Summary
This protocol details a subset of assays developed within the touchscreen platform to measure aspects of executive function in rodents. Three main procedures are included: Extinction, measuring the rate and extent of curtailing a response that was previously, but is no longer, associated with reward; Reversal Learning, measuring the rate and extent of switching a response toward a visual stimulus that was previously not, but has become, associated with reward (and away from a visual stimulus that was previously, but is no longer, rewarded); and the 5-Choice Serial Reaction Time (5-CSRT) task, gauging the ability to selectively detect and appropriately respond to briefly presented, spatially unpredictable visual stimuli. These methods were designed to assess both complimentary and overlapping constructs including selective and divided visual attention, inhibitory control, flexibility, impulsivity and compulsivity. The procedures comprise part of a wider touchscreen test battery assessing cognition in rodents with high potential for translation to human studies.
INTRODUCTION
Executive function can be conceptualized as a set of processes or mechanisms that coordinate and regulate other cognitive (sub)processes or behaviors. While there is, as yet, no precise formal definition of what executive functioning entails, it is generally thought to contribute importantly to the dynamics and organisation of flexible, goal-directed behavior, and to be distinguishable from other basic cognitive properties (e.g., memory, perception) that it governs1,2. Executive function is typically described as a collection of processes that includes selecting, updating and planning motor sequences, withholding and stopping actions, monitoring and changing behavior where appropriate and dividing, switching and sustaining attention3-5 - these processes likely share overlapping features and mechanisms while also representing distinct psychological constructs3,6. Disruption of such processes is associated with a variety of psychiatric and neurological disorders7-9, and there is considerable evidence suggesting that executive function depends, at least in part, on functional integrity of the frontal lobes10-13. It is thus increasingly important to establish preclinical assays of executive function in rodents that are standardized, reliable, practical (e.g., easy to implement) and translational, providing valid (e.g., face, predictive, construct) models of cognitive processes in humans (for recent discussions of translational validity in animal models of human cognition see 14-17).
Numerous paradigms and procedures have been designed to measure aspects of executive function. In humans, prototypical examples include the Wisconsin Card Sorting Test, the Tower of London test , the Spatial Working Memory task, the Stroop task, reversal learning paradigms and the Continuous Performance test18-22. In nonhuman primates and rodents, numerous analogues to these tests have been developed such as odor- and visual-based intradimensional/extradimensional set-shifting tasks, as well as maze and operant chamber variants of tests of spatial working memory, reversal learning and attentional control23-27. This article focuses on three appetitive procedures that have been developed within the rodent touchscreen platform to assess certain aspects of executive function: Extinction, an assay of the rate and extent of curtailing (inhibiting) a learned response that was previously, but is no longer, associated with reward; Reversal Learning, an index of the rate and extent of switching a response toward a visual stimulus that was previously not, but has become, associated with reward (and away from a visual stimulus that was previously, but is no longer, rewarded); and the 5-Choice Serial Reaction Time (5-CSRT) task, which measures the ability to selectively detect and appropriately respond to briefly presented, spatially unpredictable visual stimuli. These three tests have undergone validation work and have proven useful for assessing aspects of executive function across a variety of animal models. However, our laboratory and others are working to continually improve the current protocols as well as to develop new translational tests with which to assess executive function.
As indicated below, each of the three tasks described in the present protocol has also been implemented in non-touchscreen testing apparatus such as experimental mazes or specialized operant chambers. However, there are several advantages to using the touchscreen method for assessing cognition in rodent, as has been described previously28-31. Briefly, these include a high degree of automation and standardization, the ability to test numerous subjects simultaneously, the minimisation of possible confounds and variability due to within- or between-trial handling, the use of similar stimulus and response characteristics to those employed by analogous tasks in humans and non-human primates, the capacity to introduce novel visual elements, and the ability to cross-compare a behavioral test against a battery of cognitive tasks within identical apparatus in order to help delineate factors contributing to test performance or the effects of an experimental manipulation.
Relative weaknesses of the method include moderate to long training procedures, potential confounds due to disruptions or differences in subjects’ visual functioning, as well as limitations common to most appetitive, operant paradigms (e.g., performance may be sensitive to variations in food restriction, hedonic/motivational factors, basic learning mechanisms and motor control). Importantly, the impact of several of these latter confounds can be assessed and potentially ruled out by running appropriate control experiments or by inspecting relevant dependent variables such as trial omissions and reaction times to elicit a response or to collect rewards. Moreover, the ability to test many animals in parallel may serve to offset any detriment due to extended training periods.
A) Extinction
The ability to learn to stop making a response that no longer provides a desired or adaptive outcome can be just as important as learning to produce the behavior in the first place. Since early descriptions of Extinction32, it has become clear that it is a complex phenomenon: in many instances, behavioral changes cannot be simply explained by “forgetting” what has previously been learned, and its expression has been demonstrated to be highly sensitive to context33-34. Much of the recent literature on Extinction learning has been devoted to examining the mechanisms underlying extinction of conditioned fear responses, typically using standard operant testing apparatus35-36. Although fewer studies have investigated the neural basis of extinction of learned appetitive responses, there is evidence that it involves, at least to some extent, similar substrates as fear extinction – ventromedial regions of the prefrontal cortex in humans and infralimbic cortex in rodents, as well as relevant subcortical circuitry including the striatum and amygdala, have been implicated in extinction of appetitive responding37-38. Recent reports have further suggested that abnormal resistance to extinction of appetitive responses may, in part, underlie disorders characterized by compulsive behavior, such as addiction and obsessive compulsive disorder38-39. Thus, Extinction learning may be aligned with executive functioning through its parallels with other forms of inhibitory control, its theoretical complexity and its modulation by specific frontal brain circuitries.
In the Extinction procedure outlined here, animals are first trained to acquire a simple visually-guided response (e.g., touch a white square) in order to earn reward. Following this acquisition phase, animals are presented with similar opportunities to respond, but in the absence of rewards and associated cues. The time it takes and the extent to which animals suppress their responding provide the basic indices of extinction learning. Variants of this protocol have been used to test extinction in various mutant mouse lines40-44 and genetic strains45-46. We have only recently started using this procedure in rats, and no major issues have been observed or are expected in translating this relatively straightforward task. Indeed, quite similar methods for testing extinction can be used in most operant settings where rats or mice have learned to make a response for reward47-48.
Touchscreen extinction assays have been usefully employed to phenotypically characterize behavioral differences between inbred mouse strains. These studies have shown that some strains of mice, including the commonly used C57BL/6J and BALB/cJ mice perform well on the extinction task compared with certain other strains, such as DBA/2J. In addition, some strains such as 129S1/SvImJ have been found to show normal touchscreen extinction despite being significantly impaired on other (e.g., Pavlovian fear) measures of extinction45. While the principal brain regions mediating touchscreen extinction have not yet been identified using lesion or other techniques, studies using genetic mutant mice have provided preliminary insights into the molecular basis of the behavior. For example, gene deletion of one major glutamate receptor subtype, AMPA GluA1, significantly retarded extinction42, whereas neither deletion of the gene encoding the NMDA receptor subunit GluN2A40 nor the gene encoding the glutamate transporter, GLAST41, disrupted the behavior. Moreover, deletion mutations of two paralogs of the Discs Large homolog (Dlg) family of postsynaptic scaffold proteins resulted in reciprocal effects: extinction was retarded in Dlg2 −/− mice but significantly enhanced in Dlg3 −/− mice43. Enhanced extinction has also recently been observed in the TgCRND8 mouse model of Alzheimer’s disease (AD)-related amyloid pathology44. These findings highlight the bidirectional sensitivity of the touchscreen extinction assay and the utility of a test battery approach using multiple touchscreen tasks30, where effects on extinction can be distinguished from, and otherwise inform, effects on other executive functions.
Although the touchscreen extinction assay described here is relatively simple to implement, extinction is a complex phenomenon with numerous factors or underlying processes putatively contributing to its expression (e.g., generalization decrement, response inhibition, Pavlovian and/or instrumental learning mechanisms)49. On the one hand, this cautions that care must be taken when interpreting Extinction results, particularly with respect to the animal’s distant and recent learning history – extinction has been noted to be highly dependent on context. On the other hand, it suggests that there are a variety of behavioral probes, modifications and extensions of the current procedure (e.g., reinstatement, renewal, reacquisition) that can provide valuable additional insights46. The flexibility and breadth of stimulus control offered by the touchscreen platform make it particularly well-suited for innovating and expanding research examining extinction processes.
B) Reversal Learning
In addition to extinction learning, it is also important for organisms to be able to flexibly adjust their behavior in other ways when faced with changing environments or rules. Appetitive reversal learning procedures are widely-used assays for such flexibility. In these procedures subjects are first taught to discriminate and choose a rewarded over an unrewarded stimulus/response option. Following this discrimination learning phase, the reward associations are switched and subjects must learn not only to extinguish the previously-rewarded response, but also to choose the previously-unrewarded (now-rewarded) option. The rate and extent to which the new “reversed” discrimination is learned provides an index of flexibility. Reversal learning deficits have been observed in many neuropsychiatric disorders, including schizophrenia50, Parkinson’s disease51 and obsessive compulsive disorder52. There is also considerable evidence, across several different species, testing apparatus and protocols, linking reversal learning to a functional neural circuitry including the prefrontal cortex (PFC) – particularly orbitofrontal (OFC) regions24,53-59 – and dorsal striatum60, as well as to neuropharmacological factors such as serotonin (5-HT)61-63 and dopamine signalling64-66.
Related findings have also been observed using the rodent touchscreen visual Reversal Learning procedure, which has the translational advantage of incorporating near-identical stimulus and response characteristics to methods commonly used in humans and non-human primates. Orbitofrontal lesions in both rats67,99 and mice68 significantly retard visual reversal learning, as do lesions of the dorsolateral striatum in the mouse68. Lesions of medial PFC in rats69 and mice40 have also been observed to impair reversal, but only when visual stimuli are difficult to discriminate. In contrast, amygdala lesions in rats99 and ventromedial-specific PFC lesions in mice were found to significantly facilitate reversal learning68. Touchscreen reversal learning has further been found to be retarded following systemic treatment with methamphetamine100, a D1-agonist70, as well as in NMDA receptor subunit GluN2A KO transgenic mice40. Enhanced reversal learning has been observed in the TgCRND8 mouse model of amyloid pathology44, and following manipulations that elevate brain 5-HT content such as 5-HT transporter knockout or subchronic treatment with the serotonin-selective reuptake inhibitor, fluoxetine71. Deletion of the gene encoding the AMPA receptor subunit GluA1 also slightly improves reversal42.
As with Extinction, these findings highlight the bidirectional sensitivity of the touchscreen visual Reversal Learning procedure. Moreover, there are additional processes thought to contribute to reversal beyond those described for extinction of a previously rewarded, but currently unrewarded response option. These include overcoming the learned irrelevance and/or avoidance of a previously unrewarded response option, attending and selecting the appropriate response strategy (e.g., choosing based on visual discrimination of stimuli rather than an egocentric position bias) and acquiring a new stimulus-reward association62. The flexibility of stimulus control offered by the touchscreen platform makes it particularly well-suited for attempting to dissociate such processes (e.g., through introduction of novel “replacement” stimuli and/or new response options – see EXPERIMENTAL DESIGN for further discussion).
C) 5-Choice Serial Reaction Time task
The touchscreen 5-Choice Serial Reaction Time (5-CSRT) task is a modified version of the five-choice task originally developed for rats using the classic nine-hole operant chamber27, an analogue of the five-choice serial reaction task used to study human attentional processes72. The operant chamber method has also been modified for use in mice73-74, and a considerable amount of work has now assessed 5-CSRT phenotypic differences between various mouse strains as well as in transgenic and mutant lines75-81.
The 5-CSRT task trains rodents to report the occurrence and location of brief, visual stimuli presented pseudorandomly across five spatial locations in a horizontal array of apertures. The task can be used to evaluate various aspects of executive function: response accuracy (proportion of correct over all attempted trials) is typically interpreted as a measure of sustained, spatially-divided attention; omissions (trials when no response is made) are a putative index of global attentional processes – but also sensitive to sensory, motor or motivational changes; premature (responses before stimulus onset) or perseverative responses (extra responses after outcome feedback) are measures of inhibitory control, possibly related to constructs of impulsivity and compulsivity, respectively. Additionally, response and reward collection latencies relate to processing speed and motoric/motivational factors. These measures may reflect, to some extent, dissociable cognitive processes and have been demonstrated to be sensitive to distinct pharmacological treatments and the integrity of distinct sectors of the rodent PFC. Detailed summaries of the application and protocol of the traditional operant box versions of the 5-CSRT task have been previously described for both rats82 and mice74.
The 5-CSRT task procedure outlined here was first developed by Susan Bartko, Carola Romberg and colleagues for testing mice using the touchscreen platform. The touchscreen method has recently been used to examine executive function within various genetic mouse models. Relative to their wild-type controls, both a triple transgenic AD mouse model (3xTgAD)83 and the TgCRND8 mouse model of AD-typical amyloid pathology44 showed decreased response accuracy across the session when the attentional load of the task was increased by shortening the duration of the target stimulus. The attentional deficits in 3xTgAD mice could be ameliorated by adminstration of the cholinesterase inhibitor, donepezil (Aricept), which has procognitive effects in AD patients. Mice with homozygous deletion of the cholinergic M1 receptor (M1R−/−) showed no alterations in performance accuracy, but were found to display lower omissions, higher premature responses, and greater perseveration compared to wild-type mice84. More recently, it was found that inbred BTBR T+tf/J mice (a putative model for autism) show decreased accuracy for detection of short stimuli as well as increased impulsivity and decreased motivation relative to C57Bl/6J mice85. This method shares similar benefits and limitations as the traditional version82, with the additional advantages of superior flexibility and control over the visual target stimuli (e.g., brightness, contrast, size, spacing) and the ability to run the test in the same apparatus and behavioral setting as many other tests of cognition31,86.
