurosci. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol. Learn. Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Schultz W. Activity of dopamine neurons in the behaving primate. Sem. Neurosci. 1992;4:129–138. [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 1982;9:321–33. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Waraczynski MA. The central extended amygdala network as a propossed circuit underlying reward valuation. Neurosci. Biobehav. Rev. 2006;30:472–496. doi: 10.1016/j.neubiorev.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Ann. Rev. Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Mathur A, Tampakeras M. Rewarding brain stimulation: Role of tegmental cholinergic neurons that activate dopamine neurons. Behav. Neurosci. 1993;107:1077–1087. doi: 10.1037//0735-7044.107.6.1077. [DOI] [PubMed] [Google Scholar]
- Zahm D,S, Jensen SL, Williams ES, Martin JR., 3rd Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur. J. Neurosci. 1999;11:1119–1126. doi: 10.1046/j.1460-9568.1999.00524.x. [DOI] [PubMed] [Google Scholar]