EXPERIMENTAL DESIGN
General Considerations
As the procedures in this protocol have primarily been published using mice as subjects, the main task descriptions are based on procedures developed for the mouse. For Reversal Learning, which was first developed in the touchscreen using rats69, the major differences between mouse and rat protocols are highlighted. While Extinction and 5-CSRT task touchscreen methods have not yet been published using rats, unpublished studies recently conducted in our laboratory (K. McAllister, L. Lyon, A.C. Mar, L.M. Saksida & T.J. Bussey) suggest that no major issues are to be expected with translating these relatively straightforward and well-established tasks. It should also be noted that although the protocols described below are based on the original publications cited above, certain minor refinements have since been made to further optimize the procedures.
This protocol describes the standard procedures as currently used in our laboratory.
In all of the tasks described here, the specific research question and experimental manipulation may affect the behavioral procedure. For clarity, we will limit discussion to 4 frequently-used treatment scenarios. In Case 1, the subject receives treatment before onset of the experiment (e.g., constitutive transgenic or knock-out models, developmental manipulations). In Case 2, the subject receives treatment before task acquisition, but after pretraining (e.g., subchronic drug treatment, neurotoxic lesions). In Case 3, the subject receives treatment after acquisition to assess effects on asymptotic performance level or post-acquisition behavioural challenges using a between-subject design (e.g., neurotoxic lesions, subchronic drug treatment). In Case 4, the subject receives a transient manipulation at performance level, or during post-acquisition behavioural challenges, that can be performed within-subject (e.g., systemic pharmacological or intracerebral infusion procedures). We will refer to these cases as appropriate in the procedure. If the effects upon both acquisition and the main task are of interest, the manipulation can be made prior to the start of training (e.g., Cases 1 and 2). However, if the effect on the main task is of primary or exclusive interest, the researcher may prefer to introduce the manipulation after the acquisition phase if practical (e.g., Cases 3 and 4). It should also be noted that although the 5-CSRT task typically yields stable levels of performance and lends itself well to examination of acute manipulations (e.g., Case 4), animals typically require multiple sessions to achieve criterion in Extinction and Reversal Learning (see TIMING), and it may be difficult to assess the effects of acute manipulations on learning in these procedures.
There are also several options for determining the point at which animals can be advanced from acquisition training to post-acquisition manipulations (including Cases 3 and 4, and post-acquisition behavioral challenges including Reversal Learning, Extinction and 5-CSRT probe phases:
Fixed Training Period Train all animals on a pre-determined number of acquisition phase sessions before moving on to primary task phase or probe testing, regardless of performance level. This protocol is desirable when it is essential for all animals to be reversed at a particular timepoint or age, such as when assessing progressive disease models87. However, using this approach, some animals may receive significant overtraining while others fail to acquire the initial contingencies, which may confound interpretation of subsequent primary task or probe test effects.
Group Criterion Continually train animals on acquisition phase training (e.g., pretraining phases and/or main task phase) until all animals have reached criterion before proceeding to primary task phase or probe tests. This protocol is desirable when complete acquisition curves are required, as all animals will have the same number of training days prior to the primary task or probe tests. However, some subjects might be overtrained and/or will have obtained more rewards during the acquisition phase, and this may confound performance in the primary task or probe tests.
Individual Criterion Move each animal onto the primary task phase or probe tests on the session immediately after it has reached acquisition phase criterion. This option prevents possible carry-over effects due to overtraining during acquisition (Extinction and Reversal Learning can be particularly sensitive to such overtraining). However, as the primary task testing is likely to be staggered across animals, decisions based on group-average performance levels can be difficult to make. Moreover, depending on how long the acquisition phase lasts for some animals, there may be significant time/age difference between individual subjects or experimental groups.
Hybrid approach This is a hybrid between Group Criterion and Individual Criterion approaches. For most common experimental designs, we generally recommend this option. Using this option, animals are trained to criterion on all acquisition phases and put “on rest” until all animals have reached criterion. Then, all animals can be tested at the same time for a further one to three sessions to ensure they are all still performing at criterion before proceeding to the primary task phase or probe tests. If there is a large discrepancy between the fastest and slowest learners during acquisition, it might be useful to run occasional “reminder” sessions for the animals who have already reached criterion (e.g., one session per week). If an animal’s performance falls below criterion in a “reminder” session, that animal is trained daily until criterion is reattained. This protocol option ensures that all animals experience their initial primary task phase session on the same test day, as well as minimizing overtraining effects. However, depending on the variability of acquisition, it should be noted that some subjects may be exposed to significantly more sessions and rewards.
The three tests measuring aspects of executive function described in this protocol may be administered together and/or as part of a flexible test battery including touchscreen tasks assessing other cognitive domains (see 31,86). This battery approach can be tailored to test specific hypotheses, or used to generate general cognitive profiles for individuals or groups of animals which may aid in the interpretation of the behavioral data. The approach may be even more powerful if animals are tested within-subjects (each animal tested on all tasks of interest), however, care must be taken when determining an appropriate sequence of task presentation.
Pretraining
All three tasks in this protocol are motivated by food reward and require instrumental responses to the touchscreen. To ensure sufficient levels of motivation, animals thus are subject to mild food restriction beginning prior to pre-task training and continued throughout the course of the experiment. The purpose of pretraining is to gradually shape appropriate screen-touching behavior and normally consists of three to five stages followed by training specific to each task. The number and size of response windows and the size and type of visual stimuli used during pretraining is task dependent. For Extinction and 5-CSRT tasks, the pretraining stimulus is a solid white square. For Reversal Learning of a visual discrimination, pretraining stimuli are typically selected from a library of 40 varied black and white shapes, which are distinct from the stimuli used in the main task procedure. The rationale for using a large number of varied stimuli is to try to minimize generalization between the pretraining- and task-specific stimuli.
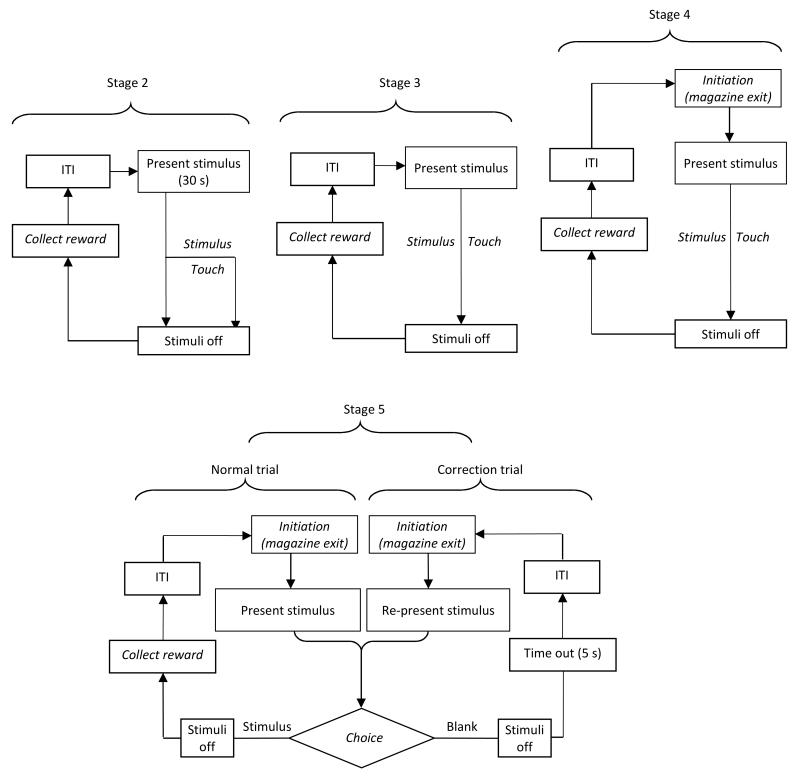
Following commencement of mild food restriction, animals are habituated to the food reward and chambers for at least 2 daily sessions (Stage 1, see PROCEDURE Steps 4 and 5). In Stage 2 (see Fig. 1), a series of trials are delivered in which a stimulus is presented within one of the response windows. If the stimulus is not touched within 30 s the stimulus is removed and its offset is concurrent with reward delivery and with activation of the magazine light and a brief (1 s, 3 kHz) tone (conditioned reinforcers). Touches to stimuli are encouraged by immediate offset of the stimulus, a triple reward delivery, and activation of the magazine light and a tone. Following reward retrieval, an intertrial interval (ITI) begins, after which the next trial is automatically initiated. For Extinction and the 5-CSRT task the ITI is typically set at 5 s. For Reversal Learning the ITI is typically set at 20 s as it has been demonstrated that longer ITIs might help facilitate learning29. Our current standard procedure is to have the houselight off during stimulus presentation and ITIs (and on for “time out” periods – see Stage 5 below), but each of these tasks have also been performed with the houselight on. Although we have not made any direct comparisons between these procedural variants, there may be some reduction in luminance contrast when the houselight is on during stimulus presentation due the higher ambient light levels, which may increase the threshold for signal detection.
Figure 1. Flowchart overview of pretraining stages 2-5.
Stage 2: A visual stimulus is presented in one of the response windows. If not touched, stimulus offset occurs after 30 s and a reward is delivered. If touched, offset is immediate and a triple reward is delivered. After reward collection and an ITI period, the next stimulus is presented in a new trial. Stage 3: Proceeds as in Stage 2, but the stimulus remains on the touchscreen until touched. Animals to be tested on Extinction can proceed directly to the Extinction procedure after reaching criterion on Stage 3. Stage 4: Proceeds as in Stage 3, but the animal must enter and exit the magazine after the ITI to initiate the next trial. Stage 5: Proceeds as in Stage 4, but touches to blank response windows (when there is a stimulus on the screen) are discouraged with a time out. Following this and the ITI the next trial may be initiated, but in pretraining for the majority of tasks this is a CT in which the previous stimulus is represented, rather than a new trial. Note that CTs are not given in Stage 5 of pretraining for the 5-CSRT. The labels in italics indicate steps in which the animal is required to perform an action.
Stage 3 (see Fig. 1) is similar to Stage 2 in all respects with the exception that subjects are required to touch the stimulus to cause reward delivery, stimulus offset and activation of the magazine light and tone. Subjects proceeding to Extinction training would do so after completing Stage 3.
Stage 4 (see Fig. 1) is similar to Stage 3, but subjects are further required to trigger stimulus presentation, referred to as trial “initiation”. The session begins with a “free” reward delivery and magazine illumination, indicating that a trial may be initiated by the subject. When the animal nose pokes into the magazine, the magazine light is extinguished and a brief (0.2 s) click sound is generated. When the animal withdraws its nose from the magazine, the stimuli are presented on the screen. Initiation is also required after the ITI between each trial.
Stage 5 (see Fig. 1) is similar to Stage 4, but subjects are additionally discouraged from touching blank response windows during stimulus presentation, via the negative feedback of immediate stimulus removal and initiation of a time out period in which the houselight illumination status is inverted (e.g., on-to-off or off-on – see Stage 2 above). This stage serves to introduce the subject to the cue signalling incorrect responses (e.g., the time out period). Only after the time out period elapses, the ITI begins, after which the next trial can be initiated. For subjects proceeding to Reversal Learning training, any trial immediately following a time out period is designated a correction trial in which the same stimulus/stimuli are re-presented in the same location(s). There is no limit on the number of correction trials that can be given consecutively, but once the subject responds correctly, the correction procedure ends and a normal, non-correction trial resumes. Correction trials do not count towards the session trial limit. The purpose of correction trials is to help counteract development of side or stimulus biases, and to ensure that subjects receive a consistent number of rewards per session. While correction trials may also be implemented for 5-CSRT task training, our current standard protocol does not include correction trials in 5-CSRT pretraining or in its main procedure.
By the end of Stage 5 of pretraining, subjects should be completing a sufficient number of trials per session (as specified in PROCEDURE), to promote completion of sessions in the subsequent task. Note that rats are typically given the opportunity to complete more trials per session than mice, e.g., 100 as opposed to 30 during pretraining. Rats readily complete a greater number of trials per session than mice, perhaps because the mouse:rat body mass ratio is smaller than the mouse:rat reward pellet size ratio (14mg:45mg).
Analysis of pretraining performance is generally minimal. The number of sessions required to complete each phase of pretraining, or the overall number of sessions required to complete pretraining, may be analyzed if desired or if matching for performance level prior to an experimental manipulation (see case 2 above).
A) Extinction
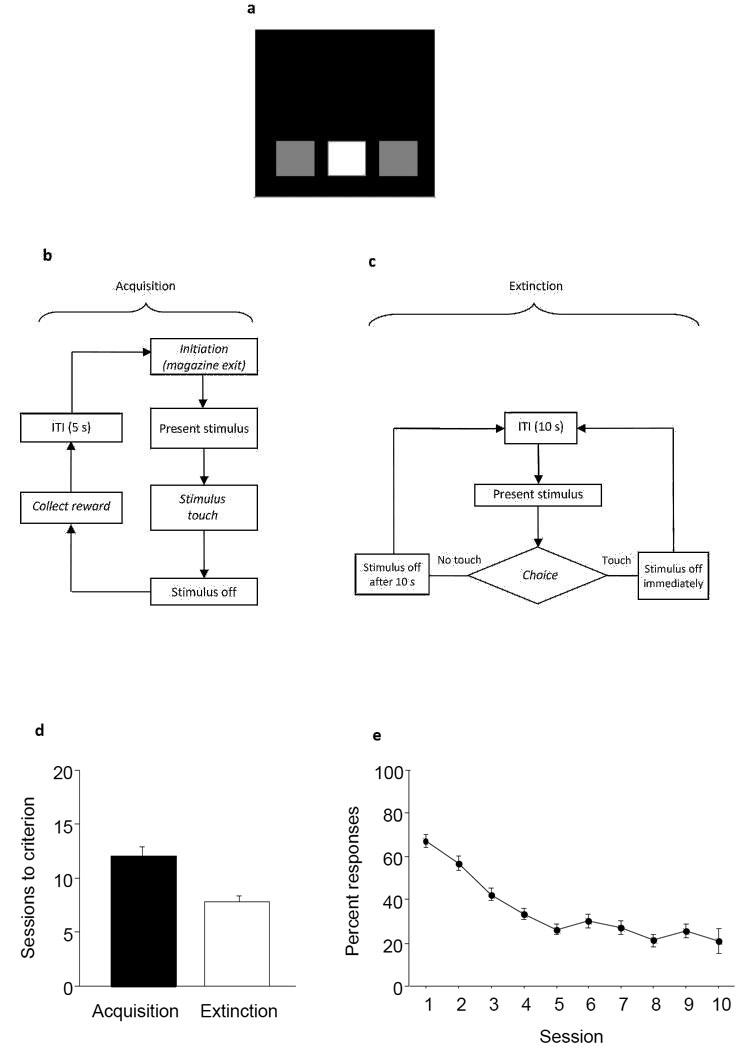
The Extinction procedure described here is the product of a series of refinements to the methods described in recent publications (discussed above); changes have generally involved simplifying the stimulus and response requirements. The trial structure of the acquisition phase of the Extinction procedure (see Fig. 2b) requires only a small modification of the standard operant pretraining paradigm (compare to pretraining Stage 4 above). During the response acquisition phase, sessions begin with a “free” reward delivery to the magazine and magazine light illumination, indicating that a trial may be initiated. Trials are initiated by the subject’s head entry into the magazine (turning off the magazine light and activating a 0.2 s auditory click), where subsequent head withdrawal from the magazine initiates presentation of a single, solid white square stimulus at a central location on the touchscreen. When the subject touches the stimulus, the stimulus is removed from the screen, a reward is delivered and the magazine light and a 1 s tone are turned on. Following reward collection, the magazine light is extinguished and a 5 s ITI commences, after which the magazine light is illuminated and subjects are again given the opportunity to initiate a new trial.
Figure 2. Extinction task.
A: Schematic of a three-hole mask used in the Extinction paradigm. B: Flowchart overview of acquisition learning phase. Following initiation, a solid white square stimulus is presented at a central location on the touchscreen. When the subject touches the stimulus, a reward is delivered, and after reward collection and an ITI, a new trial may be initiated. The labels in italics indicate steps in which the animal is required to perform an action. C: Flowchart overview of extinction learning phase. Each trial begins with a 10 s ITI, after which the single, solid white square stimulus is presented on the touchscreen. The subject is not required to initiate the trial. If the subject either touches the stimulus (response) or does not touch the stimulus within a 10 s duration (omission), the stimulus is removed and the 10 s ITI leading to the next trial is initiated. No rewards or conditioned reinforcers (e.g., traylight or tone associated with reward delivery) are delivered during the extinction phase. The labels in italics indicate steps in which the animal is required to perform an action. D: Representative data of C57BL/6J mice using a 2-choice acquisition and extinction procedure showing the typical number of sessions to criterion (data replotted from 46). E: Representative data from the same mice showing the time course of response extinction. Data are presented as means with error bars indicating S.E.M.
There are several alternatives to the response acquisition phase trial structure described above. For example, the procedure may be simplified even further, eliminating the requirement for trial initiation (e.g., proceeding directly to the extinction phase below from pretraining Stage 3 above). This would permit faster pretraining times and also share more procedural similarity with the extinction phase procedure described below. Another possibility would be to randomize the position of the stimulus across 2 or 3 screen locations (using a 2- or 3-stimulus mask – see EQUIPMENT SETUP). The acquisition phase could be programmed such that touches to blank locations cause disappearance of the stimulus, turn on the house light and commence a time out period during which animals are unable to initiate a new stimulus presentation (c.f., pretraining Stage 5 above). Indeed, an extinction procedure employing two stimulus locations on the touchscreen has been used frequently with success40-42. Although more complex, this approach can help distinguish whether subjects are acquiring a specific response to the visual stimulus or simply approaching the touchscreen. Indeed, virtually any response learning procedure may be used prior to extinction learning, with the proviso that the rate, extent and interpretation of extinction learning can vary depending on what is learned during response acquisition, or earlier. It is important to reiterate that, as expression of extinction is highly dependent on the training context, comparisons of rate of extinction learning should only be made in light of similar training histories.
In the extinction phase of the current procedure (see Fig. 2c), each trial typically begins with a 10 s ITI, after which the single, solid white square stimulus is presented on the touchscreen. The subject is not required to initiate the trial. If the subject either touches the stimulus (response) or does not touch the stimulus within a 10 s duration (omission), the stimulus is removed and the 10 s ITI leading to the next trial is initiated. No rewards or conditioned reinforcers (e.g., traylight or tone associated with reward delivery) are delivered during the extinction phase.
As animals typically require multiple sessions to achieve criterion in Extinction (see TIMING and ANTICIPATED RESULTS) it may be difficult to investigate the effects of acute experimental manipulations (an example of Case 4). However, task parameters in the extinction phase may be adjusted (e.g., decrease the criterion number of trials and/or increase the session length) to help facilitate investigations of acute manipulations. Various post-training probe tests may also be carried out after the extinction phase (e.g., reinstatement procedures13 – see PROCEDURE) to further assess aspects of extinction learning that may be amenable to acute experimental manipulations. The most well-established post-extinction probes include various forms of relapse – the re-occurance of the relevant behavior learned prior to Extinction training33,88. For example, Reacquistion may be assessed by examining recovery of pre-Extinction behavior after the unconditioned reinforcer (i.e., food reward) and/or conditioned reinforcers (i.e., magazine light and brief tone) are reintroduced in a manner fully contingent on presentation of, and the subject’s response to, the visual stimulus (i.e., identical to the learning stage prior to removal of these reinforcers during Extinction). If Reacquisition is only partial (e.g., limited in time or number of reinforcers) or reexposure to the unconditioned stimulus is not delivered contingently on previously learned stimuli or responses, the probe procedure is typically described as Reinstatement46. Reinstatement may also be assessed after contingent or noncontingent exposure to only the conditioned reinforcer(s) (e.g., without the unconditioned reinforcer), although such procedures which reintroduce elements of the pre-Extinction learning context are more commonly referred to as Renewal49,88. These post-extinction probes may be used to evaluate propensity to relapse and also the nature and extent of extinction processes; for example, the strength of reinstatement is believed to be an indicator of the strength of contextual conditioning89-90. For a fuller theoretical discussion of such post-extinction probes, see 33,49.
The primary measures of Extinction learning are the number of sessions, responses and trials required to reach criterion. Additionally, response latencies and rates of responding can also be examined. Post-extinction probe sessions are typically analyzed by calculating the difference between responding during the last extinction session(s) and during the post-extinction probe session(s).
B) Reversal Learning
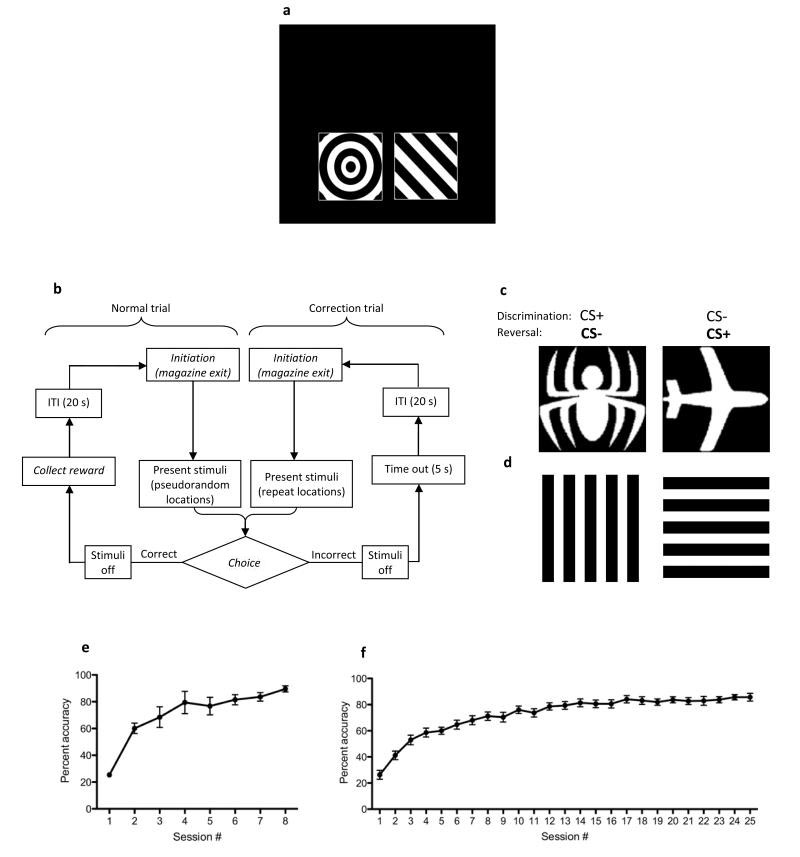
The trial structure of the visual Reversal Learning task requires a simple adjustment of the Visual Discrimination task (c.f., 31) and is illustrated in Figure 3b. Each session begins with a “free” reward delivery to the magazine and magazine light illumination, indicating that a trial may be initiated. A trial is initiated when the subject enters (signalled by a 0.2 s auditory click and the magazine light turning off) and withdraws its head from the reward magazine; head withdrawal is followed immediately by the appearance of two stimuli in two distinct, predefined locations on the touchscreen (see EQUIPMENT SETUP). One of the stimuli is designated as correct (CS+) while the other is incorrect (CS−). The location of the CS+ and CS− is determined pseudorandomly for each trial, with the constraint that the stimuli not are displayed in the same locations for more than 3 consecutive trials (excluding correction trials – see below). If the subject touches the correct stimulus, both stimuli are removed, the magazine light and a 1 s tone are turned on, and a reward is delivered. When the subject makes a magazine entry to collect the reward, a 20 s ITI is initiated. If the subject touches the incorrect stimulus, both stimuli are removed and the houselight is turned on for a 5 s time out period (no reward is delivered). When the time out period has elapsed, the house light is switched off and the 20 s ITI is initiated. When the ITI period ends after either a correct or incorrect trial, the magazine light is turned on allowing the opportunity for the subject to initiate a new trial. An incorrect trial causes the subsequent trial to be a correction trial - presented in identical manner to the trials described above, with the constraint that the two stimuli are presented in same locations as the preceding trial. Correction trials continue to be presented until the animal touches the CS+ and do not add to the total trial count for the session. Acquisition and reversal sessions of visual discrimination learning are identical, except that during reversal learning, the previously correct stimulus (CS+) becomes the incorrect stimulus (CS−), while the previously incorrect stimulus (CS−) becomes correct (CS+).
Figure 3. Reversal Learning task.
A: Schematic of a two window mask and stimuli used in the Reversal Learning paradigm. B: Flowchart overview of the Reversal Learning procedure. Following initiation, a pair of stimuli (CS+, CS−) is presented on the screen, in pseudorandom locations. Correct responses (to CS+) are rewarded, and after reward collection and an ITI, a new trial may be initiated. Incorrect responses (to CS−) are discouraged with a time out, then after an ITI and initiation the previous trial type is represented (a correction trial). The correction trial loop will continue until a correct response is made. The labels in italics indicate steps in which the animal is required to perform an action. C: Spider versus plane stimuli typically used for Visual Discrimination and Reversal Learning in rats (reproduced from 29 with permission). D: Horizontal versus vertical pattern stimuli which rats acquire more readily. E: Typical Reversal Learning performance in rats (n = 10, with a history of Object-Location Paired Associates Learning and Trial-Unique Nonmatching-to-Location training) using “castle” versus “face” photographic stimuli (C.A. Oomen, unpublished results). F: Typical Reversal Learning performance in mice (n = 17, of mixed background (approximately 1:15 CBA/ca:C57BL/6J), using “marble” versus “fan” stimuli (A.E.H., unpublished results). Data are presented as mean +/− S.E.M.
Reversal Learning is typically assessed using the number of sessions, trials and errors (incorrect responses on non-correction trials) required to reach criterion. Additionally, latencies, percentage of bias and perseveration score may be analyzed (see step 10B vi of PROCEDURE)
Several reversal procedure parameters can be modified to affect performance and provide insight into underlying processes. One example is the use of stimuli that are harder to discriminate relative to standard shape images (e.g., stimuli that have overlapping features of both the correct and incorrect stimuli (blended, morphed or complex photographs)31,91. This may increase the potential of detecting certain experimentally-induced performance improvements through extension of the reversal training period. Alternatively, use of luminance-matched patterns instead of shape stimuli has been observed to speed visual discrimination criterion attainment (Mar, et al., Measuring Behaviour Conference, Utrecht, August 2012 – see Fig. 3d).
Post-reversal experimental manipulations include the testing of serial reversals, which may be used to supplement67 as well as to add distinct information62 to that observed for the first reversal, or multiple repetitions of acquisition and reversal (with novel stimuli sets), which may reduce the amount of learning transfer from previous reversals (see PROCEDURE). Both of these forms of repeated reversal learning may recruit separate or additional learning mechanisms to those employed on first reversal exposure. Another post-reversal experimental manipulation is the replacement of either CS+ or CS− stimuli with a novel stimulus during the reversal phase. When used in conjunction with multiple reversals, this manipulation may help dissociate processes such as perseveration toward the previously rewarded option (e.g., replacing CS+ with a novel stimulus during reversal) or avoidance of the previously non-rewarded option (e.g., replacing CS− with novel stimulus), that can affect Reversal Learning performance. This strategy has been successfully used in a touchscreen visual discrimination and reversal paradigm in marmoset monkeys62.
Variants of the Visual Discrimination and Reversal Learning procedure employing more than two stimuli and/or response windows are also in development. The basic procedure for these is similar to that for the standard task. For example, a 3-stimulus version has been performed recently in rats (J. Alsiö, A.C. Mar, D.E. Theobald and T.W. Robbins, unpublished data). In lieu of using two stimuli in two locations, three different stimuli (one stimulus designated as CS+, and the other two stimuli both designated as CS−) are presented across three different locations during visual discrimination learning (using a 3-window mask). Following reversal, the previous CS+ becomes a CS−, and one of the previous CS− stimuli becomes the new CS+. The third stimulus remains a CS− during both discrimination and reversal learning. This 3-stimulus task is more difficult than the 2-stimulus version and may thus provide greater opportunity for detection of certain pro-cognitive effects. A 3-stimulus task also permits separation of errors of perseveration (i.e., responses to the previous CS+) from errors that occur using other search strategies (i.e., spatial response bias, including responses to the constant CS−). Similar effects may be observed if the constant CS− stimulus is instead included as a neutral stimulus (e.g., recorded, but no programmed consequences upon touching the neutral CS stimulus) in both discrimination and reversal phases. Another example is retaining the use of only two stimuli, but increasing the number of locations to 3 or 4 (using an appropriate 3- or 4-window mask), thereby increasing the ratio of locations to stimuli. This procedure increases the difficulty of the task and helps control or rule out the possibility of subjects solving the discrimination or reversal using simple configural learning strategies (e.g., when CS+ left/CS− right, touch left; when CS+ right/CS− left, touch right). This may also help reduce development of spatial biases.
C) 5-Choice Serial Reaction Time task
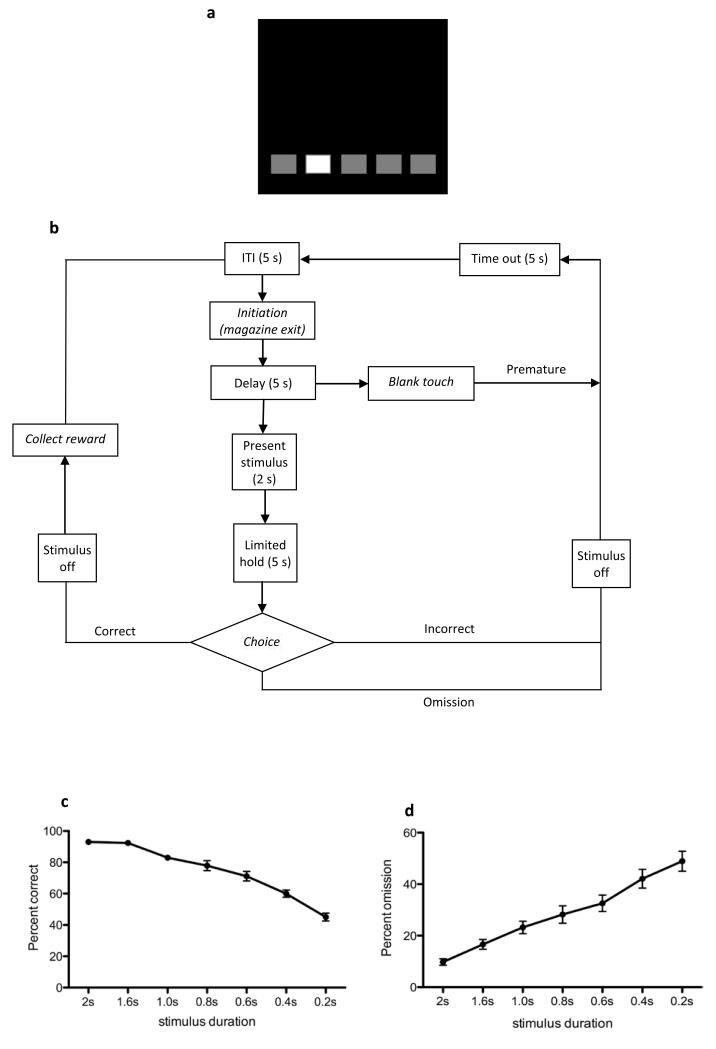
The basic 5-CSRT task requires the animal to sustain and divide its attention across a row of five screen locations in order to detect and respond to a brief visual stimulus (see Fig. 4a-b). Variations of the mouse touchscreen 5-CSRT task procedure outlined here have been previously described with the main protocol featuring several key differences from the standard operant procedure in the rat82. Briefly, each session consists of 40-60 trials for which a maximum of 60 mins is allowed, whereas for rats, the number of trials is typically 100+ in 30-60 mins. Each session begins with delivery of a “free” reward and illumination of the magazine light. Upon reward collection from the magazine, the magazine light is extinguished and a 5 s ITI period commences. After the ITI elapses, the magazine light is again illuminated to indicate that a trial can now be initiated. This 5 s ITI and second magazine entry requirement following reward collection was specifically introduced into the mouse touchscreen procedure to space trials further apart in order to permit mice a longer time to consume their rewards and to add a self-pacing element to help counteract difficulties that mice sometimes experience with the pace of the more traditional version (e.g., resulting in a high level of trial omissions). This additional ITI and/or initiation requirement can be removed to render the procedure more equivalent to standard versions of the 5-CSRT task82 for use in testing rats or to increase the difficulty of the current mouse protocol.
Figure 4. 5-Choice Serial Reaction Time (5-CSRT) task.
A: Schematic of a five-hole mask used in the 5-CSRT paradigm. B: Flowchart overview of the 5-CSRT task. A trial is initiated when the subject enters and withdraws its head from the illuminated magazine. After a 5 s delay, a white square stimulus is briefly presented in one of the five response windows. Touching the white-square location either when the stimulus is present (or during a short subsequent limited-hold period) is recorded as a correct trial and rewarded. Collection of reward initates a 5 s ITI. A response in any other response window is recorded as an incorrect trial, and results in a time out period, before the beginning of the 5s ITI. Failure to respond at the screen during the stimulus presentation or the limited-hold period is recorded as an omission and also leads to a time out. Response(s) before the onset of the stimulus is recorded as a premature response, and lead to a time out, but premature trials do not contribute to the session trial count. After the ITI has elapsed, the magazine is illuminated and the subject can initate another trial. C and D: Representative data illustrating the dependence of response accuracy and omission, respectively, on stimulus duration in adult C57Bl/6J mice (S. Nilsson, L Saksida, T Bussey, unpublished data). Data are presented as mean +/− S.E.M.
After the 5 s ITI has elapsed, trials can be initiated by magazine head entry (signalled by turning off the magazine light and by a 0.2 s auditory click) and exit from the reward magazine. A solid white square stimulus then appears briefly (e.g., 2 s) in one of the five screen locations after a fixed 5 s “delay” period. The stimulus position is chosen pseudorandomly such that it is equally presented at each location throughout the session. There are no correction trials implemented after incorrect responses or omissions. There are four possible trial outcomes. Touches to one of the screen locations during stimulus presentation or during a 5 s “limited hold” time period after the stimulus is removed results in either a “correct” or “incorrect” trial. “Correct” trials are recorded if the response is in the same location as the visual stimulus while “incorrect” trials are recorded if one of the other four “blank” locations is touched. If no touch response is made, the trial is classified as an “omission”. If a location is touched during the delay prior to stimulus onset, the trial is deemed as “premature”. Premature trials are recorded but are not included in the total trial count for the session (e.g., similar to correction trials in other procedures). Following premature responses, the same trial is repeated until a correct, incorrect or omission trial is performed. The stimulus is removed from the screen immediately if still present following a correct or incorrect response. Correct trials are followed by illumination of the magazine light and delivery of a 5 s tone and a reward. Incorrect trials, premature trials and trial omissions are all followed by a 5 s time out period in which the houselight is turned on. Upon reward collection or after the time out period has elapsed, the 5 s ITI period preceding the next trial commences. Baseline 5-CSRT parameters are reached by training animals through a series of stages in which the stimulus duration is gradually reduced to increase task difficulty (see Table 2). When animals are performing stably at baseline, a variety of probes can be used to further assess performance including variations in stimulus duration, stimulus brightness, delay prior to stimulus presentation, and number of trials/session length. These alternatives are discussed in more detail in the PROCEDURE. Other manipulations have also been implemented in the standard operant apparatus that may also serve to increase the attentional demands of the touchscreen task. Examples include removal of the requirement for trial initiation (e.g., target presentation is experimenter-paced rather than self-paced) and alteration of the rate of presentation of the stimuli (e.g., high event rate of 1 s or low event rate of 20 s)101-102
Table 2. 5-Choice Serial Reaction Time task training procedure.
| Step | Training Stage | Session length (mins) | Trials per session (max) | Delay (s) | ITI (s) | Stimulus Duration (s) | Criterion | Timing |
|---|---|---|---|---|---|---|---|---|
| 10C i-iv | 5-CSRT training | 60 | 40-60 (mice) 100 (rats) | 5 | 5 | Progression from 8 to 4 to 2 (or lower) | Complete all trials, >80% accuracy and <20% omissions for 3 out of 4 consecutive sessions at each duration | 60 mins, ~12-30 sessions |
| 10C v | 5-CSRT probes Variable stimulus durations, (between-session) | 60 | 40-60 (mice) 100 (rats) | 5 | 5 | Consecutive (e.g., descending) or counterbalanced sessions at each duration e.g., 2.0, 1.0, 0.8, 0.6, 0.4, 0.2 | n/a | 2 sessions per probe with 2 sessions baseline in between |
| 10C v | 5-CSRT probes Variable stimulus durations, (within-session) | 60 | 40-60 (mice) 100 (rats) | 5 | 5 | Variable within-session stimulus durations presented in pseudorandom order e.g., 2.0, 1.0, 0.8, 0.6, 0.4, 0.2 | n/a | 2 sessions per probe |
| 10C v | 5-CSRT probes Increased trials and session length | 90 | 100-200 (mice) 150-300 (rats) | 5 | 5 | Typically baseline (e.g., 2 s) | n/a | 1-2 sessions for baseline, 2 sessions for probe |
| 10C v | 5-CSRT probes Variable ITI (between and within-session) | 60 | 40-60 (mice) 100 (rats) | 2-14 | 5 | Typically baseline (e.g., 2 s) | n/a | 1-2 sessions for baseline, 2 sessions per probe |
| 10C v | 5-CSRT probes Reducing stimulus brightness | 60 | 40-60 (mice) 100 (rats) | 5 | 5 | Typically baseline (e.g., 2 s) | n/a | 1-2 days for baseline, 2 days per probe |
MATERIALS
REAGENTS
Rats or mice (see REAGENT SETUP)
Animal housing (see REAGENT SETUP)
Rodent food pellets (e.g., Rodent Pellets, Special Diets Services, UK)
Food rewards: Either solid (e.g., Bio-Serv® purified rodent Dustless Precision Pellets®, 45 mg (rat)/14 mg (mouse), through Sandown Scientific, Esher, UK) or liquid (Yazoo® strawberry milkshake, FrieslandCampina UK Ltd)
Cleaning materials (e.g., TriGene®, 70% ethanol solution, stiff brush)
Critical Step Reward pellets generally work well for rats. We use either liquid or solid for mice. In some cases, liquid rewards may be the better option, e.g., when using manipulations that result in motoric changes that could affect chewing, cause dry mouth, or reduce motivation. Liquid rewards also afford more flexibility to vary reinforcement value (e.g., concentration, volume).
Caution When filling reward dispenser with Dustless Precision Pellets®, take care to discard dust, as this can potentially clog dispensers.
Caution All liquid reward containers and delivery lines should be thoroughly rinsed at the end of each testing day to prevent clogging and/or growth of potentially harmful micro-organisms.
EQUIPMENT
Sound- and light-attenuating box with ventilation system, enclosing an operant chamber and reward delivery system.
Touchscreen operant chambers (from, e.g., Campden Instruments Ltd., Med Associates Inc., other commercial suppliers; or custom-made operant system). Note that these are species-specific. See EQUIPMENT SETUP.
Camera above chamber, connected to closed circuit monitor and digital video recording device, to monitor and record animals’ behavior (optional but recommended)
Controlling software and devices (generally available from operant chamber supplier)
Black plastic masks with response windows (the number and size of which differ between tasks – see EQUIPMENT SETUP)
Shelf for rat chamber (for some tasks, see EQUIPMENT SETUP)
Appropriate data analysis software
Personal protection equipment (e.g., disposable medical gloves, lab coat or coverall, FFP2 mask) should always be worn when handling or working near animals, to minimize allergen exposure.
Caution Completely power off and take care not to damage touchscreens whenever inserting or removing response window masks. Failure to do so may require touchscreen recalibration and/or touchscreen maintenance or replacement.
REAGENT SETUP
Rodents
Laboratory-bred or commercially available rats/mice are generally used for testing. There are some advantages to testing male rodents, such as avoiding potential estrus cycle-related performance variability in females, and potentially increased inter-male aggression when males must be tested in the same apparatus as females92-93. Most commonly, we use Lister Hooded rats and mice on the C57BL/6 or 129 substrain genetic backgrounds. We prefer to begin training when rodents are young adults, e.g., mice 10-14 weeks old. However, females, aged rodents and various strains have also been successfully tested29,94.
Caution All experiments using live animals must be approved by national and institutional bodies, and performed according to their regulations.
Caution If animals are not fully grown when food restriction begins, they must be allowed to gain sufficient weight as they continue to grow. Standard strain growth curves are available for guidance (e.g., http://jaxmice.jax.org/support/weight/index.html). See Step 3 of the PROCEDURE for further details.
Animal housing
Rats and mice should be housed in groups (e.g., 2-5), with sawdust, bedding and (optional, although recommended) some form of shelter (e.g., plastic or cardboard tube). Cages should be cleaned and bedding etc. changed weekly. The housing room should be maintained at a constant temperature (20-24 °C) and humidity (55 ± 10%). Lighting is usually on a 12-hour light-dark cycle, with lights off at 7:00 AM or 7:00 PM (we favor lights off at 7:00 AM, so that rodents can be tested in the active period of their circadian cycle).
EQUIPMENT SETUP
Rodent touchscreen operant chambers
Rodent touchscreen operant chambers made by different companies may vary, but share many common features. The specific model used depends on the experimenter’s needs and preference. Here, we will describe mouse and rat chambers from Campden Instruments Ltd. and our in-house assembled boxes.
Campden chambers
Housed inside a dense fiberboard box, these are equipped with a fan (for ventilation and masking extraneous noise), touchscreen monitor (rat: 15.0 inch, screen resolution 1024 × 768 (rotated); mouse: 12.1 inch, screen resolution 600 × 800), tone and click generator, houselight (LED), magazine unit (with light and infrared beam to detect entries; in the standard configuration this is outside the testing arena, on the wall opposite the touchscreen) and pellet dispenser and/or pump connected to bottles of liquid reward (see Fig. 5a for rat chamber). The chambers have a trapezoidal shape [rat: 30 h × 33 l (screen-magazine) × 25 w (at screen) or 13 w (at magazine) cm; mouse 20 h × 18 l × 24 or 6 w cm; excluding space between floor], composed of 3 black plastic walls opening on to the touchscreen, intended to help focus the animal’s attention to the touchscreen and reward delivery area. The touchscreen uses optical infrared sensors and therefore does not require the subject to exert any pressure in order for responses to be registered. We have found that this feature greatly facilitates performance compared to other types of touchscreen and that they are particularly robust and reliable (e.g., unaffected by minor screen surface scratches or dirt that may accumulate during testing). Access to the chamber is through a transparent lid, which can be secured to the trapezoidal walls with latches during animal testing. The floor is perforated stainless steel, raised above a tray lined with filter paper. Two additional photobeams extend between the side walls of the arena, parallel to the screen, to detect the movement of an animal in the front (rat: ~6 cm from the screen; mouse: ~7 cm) or the rear (rat: ~5 cm from the magazine; mouse: ~3.5 cm) parts of the arena. A small infrared camera can be installed above the chamber to monitor animals’ behavior (optional, but recommended). In rat chambers, attaching a shelf to the mask has proved to be effective at reducing impulsive responses and improving attention directed to the stimuli, by forcing the rat to rear up before making a choice28. In Campden rat chambers, a spring-hinged “shelf” (24 w × 6 l cm) can be attached 15 cm above the floor at a 90° angle to the screen and mask, for some tasks. Campden Instruments Ltd. provide advice on setting up the touchscreen equipment, including touchscreen and reward dispenser calibration.
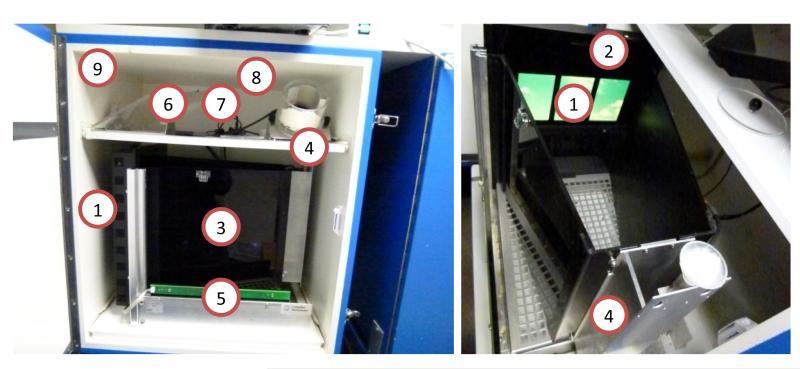
Figure 5. Annotated photographs of a Campden Instruments rat touchscreen chamber.
(1) Touchscreen, (2) black plastic mask covering touchscreen except for response windows, (3) black Perspex walls, (4) pellet dispenser (optional), (5) infrared beam assembly, (6) houselight positioned above chamber, (7) infrared camera positioned above chamber, (8) tone and click generator, (9) sound/light-attenuating box with ventilation fan fitted.
Our in-house chambers
Housed inside a melamine box, chambers (modified in our lab from Med Associates operant chambers) are equipped with a fan, infrared touchscreen monitor (rat: 29.0 h × 23.0 w cm; mouse: 16.0 h × 21.2 w cm; Craft Data Limited, Chesham, UK), tone generator, click generator, houselight (3 W), magazine (with light and infrared beam) and pellet dispenser. As with the Campden system, the optical infrared touchscreen does not require the subject to exert any pressure in order for touches to be registered. The chambers have a rectangular shape, consisting of a metal frame with clear Perspex walls (rat: 29 h × 31 l × 24 w cm; mouse: 13 h × 25 l × 19 w cm; excluding space below floor). Access is through a hinged side wall, secured with a latch during testing. The floor is stainless steel bars spaced 1 cm apart, above a tray lined with filter paper. The magazine is equipped with a light (3 W) and a photocell nose poke detector. A spring-hinged “shelf” (20.5 w × 6 l cm) is also fitted in rat chambers 14.0 cm above the floor, at a 90° angle to the screen and mask.
Masks and stimuli
A black plastic mask (rat in-house: 38.7 h × 30.0 w cm; rat Campden: 35.8 h × 28.0 w cm; mouse in-house: 11.8 h × 22.8 w cm; mouse Campden: 24.3 h × 28.0 w cm) with response windows is fitted in front of the touchscreen to reduce accidental screen touches and make response locations clearly identifiable from the background. These have varying numbers and sizes of response windows, depending on the task. For Extinction, the mask should ideally be constructed to block the entire touchscreen except for a central response window (for mice, typically 5.0-7.1 × 5.0-7.1 cm, 1.5 cm above the floor; for rats, window size can be of similar or larger dimensions, 2.5 cm from floor without shelf, 16.0 cm above floor if shelf is included). Other mask types may also be used, depending on the procedure used (e.g., 2-window Reversal Learning mask or 3-window Object-Location Paired-Associates Learning mask; see Figs. 2a, 3a and 31). The visual stimulus is typically a solid white square of similar dimensions and position as the mask response window.
For Reversal Learning, a mask with two response windows is used (see Fig. 3a). For mice, the two response windows are typically 7.0 × 7.5 cm, positioned centrally with windows 0.5 cm apart, 1.5 cm above the grid floor. For rats, the two response windows are typically 10.0 × 10.0 cm, positioned centrally with windows 1.0 cm apart, either 2.5 cm from floor without shelf or 16.0 cm above floor if shelf is included. (Other mask types might be used for reversal task variants, e.g., 3-window Object-Location Paired-Associates Learning mask). The visual stimuli are typically selected from a set of custom-made images (e.g., see Fig. 3c and 31). Recent development work has demonstrated that using patterns as stimuli (see, e.g., Fig. 3d) can enhance the speed and extent of visual discrimination (Mar et al., Measuring Behaviour Conference, Utrecht, August 2012).
For the 5-CSRT task, a five-choice mask should be inserted in front of screen (see Fig. 4a). For mice, the five response windows are typically 2.0-4.0 × 2.0-4.0 cm squares, positioned centrally with windows spaced 1.0 cm apart, 1.5 cm above the grid floor. For rats, the five response windows are typically 2.0-3.0 × 2.0-3.0 cm squares, positioned centrally with windows spaced 1.0-1.5 cm apart, 1.5-2.0 cm from the grid floor. For rats, the shelf is not used. The visual stimulus is typically a solid white square of similar dimensions and position as the mask response windows.
Controlling software and devices
Controlling software can be purchased from the suppliers of the operant chambers, e.g., “Whisker Server”95; ELO software (ELO Touchsystems Inc.). Multiple chambers may be controlled by a single computer, although it is important to check that minimum system requirements are met (e.g., memory and graphics cards) to prevent delays in stimuli presentation and chamber responses. All task software is based on earlier publications and is available (excluding, in some cases, recent modifications) with User’s guide and technical support from Campden Instruments Ltd., and in some cases from Med Associates Inc.(K-Limbic) or other suppliers. Alternatively, software may be programmed using common programming languages, e.g., Visual Basic 6.0 (Microsoft, Redmond, WA).
PROCEDURE
Preparation for pretraining
1. If it is not necessary to transport animals to the facility from an external source, proceed directly to Step 2. Otherwise, transported animals should be permitted an acclimatization period of at least 7 days, with free access to food and water, before proceeding to Step 3. Begin basic handling and weighing procedures (Step 2) after 4 days of acclimatization.
Critical Step We advise consulting with your institutional animal care regulatory body regarding all aspects of animal husbandry when planning and designing experiments with rodents.
Critical Step Some cohorts of mice have relatively high between-subject variability, and so larger sample sizes are required. Where possible, calculation of sample size should be based on a power calculation derived from previous work with that strain of animal, ideally from the same animal supplier. We advise minimising the age range of the cohort(s) tested to reduce potential age-related variability.
Critical Step For touchscreen naive animals to be tested on Extinction, follow preparation and pretraining Steps 1-7 before proceding to Extinction training step 10A. For touchscreen naive animals to be trained on Reversal Learning or the 5-CSRT task, follow preparation and all pretraining Steps 1-9 before proceeding to Reversal Learning training (Step 10B) or 5-CSRT training (Step 10C). If subjects have previously been trained and tested on another instrumental touchscreen task in the battery, maintain food restriction and begin training at the highest pretraining Step previously run.
2. Weigh each animal for three consecutive days while receiving ad libitum food and water, and calculate the mean free-feeding weight of each animal.
Critical Step Ensure that each animal can be stably and reliably identified throughout the course of the experiment.
3. Begin food restriction, adhering to institutional and national guidelines. Slowly reduce (e.g., over 3-7 d) the weight of individual animals down to the goal weight, which will be a percentage of the measured free-feeding weight (e.g., we use 85-95%, which is inline with our institutional and national guidelines) by controlling the daily amount of food they are given (e.g., for rats, ~7 g food per 100 g body weight; for mice, ~2-3 g food per 25-35g mouse). Start Step 4 when animals are close to their goal weights. Maintain food restriction throughout touchscreen testing.
Critical Step It is important to check the weight of animals daily (mice) or twice a week (rats) until the target weight is reached. This also helps habituate the animals to being handled. Aim to avoid weight reduction of greater than 5% per day, and weight reduction below 85% of free-feeding.
Critical Step If animals are not fully grown when food restriction begins, the target weight should be adjusted upward week-by-week based on known growth curves for that species/strain to account for additional weight gain sufficient to ensure normal development (see REAGENT SETUP).
4. Introduce reward (pellets or milkshake) inside the cage to habituate the animals for 1-3 days. Solid rewards may be scattered on the cage floor; liquid rewards should be poured into a wide, shallow dish.
Pretraining
5. Set up the apparatus (see MATERIALS) for the pretraining stage (EXPERIMENTAL DESIGN – Stage 1), with all electronic components on so that subjects may habituate to these. In this and all subsequent steps, use the touchscreen masks and stimuli as appropriate for the task (see EQUIPMENT SETUP). Although it is not necessary to run any software during Stage 1, we recommend recording subjects’ activity if the necessary apparatus and software is available. If the computer program does not automatically do so, manually place ~10 reward pellets or 0.2 ml liquid reward into the magazine of each chamber prior to the session. Place each rodent into its assigned chamber for 30 min. Remove the rodent and check that all reward has been consumed. Return each animal to their respective home cage. The criterion for advancing to Step 6 is consuming all reward within a session for 2 consecutive sessions.
Critical Step Animals acheiving criterion can generally be moved to Step 6 on the subsequent testing session unless there are other experimental reasons for adjusting the pretraining progression (see EXPERIMENTAL DESIGN). Excessive habituation may serve to retard operant learning in some animals.
Critical Step Animals require fewer standard rodent food pellets when receiving rewards during training; adjust daily food allowance as appropriate to maintain goal weight. Maintain the animal weighing routine described in Step 3. If large disparities in weights between animals within a cage exist prior to, or emerge during the course of, behavioural testing, consider feeding individual animals separately before returning them to their home cage to better control the daily intake of individual animals.
Critical Step As the behavioral performance of animals can be influenced by changes in the experimental context, aim to train, weigh and feed each animal at approximately the same time each day, use the same operant box for each animal and have the same experimenter or set of experimenters conduct the experiment. Attempt to counterbalance chambers and testing times across experimental groups.
Critical Step Operant chambers should be cleaned regularly (e.g., once a week, or more) to avoid context change during sensitive task phases, to ensure the touchscreen and infrared photobeams retain maximum sensitivity, and to prevent accumulation of dirt and excrement. We typically dismantle inner chambers (as far as possible), and clean with surface disinfectants (e.g., TriGene® and 70% ethanol) using paper towel or a stiff brush.
6. Set up the apparatus as detailed in MATERIALS and the software program for stage 2, with settings as detailed in EXPERIMENTAL DESIGN. For Extinction using a single location, only that single location should be used to display stimuli. Place each subject in its assigned chamber, and start the session. The session finishes after 60 min or 100 trials (rat)/30 trials (mouse) are completed, whichever comes first. After session termination, return each animal to their respective home cage. Advance individual subjects to the next training phase when they have achieved a criterion of completing all 30 trials (mice) or 60 trials (rats) within the 60 min session. Animals can be moved to Step 6 for the session immediately after acheiving criterion.
Critical Step Animals acheiving criterion can generally be moved to Step 7 on the subsequent testing session unless there are other experimental reasons for adjusting the pretraining progression (see EXPERIMENTAL DESIGN). Excessive pavlovian training exposure may serve to retard later operant learning in some animals.
Critical Step At the end of each session, record critical data for each subject (e.g., number of correct responses, number of trials completed), in case of computer malfunction. However, most software programs will log many other measures (see EXPERIMENTAL DESIGN).
Critical Step If testing the effects of a manipulation conducted before onset of the experiment (see Case 1 in EXPERIMENTAL DESIGN), ensure that animals in experimental and control groups complete comparable numbers of trials per session by limiting the number of trials given per session to accommodate the lowest responders.
7. Repeat the procedure in Step 6 using the software program for stage 3, with settings as detailed in EXPERIMENTAL DESIGN. For animals to be tested on Extinction, after reaching criterion on this Step, proceed to Step 10.
8. If your program does not do this automatically, manually provide a single free reward into the magazine prior to running the software for this step. Repeat the procedure in Step 6 using the software program for stage 4 as detailed in EXPERIMENTAL DESIGN.
9. Proceed as in Step 8 using a different software program for Stage 5 (see EXPERIMENTAL DESIGN). The criterion for completing this stage is completing all trials with ≥ 80% correct (not including correction trials) within 60 min (rat), or with ≥ 75% correct within 35 min (mouse), on 2 consecutive sessions. For animals to be trained on the 5-CSRT task, if no ITI is desired within the final 5-CSRT task protocol (e.g., either to increase task difficulty or if rats are used as subjects (see EXPERIMENTAL DESIGN), set ITI to 0 s when performing this and all pretraining steps. In addition, as no corrections trials are given during 5-CSRT training (Step10C), they may also be removed from the current step as desired.
Critical Step There is likely to be variation in the number of days that animals require to complete pretraining. We suggest “resting” animals when they reach criterion at step 9, with “reminder” sessions, then rebaselining all subjects so that the entire group can advance to a specific touchscreen task on the same day, unless there is a specific experimental reason to do otherwise (see EXPERIMENTAL DESIGN).
Critical Step If subjects are scheduled to receive experimental treatments after pretraining but prior to task acquisition (Case 2, EXPERIMENTAL DESIGN), perform these now (after completing Step 9). Rebaseline on Step 9 (Stage 5, EXPERIMENTAL DESIGN) before task-specific training.
Critical Step Attempt to counterbalance experimental groups according to the number of sessions required to complete pretraining.
Task
10. Proceed to Extinction (Option A), Reversal Learning (Option B) or 5-Choice Serial Reaction Time task(Option C).
A) EXTINCTION
i. Acquisition
Begin training on once-daily sessions of acquisition, 5-7 days per week. Maintain the same equipment setup as during pretraining, and use the software program for this stage with settings as detailed in EXPERIMENTAL DESIGN. Place each subject into their assigned chamber and start the session. For mice, the session finishes either after 30 mins or 30 trials are completed (whichever comes first). For rats, a greater number of trials per session (e.g., 60+) may be employed. After session termination, return each animal to their respective home cage.
If a shorter acquisition procedure is desired prior to the extinction phase, simply continue training subjects on Step 7 (pretraining Stage 3, EXPERIMENTAL DESIGN) until criterion for this acquisition phase is reached and proceed to step 10Aiii.
Critical Step If a multiple response window mask is used (see EQUIPMENT SETUP) an additional task acquisition phase may be programmed such that touches to blank locations result in stimulus offset and a time out period (c.f., step 9, and pretraining stage 5 in EXPERIMENTAL DESIGN). Touches to the blank (i.e., containing no stimulus) response windows should be measured here and in all subsequent experimental phases. This approach can help ascertain whether animals are acquiring a specific response to the visual stimulus, or simply approaching the touchscreen.
Critical Step If testing the effects of a manipulation conducted before onset of the experiment or task acquisition (Cases 1 and 2, EXPERIMENTAL DESIGN), ensure that animals in experimental and control groups complete comparable numbers of trials per session throughout task acquisition.
Critical Step At the end of each session, record critical data for each subject (e.g., number of trials completed, time required), in case of computer malfunction. However, most software programs will log many other measures (see EXPERIMENTAL DESIGN).
ii. Continue acquisition training
The acquisition criterion for this task is the completion of all trials in 12.5 mins (mice) over five consecutive sessions. For rats, allow more time, e.g., 25 mins. In the majority of experimental situations (e.g., Cases 1 and 2), we suggest advancing subjects individually to extinction training (Step 10Aiii.) when they have attained this criterion (see EXPERIMENTAL DESIGN for discussion and alternatives). If subjects are scheduled to receive experimental treatments after acquisition but before Step 10Aiii. (e.g., Case 3, see EXPERIMENTAL DESIGN), we suggest performing these when all animals have reached criterion once, counterbalancing control and experimental groups according to acquisition performance, then rebaseline (see EXPERIMENTAL DESIGN).
iii. Extinction
Proceed as in Step 10Ai., but using the software program for the extinction phase as described in EXPERIMENTAL DESIGN. For mice, sessions terminate after a maximum of 30 trials (or approx.10 mins) have elapsed. For rats, a greater number of trials (e.g., 60+), and therefore time, may be employed.
iv. Continue extinction training
for each subject until they attain a criterion of two consecutive sessions with ≥77% omissions (i.e. for mice, at least 23 out of 30 trials). If post-extinction experimental manipulations are not of interest, we suggest testing subjects for no less than ten extinction sessions so that a group extinction curve may be plotted. If they are of interest, advance subjects individually to Step 10 A v. when extinction criterion is met.
Critical Step At the end of each extinction session, record critical data for each subject (e.g., number of trials completed, number of omissions), in case of computer malfunction. Analyse these daily to ascertain if criterion has been met for each subject.
Critical Step In addition to withholding food reward, the light and tone “conditioned reinforcers” should also be withheld, as they may be sufficient to maintain responding during extinction. Variants of the protocol can be applied to test the ability of the conditioned reinforcer stimuli to maintain responding in the absence of food reward by presenting the stimuli, but omitting food reward, during extinction.
v. Post-extinction experimental manipulations
Several post-extinction manipulations may be conducted. (see EXPERIMENTAL DESIGN). Some published examples of reinstatement procedures are described below46. Proceed in a similar manner to Step 10Aiii., but with minor modifications to the basic extinction software program:
Reinstatement of instrumental responding through contingent partial re-exposure of the rewarding outcomes. Assess this using a software program in which rewards and all conditioned reinforcers (e.g., tone, magazine light) are delivered immediately following appropriate touch responses over the first few trials (e.g., 6) of a session, with no rewards or conditioned reinforcers over the remaining trials.
Reinstatement of instrumental responding through noncontingent partial re-exposure of the rewarding outcomes. Assess this using a software program in which rewards and conditioned reinforcers are delivered with some delay (e.g., 4 s) following stimulus offset over the first few trials (e.g., 6) of a session, with no rewards or conditioned reinforcers over the remaining trials.
Reinstatement of instrumental responding through contingent re-exposure of only the conditioned reinforcers. Assess this using a software program in which conditioned reinforcers (e.g., tone, magazine light, but no reward) are delivered immediately following appropriate touch responses, across all trials of the session.
Reinstatement of instrumental responding through noncontingent re-exposure of the conditioned reinforcers. Assess this using a software program in which conditioned reinforcers (e.g., tone, magazine light, but no reward) are delivered with some delay (e.g., 4s) following stimulus offset, across all trials of the session.
vi. Data analysis
Analyze the following performance measures for acquisition and extinction phases:
the number of responses (and conversely, omissions) in each extinction session
the response rate per unit time (e.g., per minute)
time taken to complete a session
the number of trials and/or sessions required to reach criterion
time taken (response latency) to respond to the stimulus
time taken (magazine latency) to retrieve the reward (acquisition) or check the food magazine (extinction)
if applicable, reinstatement of instrumental responding can be determined by calculating the difference between responding during reinstatement and responding during the last extinction session.
B) REVERSAL LEARNING
i. Visual Discrimination acquisition training
When subjects are ready for task training to begin, counterbalance stimulus reward contingencies (such that approximately half of each group receive a given stimulus as CS+ and the other as CS−, and the rest the reverse), according to the number of sessions required to complete pretraining. Begin training on once-daily sessions of Visual Discrimination acquisition, 5-7 days per week. Provide a single free reward (if your program does not do this automatically). Set up the apparatus as detailed for this task in MATERIALS and the software program for this stage with settings as detailed in EXPERIMENTAL DESIGN with reward contingency as appropriate for each subject. Place each subject in its assigned chamber, and start the session. The session finishes either after 60 min or 100 trials (rat)/30 trials (mouse) are completed (whichever comes first). After session termination, return each animal to their respective home cage.
Critical Step Give careful consideration to the stimulus set you choose. Any novel combinations of stimuli should be pre-assessed for stimulus biases - see EXPERIMENTAL DESIGN and 31.
Critical Step Carefully monitor visual stimulus biases on the first day of testing (see data analysis, Step 10Bvi.). If animals show strong stimulus biases, consider revising the stimuli.
Critical Step Given that performance will be at chance at the start of training, limit sessions to 50 trials (rat)/15 trials (mouse) in 60 min, for at least 2 sessions. Continue until subjects can complete this in 30 min. Give each subject an even number of these reduced sessions, such that they can be combined into full 100 (or 30)-trial sessions for analysis. If the subject completes fewer trials than required, the missed trials may be added on to the trials required in the next session (if less than ~10), or given in a new session.
Critical Step If testing the effects of a manipulation conducted before onset of the experiment or task acquisition (Cases 1 and 2, see EXPERIMENTAL DESIGN), ensure that animals in experimental and control groups complete comparable numbers of trials per session throughout task acquisition. Cap the number of trials given per session to accommodate the lowest responders.
Critical Step At the end of each session, record critical data for each subject (e.g., number of correct responses, number of trials completed), in case of computer malfunction. However, most software programs will log many other measures (see EXPERIMENTAL DESIGN).
Critical Step As different strains perform differently on this task, the performance criteria can be adjusted accordingly.
ii. Continue acquisition training
until the acquisition criterion is reached. The acquisition criterion for this task is the completion of all trials with an accuracy of ≥ 80%, or alternatively, 85% (e.g., ) (excluding correction trials) for two consecutive sessions. Reversal Learning is likely to be of primary interest, so regardless of experimental manipulation (e.g., Cases 1-4, see EXPERIMENTAL DESIGN), we suggest “resting” animals when they reach criterion, with “reminder” sessions, until the entire group has achieved criterion, upon which the entire group may be rebaselined before progressing to StepBiii (see EXPERIMENTAL DESIGN for details and alternatives). If subjects are scheduled to receive experimental treatments after acquisition but before Reversal Learning (Case 3, see EXPERIMENTAL DESIGN), perform these when all animals have reached criterion (at least) once, counterbalancing control and experimental groups according to acquisition performance, then rebaseline.
iii. Reversal Learning
Proceed as for Visual Discrimination acquisition (Step 10Bi., above). Note that the reward contingency in the software program should be reversed for each subject.
Critical Step When challenged with a reversal of the reward contingencies, the performance levels of subjects can drop to an accuracy of 20% or less in the initial reversal sessions. These low rates of reinforcement often lead to fewer responses and lower number of completed trials per session. This can cause considerable variation in the number of completed trials per session across animals making accuracy analyses difficult. To account for this, sessions can be limited to 50 trials (rat)/15 trials (mouse) in 60 min, for at least 2 sessions. Continue until subjects can complete all trials in 30 min. Give each subject an even number of these reduced sessions, such that they can be combined into full 100 (or 30)-trial sessions for analysis. If the subject completes fewer trials than required, the missed trials may be added on to the trials required in the next session (if less than ~10), or given in a new session.
iv. Continue Reversal Learning training
for each subject until they attain a criterion of completing all trials with ≥80% accuracy (e.g., for mice, 24 correct responses over 30 trials), not including correction trials, for two consecutive sessions. If post-reversal experimental manipulations are not of interest, we suggest testing subjects for a minimum number of sessions (e.g., 20 for mice) so that a group reversal curve may be plotted. If they are of interest, either advance subjects individually to Step 10Bv when reversal criterion is met, or “rest” animals when they reach criterion, with “reminder” sessions, until the entire group has achieved criterion, then rebaseline before progressing to Step 10Bv (see EXPERIMENTAL DESIGN for details and discussion of alternatives).
v. Post-reversal experimental manipulations
Several options exist, including:
Serial or multiple reversal learning. After initial completion of the reversal phase (Steps 10Bii-iv), repeat the reversal phase to criterion using the same stimuli. Such serial reversals may be used to supplement67 as well as add distinct information96 to that observed for the first reversal. Alternatively, both the discrimination and reversal (Steps 10Bi-iv) may be successively repeated using a novel set of stimuli for each tandem repeat. This latter approach may be particularly useful if within-subject manipulations (e.g., drug treatments) are of interest. Animals should be appropriately counterbalanced across reversal order and experimental treatments.
Stimulus replacement. Replace either the CS+ or the CS− with a novel stimulus during the reversal phase. When performed in conjunction with multiple reversals (above), with appropriate counterbalancing, this approach may help dissociate processes such as perseveration toward the previously rewarded option or avoidance of the previously non-rewarded option that can affect reversal learning performance62 (See EXPERIMENTAL DESIGN)
vi. Data analysis
Analyze performance measures for Visual Discrimination and Reversal Learning phases, separately:
Percentage accuracy, expressed as the number of correct trials divided by the total number of trials multiplied by 100. This may be in the form of an acquisition or reversal curve, if all subjects complete a certain minimum number of sessions, e.g., 5, 10, 20.
Sessions, trials, correct and incorrect responses required to reach criterion.
Average correct and incorrect response latency to respond to the screen after the appearance of stimuli.
Average magazine latency to retrieve reward following a correct response.
Percentage of bias to stimuli and/or response locations (particularly in the first session for each animal).
Perseverative responses, expressed as the average number of correction trials per incorrect response97.
In the reversal phase, other measures can also be analysed:
Number of perseverative and learning errors, split into learning phases classified using various criteria. In some cases, incorrect responses made before achieving > 50 % correct responding in a 10-trial bin is coded as perseverative errors, while incorrect responses made after achieving ≤ 50 % correct responses in a 10-trial bin are coded as learning errors. We generally recommend an approach which defines the perseverative and new learning phases based on chance performance as determined by binomial distribution probabilities (e.g., errors during sessions with < 40 (mouse) or 100 (rat) correct trials are coded as perseverative, and errors during sessions with ≥ 40 (mouse) or 100 (rat) correct trials are coded as “new learning”)67. Moreover, where feasible, a related approach based on signal detection theory can be used to divide reversal learning into 3 phases96. Measures of discrimination (d’) and bias (c) can be calculated and compared to the normal cumulative distribution function (CDF) at two-tailed criterion values (p<0.05). Errors within sessions where CDF(d’) < 0.05 can be coded as perseverative, between 0.05 ≤ CDF(d’) ≤ 0.95 can be coded as chance and CDF(d’) > 0.95 can be coded as learning.
Percent Win-stay and Lose-shift98. Win-stay proportion is expressed as the number of correct responses followed by a second correct response, divided by the total number of rewarded responses. Lose-shift is expressed as the proportion of incorrect responses followed by correct response divided by the total number of incorrect responses.
C) 5-CHOICE SERIAL REACTION TIME TASK
i. 5-CSRT training
Begin training on once-daily sessions of 5-CSRT training, 5-7 days per week. Provide a single free reward (if your program does not do this automatically). Set up the apparatus as detailed for this task in MATERIALS and the software program for this stage with settings as detailed in EXPERIMENTAL DESIGN. Set the appropriate initial testing parameters (see Table 1): stimulus duration, 8 s; ITI, 5 s or 0 s; Limited Hold, 5 s; time out, 5 s. Place each subject in its assigned chamber, and start the session. The session usually finishes either after 60 min or 100 trials (rat)/40-60 trials (mouse) are completed (whichever comes first). After session termination, return each animal to their respective home cage.
Table 1. Troubleshooting.
| Problem | Possible Reason | Solution |
|---|---|---|
| Incomplete consumption of reward | Animal insufficiently habituated to reward | Provide reward in home cage for additional days |
| Animal insufficiently food restricted | Decrease daily food allowance to decrease animal weights as regulations permit | |
| Unstable or poor performance | Low or excessive motivation | Pay closer attention to weight control; consider temporary feeding separation to help equilibrate daily food intake |
| Aversion to mask or touchscreen | Increase exploration of the mask and screen by applying food reward on the mask (e.g. peanut butter, pellets or other) | |
| Excessive fighting in home cage | Monitor home cage and general health of animal. Consider temporary feeding separation or permanent separation of animals (Note, however, that social isolation is a stressor for the animal) | |
| Stressors in housing room (e.g. noise) | Make frequent observations of room and cage, move as necessary | |
| Poor learning ability | Exclusion from the experiment may be necessary | |
| Abrupt decline in performance and/or trial completion | Touchscreen error (e.g. non-responsiveness, not displaying images) | Reboot the system; run test program (if available); check physical connections; clean screen and infrared array; recalibrate screen; contact manufacturer |
| Reward delivery ceased or inconsistent | Check for physical blockage/disconnection; check for computer interface error; replace | |
| Initiation not detected | Clean magazine photobeam; check physical connections; replace if faulty | |
| Controlling system error (software or hardware) | Reboot the system; check physical connections; replace hardware if necessary, contact supplier | |
| Animal appears to make unusually low/high number of locomotor beam crosses (e.g., Campden) | Infrared beam failure | Clean infrared photobeams; check position of infrared emitter and detector; replace faulty beams |
Critical Step If testing the effects of a manipulation conducted before onset of the experiment or task acquisition (Cases 1 and 2, see EXPERIMENTAL DESIGN), ensure that animals in experimental and control groups complete comparable numbers of trials per session throughout 5-CSRT training. Cap the number of trials given per session to accommodate the lowest responders.
Critical Step At the end of each session, record critical data for each subject (e.g., number of correct responses, number of trials completed), in case of computer malfunction. However, most software programs will log many other measures (see data analysis, Step 10C vi).
Critical Step As a visual signal detection task, 5-CSRT performance is sensitive to parameters that affect perception of the visual stimuli (e.g., screen brightness, contrast or glare, ambient chamber illumination, stimulus size and spacing). The precise combination of these parameters may make performance systematically more or less difficult. The performance criterion at this and later stages may thus need to be slightly modified, accordingly.
Critical Step Certain strains or experimental groups of animals may differ in their perceptual abilities. The performance criterion at this and later stages may thus need to be modified, accordingly.
ii. Advance 5-CSRT training stage
When individual subjects attain a criterion of completing all trials with ≥80% response accuracy and ≤20% trial omissions (with 8 s stimulus duration), continue training as in Step 10C i, with a reduced stimulus duration of 4 s.
iii. Advance 5-CSRT training stage
When individual subjects attain a criterion of completing all trials with ≥80% response accuracy and ≤20% trial omissions (with 4 s stimulus duration), continue training as in Step 10C i, with a reduced stimulus duration of 2 s.
iv. Continue 5-CSRT training
5-CSRT training is usually conducted as a precursor to post-training probe manipulations, so it is important to ensure a stable level of performance at this stage. In most experimental cases we suggest synchronising animals before advancing to probes (see EXPERIMENTAL DESIGN). Therefore, when individual subjects attain a criterion of completing all trials with ≥80% response accuracy and ≤20% trial omissions (with 2 s stimulus duration), we suggest “resting” them with “reminder” sessions until the entire group has attained criterion (at least) once, upon which the entire group may be rebaselined before progressing to probes (see EXPERIMENTAL DESIGN for details and alternatives). If subjects are scheduled to receive experimental treatments after training but before probe tests (e.g., Case 3, see EXPERIMENTAL DESIGN), perform these when all animals have attained criterion (at least) once, counterbalancing control and experimental groups according to training performance, then rebaseline. To rebaseline, we suggest continuing training until subjects show stable performance across at least 3 consecutive sessions (≥80% accuracy and ≤20% of omissions).
v. Post-training probes
Depending on the aims of the experiment, different variations of the basic 5-CSRT procedure can be implemented in order to probe aspects of attention and inhibitory control. We describe some of the common manipulations of the basic task. To conduct these post-training probe tests, proceed as in Step 10C iv., but with modifications to the software and/or experimental parameters, as detailed below. Transient treatments may be performed in an appropriately controlled way (e.g., Case 4, Latin square design, EXPERIMENTAL DESIGN).
Variable stimulus durations: The attentional demands of the task may be challenged by reducing the stimulus duration, e.g., from 2 s (baseline stimulus duration) to 1.5, 1, 0.8, 0.6, 0.4 or 0.2 s. A between-session approach can be used wherein a single stimulus duration is presented per session (or 2 consecutive sessions), followed by at least 2 “baseline” sessions with 2 s stimulus duration to ensure that stable baseline performance is reattained before the subsequent challenge sessions. These interpolated baseline sessions also serve to counteract order effects, if stimulus durations are given in sequential (e.g., descending) order, rather than counterbalanced (e.g., Latin square design). Alternatively, a within-session approach can be used wherein several different stimulus durations are presented randomly across trials within a session (e.g., 0.8, 0.6, 0.4 and 0.2 s), with an approximately equal number of presentations of each per session.
Increased trials and session length: To further tax the attentional requirements of the 5-CSRT task, use a predetermined stimulus duration (e.g., 2 s baseline), but increase the number of trials in a session to 100-200 for mice (150-300 for rats), and extend the maximum session duration from 60 min to 90 min.
Variable delays: The attentional demands of the task can be increased by varying the delay prior to target presentation, changing the predictability of target occurrence. This manipulation can also be used to to observe respective alterations in response bias (e.g., trial omissions or premature responding)103. For variable delays, use a predetermined stimulus duration (e.g., 2 s baseline), but decrease (e.g., 2-4 s) or increase (e.g., 8-10 s) the delay period prior to stimulus onset. As for variable stimulus duration above, variable delays can be implemented in a between-session or within-session manner. As animals readily adapt to this manipulation, it is important to space repeated presentations of each probe session by an adequate number of “baseline” sessions (e.g., 3-4).
Reducing stimulus brightness: To increase attentional demand by decreasing the detectability of the stimulus, use a predetermined stimulus duration (e.g., 2 s baseline), but decrease the brightness of the white square stimulus. The contrast of the stimulus may also be altered to similar effect.
Critical Step During probe sessions it is important that subjects in all experimental groups complete comparable numbers of trials. Cap the number of trials given per session to accommodate the lowest responders.
vi. Data analysis
Analyze performance measures for 5-CSRT training and probe performance, for individual sessions and/or the average of multiple consecutive days of a particular trial type.
Accuracy: percentage correct, calculated from all trials on which a response was made to a correct location, divided by the total number of both correct and incorrect trials. This measure is typically used as the main index of attentional control.
Omissions: percentage of all trials on which the animal made no response. Omissions are often used as a measure of global attentional processes but may also be sensitive to differences in sensory, motor or motivational factors.
Correct and Incorrect responses latencies: the time between the stimulus appearing on the screen and the animal making a correct or incorrect touch response. Omitted trials are not included. These latencies are often used to assess cognitive processing speed, but can also be influenced by motivation and motoric factors.
Magazine latency: time to collect reward after a correct response. This variable is used to assess motivation and/or motor control.
Premature responses: touches to the screen during the delay period, prior to the stimulus appearing. Typically used as a measure of “waiting” impulsivity.
Record the number of sessions it takes each subject to reach the criterion performance at each stage of pretraining and for each stimulus duration presented during the 5-CSRT training. This can be particularly useful if gross deficits are seen on main 5-CSRT performance.
Attentional performance across the session can be assessed by splitting the session into 10-trial blocks and calculating percentage accuracy or omissions for each block.
Perseverative correct responses are additional screen touches made after a correct response, prior to collecting the reward; these are typically used as a measure of compulsivity.
Perseverative incorrect responses are additional responses made after an incorrect response, during the time out; these are typically used as a measure of compulsivity.
TIMING
As a rule, allow up to ~80 min (e.g., 60 min testing time, plus an additional 20 minutes for transporting animals from to the testing-room, setting up software, etc.) per testing session from Step 5 onwards. Less time would be required for Extinction sessions (e.g., under 30 mins during Acquisition Steps 10A i-iii), and under 15 mins during Extinction Steps (for mice). Approximate timing for each procedural step is indicated below in number of sessions. These timings are estimates based on our experience, and reflect an average cohort of animals on each stage of the task. These estimates are based on daily training, 5-7 days per week. These estimates will vary depending on several factors, including strain, age, level of food deprivation, and previous testing experience.
Preparation for pretraining, Steps 1-4
~6 or 10 (3+7) days. Timing depends on whether animals are acquired from an external source, in which case a 7 day acclimatization period is required, before onset of food restriction. After acclimatization, allow for approximately 3 days of initial food restriction before start of Stage 1 pretraining. Regular handling and weighing of animals can be started ~2 days after arrival. Reserve an average time per animal per day of ~5 min.
Pretraining, Steps 5-9
~10-15 sessions. Note that pretraining may take longer (e.g., ~10-30 sessions) when a mask with small response windows (e.g., < 3.0 × 3.0 cm) is used and/or if rebaselining is necessary. Also note that full pretraining is only necessary prior to the first instrumental task that an animal is tested on. Before testing on additional touchscreen tasks, animals should usually be tested from the highest pretraining stage previously attained. Being well-trained, they may progress beyond this stage after relatively few sessions.
Extinction (Acquisition phase), Steps 10A i-ii
Option i.-a.: 5-20 sessions; Option i.-b.: 5-10 sessions.
Extinction (Extinction phase), Steps 10Aiii-iv
6-20 sessions.
Extinction (Post-extinction experimental manipulations), Step10Av
These involve a limited number of sessions but some may require periods of up to several weeks without testing prior to the probe.
Visual discrimination acquisition, Steps 10Bi-ii
The average number of sessions required to reach acquisition criterion with standard visual stimuli is ~5-6 (rat)/~8-10 (mouse). Note that additional sessions may be required if “resting” and “rebaselining” are necessary (e.g., before Step 10Biii).
Reversal Learning, Steps 10Biii-iv
To reach criterion during reversal, mice typically require 400-500 trials (13-16 sessions), and Lister Hooded rats approximately 600-700 trials (6-7 sessions).
5-CSRT training, Steps 10Ci-iv
12-30 sessions.
5-CSRT probes, Step 10Cv
For rats, approximately 1-21 sessions. Mice may require more sessions for some of the longer training protocols (e.g., >30) (see PROCEDURE)
TROUBLESHOOTING
For general troubleshooting advice on the touchscreen operant chamber testing method see Table 1. Animals that fail to pass pretraining or acquisition phases, possibly due to poor motivation, impaired vision or severe motoric deficits, cannot be tested in post-acquisition phases, e.g., Extinction and Reversal Learning. Investigators may wish to set a maximum number of sessions to pass these stages (e.g., 20 sessions for Step 2, 20 sessions for Steps 3-4), and exclude, but note and report, animals that fail to reach performance criterion by these thresholds.
High omissions or non-completion of all available trials in 5-CSRT training and probe sessions may indicate a lack of motivation. During training, if you suspect over-feeding may be causing low levels of motivation, you may delay feeding the subject and re-test 2 hours later. If the problem was indeed low motivation, performance should be improved. Having confirmed this, be careful to not to overfeed again and perpetuate the cycle of low motivation the following day.
ANTICIPATED RESULTS
Extinction
For the C57BL/6J mouse strain (or mutant mice crossed to C57BL/6J), acquisition typically takes 5-20 sessions, and extinction less than 10 sessions on average (see Fig. 2d-e and 13). However, these performance profiles can vary dramatically across different mouse strains. For example, another commonly used inbred strain, DBA/2J, exhibits much slower extinction on this task than the C57BL/6J strain. These strain differences should be taken into consideration when assaying extinction in mutant mice that are not backcrossed onto a congenic C57BL/6J background. Such differences could also be a factor when using different rat strains on this task.
Reversal Learning
Figure 3 shows typical reversal performance in rats (e) and mice (f). Note that performance varies depending on strain, age, stimuli used and previous testing experience.
5-CSRT
See Figure 4c-d for examples.
ACKNOWLEDGEMENTS
The protocols described here are those currently used in our laboratory, and were written by current members of the group. However many researchers have contributed to the development of touchscreen tasks and we would like to gratefully acknowledge their contribution. They include Susan Bartko, Jonathan Brigman, Suzanna Forwood, Carolyn Graybeal, Alicia Izquierdo, Louisa Lyon, Anelise Marti, Katie McAllister, Stephanie McTighe, Jess Nithianantharajah, Carola Romberg, John Talpos and Boyer Winters. Thanks also to Dr Martha Hvoslef-Eide for her assistance in creating and modifying the mask schematics and flow charts.
The research leading to these results has received support from the Innovative Medicine Initiative Joint Undertaking under grant agreement n° 115008 of which resources are composed of EFPIA in-kind contribution and financial contribution from the European Union’s Seventh Framework Programme [FP7/2007-2013]; the Wellcome Trust/MRC [089703/Z/09/Z] and Alzheimer’s Research UK [ART/PG2006/5]. A.E.H. receives funding from the European Union Seventh Framework Programme under grant agreements No. 241995 (Project “GENCODYS”) and No. 242167 (Project “SYNSYS”). J.A. was supported by the Swedish Academy of Pharmaceutical Sciences. A.H. was supported by the NIAAA Intramural Research Program (ZIA AA000411).
Footnotes
COMPETING FINANCIAL INTERESTS: L.M.S. and T.J.B. consult for Campden Instruments Ltd. A.E.H. is an employee of Synome Ltd.
Contributor Information
Adam C. Mar, Department of Psychology, University of Cambridge, Cambridge, UK & Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, UK; am682@cam.ac.uk Tel: 01223 766157; Fax: 01223 333564
Alexa E. Horner, Synome Ltd., Moneta Building, Babraham Research Campus, Cambridge, UK & Department of Psychology, University of Cambridge, Cambridge, UK & Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, UK
Simon R.O. Nilsson, Department of Psychology, University of Cambridge, Cambridge, UK & Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, UK
Johan Alsiö, Department of Psychology, University of Cambridge, Cambridge, UK & Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, UK.
Brianne A. Kent, Department of Psychology, University of Cambridge, Cambridge, UK & Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, UK
Chi Hun Kim, Department of Psychology, University of Cambridge, Cambridge, UK & Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, UK.
Andrew Holmes, Laboratory of Behavioral and Genomic Neuroscience, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, Maryland, USA.
Lisa M. Saksida, Department of Psychology, University of Cambridge, Cambridge, UK & Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, UK
Timothy J. Bussey, Department of Psychology, University of Cambridge, Cambridge, UK & Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, UK
REFERENCES
- 1.Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol. Rev. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- 2.Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyake A, et al. The Unity and Diversity of Executive Functions and Their Contributions to Complex ‘Frontal Lobe’ Tasks: A Latent Variable Analysis. Cognit. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 4.Robbins TW, Arnsten AFT. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mar AC, Dalley JW. Cognition: Attention and impulsivity. In: Koob GF, Le Moal M, Thompson RF, editors. Encycl. Behav. Neurosci. Vol. 1. Academic Press; 2010. pp. 262–271. [Google Scholar]
- 6.Roca M, et al. Executive function and fluid intelligence after frontal lobe lesions. Brain J. Neurol. 2010;133:234–247. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott R. Executive functions and their disorders. Br. Med. Bull. 2003;65:49–59. doi: 10.1093/bmb/65.1.49. [DOI] [PubMed] [Google Scholar]
- 8.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chudasama Y. Animal models of prefrontal-executive function. Behav. Neurosci. 2011;125:327–343. doi: 10.1037/a0023766. [DOI] [PubMed] [Google Scholar]
- 10.Robbins TW. Dissociating executive functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1996;351:1463–1470. doi: 10.1098/rstb.1996.0131. Discussion 1470–1471. [DOI] [PubMed] [Google Scholar]
- 11.Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 13.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacol. 2009;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadman KK, Yang M, Crawley JN. Criteria for validating mouse models of psychiatric diseases. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009;150B:1–11. doi: 10.1002/ajmg.b.30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeler JF, Robbins TW. Translating cognition from animals to humans. Biochem. Pharmacol. 2011;81:1356–1366. doi: 10.1016/j.bcp.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Homberg JR. Measuring behaviour in rodents: towards translational neuropsychiatric research. Behav. Brain Res. 2013;236:295–306. doi: 10.1016/j.bbr.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Berg EA. A simple objective technique for measuring flexibility in thinking. J. Gen. Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- 19.Shallice T. Specific impairments of planning. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 20.Stroop RJ. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- 21.Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- 22.Rosvold H, et al. A continuous performance test of brain damage. J. Consult. Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- 23.Roberts AC, Robbins TW, Everitt BJ. The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. Q. J. Exp. Psychol. B. 1988;40:321–341. [PubMed] [Google Scholar]
- 24.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olton DS, Samuelson RJ. Remembrance of places passed: Spatial memory in rats. J. Exp. Psychol. Anim. Behav. Process. 1976;2:97–116. [Google Scholar]
- 26.Krechevsky I. Antagonistic visual discrimination habits in the white rat. J. Comp. Psychol. 1932;14:263–277. [Google Scholar]
- 27.Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav. Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- 28.Bussey TJ, Muir JL, Robbins TW. A novel automated touchscreen procedure for assessing learning in the rat using computer graphic stimuli. Neurosci. Res. Commun. 1994;15:103–110. [Google Scholar]
- 29.Bussey TJ, et al. The touchscreen cognitive testing method for rodents: How to get the best out of your rat. Learn. Mem. 2008;15:516–523. doi: 10.1101/lm.987808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bussey TJ, et al. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–1203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horner AE, et al. The touchscreen operant platform for testing learning and memory in rats and mice. Nat. Protoc. 2013;8(10):1961–84. doi: 10.1038/nprot.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlov IP. Conditioned reflexes. Oxford University Press; 1927. [Google Scholar]
- 33.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 34.Myers KM, Davis M. Mechanisms of fear extinction. Mol. Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 35.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci. Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 36.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacol. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn. Mem. Cold Spring Harb. N. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn. Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Brigman JL, et al. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn. Mem. Cold Spring Harb. N. 2008;15:50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlsson R-M, et al. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacol. 2009;34:1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barkus C, et al. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2012;62:1263–1272. doi: 10.1016/j.neuropharm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nithianantharajah J, et al. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat. Neurosci. 2013;16:16–24. doi: 10.1038/nn.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romberg C, Horner AE, Bussey TJ, Saksida LM. A touch screen-automated cognitive test battery reveals impaired attention, memory abnormalities, and increased response inhibition in the TgCRND8 mouse model of Alzheimer’s disease. Neurobiol. Aging. 2013;34:731–744. doi: 10.1016/j.neurobiolaging.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hefner K, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J. Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lederle L, et al. Reward-related behavioral paradigms for addiction research in the mouse: performance of common inbred strains. Plos One. 2011;6:e15536. doi: 10.1371/journal.pone.0015536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer AC, et al. Genetics of novelty seeking, amphetamine self-administration and reinstatement using inbred rats. Genes Brain Behav. 2010;9:790–798. doi: 10.1111/j.1601-183X.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lattal KM, Lattal KA. Facets of Pavlovian and operant extinction. Behav. Processes. 2012;90:1–8. doi: 10.1016/j.beproc.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouton ME. Context and behavioral processes in extinction. Learn. Mem. Cold Spring Harb. N. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 50.Leeson VC, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol. Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb. Cortex New York N 1991. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 52.Remijnse PL, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2006;63:1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- 53.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 54.Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol. Learn. Mem. 2008;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Mar AC, Walker ALJ, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J. Neurosci. 2011;31:6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 57.Riceberg JS, Shapiro ML. Reward stability determines the contribution of orbitofrontal cortex to adaptive behavior. J. Neurosci. 2012;32:16402–16409. doi: 10.1523/JNEUROSCI.0776-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- 59.Walton ME, Behrens TEJ, Buckley MJ, Rudebeck PH, Rushworth MFS. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J. Cogn. Neurosci. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- 61.Rogers RD, et al. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology. 1999;146:482–491. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- 62.Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb. Cortex New York N 1991. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 63.Bari A, et al. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacol. 2010;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cools R, Lewis SJG, Clark L, Barker RA, Robbins TW. L-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson’s disease. Neuropsychopharmacol. 2007;32:180–189. doi: 10.1038/sj.npp.1301153. [DOI] [PubMed] [Google Scholar]
- 65.Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus. J. Neurosci. 2011;31:4290–4297. doi: 10.1523/JNEUROSCI.5066-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clatworthy PL, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J. Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J. Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graybeal C, et al. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat. Neurosci. 2011;14:1507–1509. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav. Neurosci. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- 70.Izquierdo A, et al. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav. Brain Res. 2006;171:181–188. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 71.Brigman JL, et al. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb. Cortex New York N 1991. 2010;20:1955–1963. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leonard JA. Five-choice serial reaction apparatus. Med. Res. Coun. Appl. Psychol. Res. Unit. 1959;326 [Google Scholar]
- 73.Humby T, Laird FM, Davies W, Wilkinson LS. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur. J. Neurosci. 1999;11:2813–2823. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- 74.Humby T, Wilkinson L, Dawson G. Assaying aspects of attention and impulse control in mice using the 5-choice serial reaction time task. Curr. Protoc. Neurosci. 2005 doi: 10.1002/0471142301.ns0805hs31. Chapter 8, Unit 8.5H. [DOI] [PubMed] [Google Scholar]
- 75.Young JW, et al. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacol. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- 76.Patel S, Stolerman IP, Asherson P, Sluyter F. Attentional performance of C57BL/6 and DBA/2 mice in the 5-choice serial reaction time task. Behav. Brain Res. 2006;170:197–203. doi: 10.1016/j.bbr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 77.Lambourne SL, et al. Impairments in impulse control in mice transgenic for the human FTDP-17 tauV337M mutation are exacerbated by age. Hum. Mol. Genet. 2007;16:1708–1719. doi: 10.1093/hmg/ddm119. [DOI] [PubMed] [Google Scholar]
- 78.Siegel JA, Benice TS, Van Meer P, Park BS, Raber J. Acetylcholine receptor and behavioral deficits in mice lacking apolipoprotein E. Neurobiol. Aging. 2011;32:75–84. doi: 10.1016/j.neurobiolaging.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan TC, et al. Performance deficits of NK1 receptor knockout mice in the 5-choice serial reaction-time task: effects of d-amphetamine, stress and time of day. Plos One. 2011;6:e17586. doi: 10.1371/journal.pone.0017586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fletcher PJ, Soko AD, Higgins GA. Impulsive action in the 5-choice serial reaction time test in 5-HT c receptor null mutant mice. Psychopharmacology. 2013;226:561–570. doi: 10.1007/s00213-012-2929-0. [DOI] [PubMed] [Google Scholar]
- 81.Oliver YP, Ripley TL, Stephens DN. Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology. 2009;204:679–692. doi: 10.1007/s00213-009-1500-0. [DOI] [PubMed] [Google Scholar]
- 82.Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat. Protoc. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- 83.Romberg C, Mattson MP, Mughal MR, Bussey TJ, Saksida LM. Impaired attention in the 3xTgAD mouse model of Alzheimer’s disease: rescue by donepezil (Aricept) J. Neurosci. 2011;31:3500–3507. doi: 10.1523/JNEUROSCI.5242-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartko SJ, et al. Intact attentional processing but abnormal responding in M1 muscarinic receptor-deficient mice using an automated touchscreen method. Neuropharmacology. 2011;61:1366–1378. doi: 10.1016/j.neuropharm.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McTighe SM, Neal SJ, Lin Q, Hughes ZA, Smith DG. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. Plos One. 2013;8:e62189. doi: 10.1371/journal.pone.0062189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oomen CA, et al. The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat. Protoc. 2013;8(10):2006–21. doi: 10.1038/nprot.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morton AJ, Skillings E, Bussey TJ, Saksida LM. Measuring cognitive deficits in disabled mice using an automated interactive touchscreen system. Nat. Methods. 2006;3:767. doi: 10.1038/nmeth1006-767. [DOI] [PubMed] [Google Scholar]
- 88.Bouton ME, Winterbauer NE, Todd TP. Relapse processes after the extinction of instrumental learning: renewal, resurgence, and reacquisition. Behav. Processes. 2012;90:130–141. doi: 10.1016/j.beproc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bouton ME, King DA. Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J. Exp. Psychol. Anim. Behav. Process. 1983;9:248–265. [PubMed] [Google Scholar]
- 90.Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–758. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- 91.Talpos JC, Fletcher AC, Circelli C, Tricklebank MD, Dix SL. The pharmacological sensitivity of a touchscreen-based visual discrimination task in the rat using simple and perceptually challenging stimuli. Psychopharmacology. 2012;221:437–449. doi: 10.1007/s00213-011-2590-z. [DOI] [PubMed] [Google Scholar]
- 92.Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav. Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- 93.Meziane H, Ouagazzal A-M, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 94.Clelland CD, et al. A Functional Role for Adult Hippocampal Neurogenesis in Spatial Pattern Separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cardinal RN, Aitken MRF. Whisker: a client-server high-performance multimedia research control system. Behav. Res. Methods. 2010;42:1059–1071. doi: 10.3758/BRM.42.4.1059. [DOI] [PubMed] [Google Scholar]
- 96.Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 97.Chudasama Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur. J. Neurosci. 2001;14:1009–1020. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- 98.Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J. Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Izquierdo A, et al. Basolateral Amygdala Lesions Facilitate Reward Choices after Negative Feedback in Rats. J. Neurosci. 2013;33:4105–4109. doi: 10.1523/JNEUROSCI.4942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kosheleff AR, Rodriguez D, O’Dell SJ, Marshall JF, Izquierdo A. Comparison of single-dose and extended methamphetamine administration on reversal learning in rats. Psychopharmacology. 2012;224:459–467. doi: 10.1007/s00213-012-2774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology. 1998;138:266–274. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- 102.Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology. 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- 103.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]