Abstract
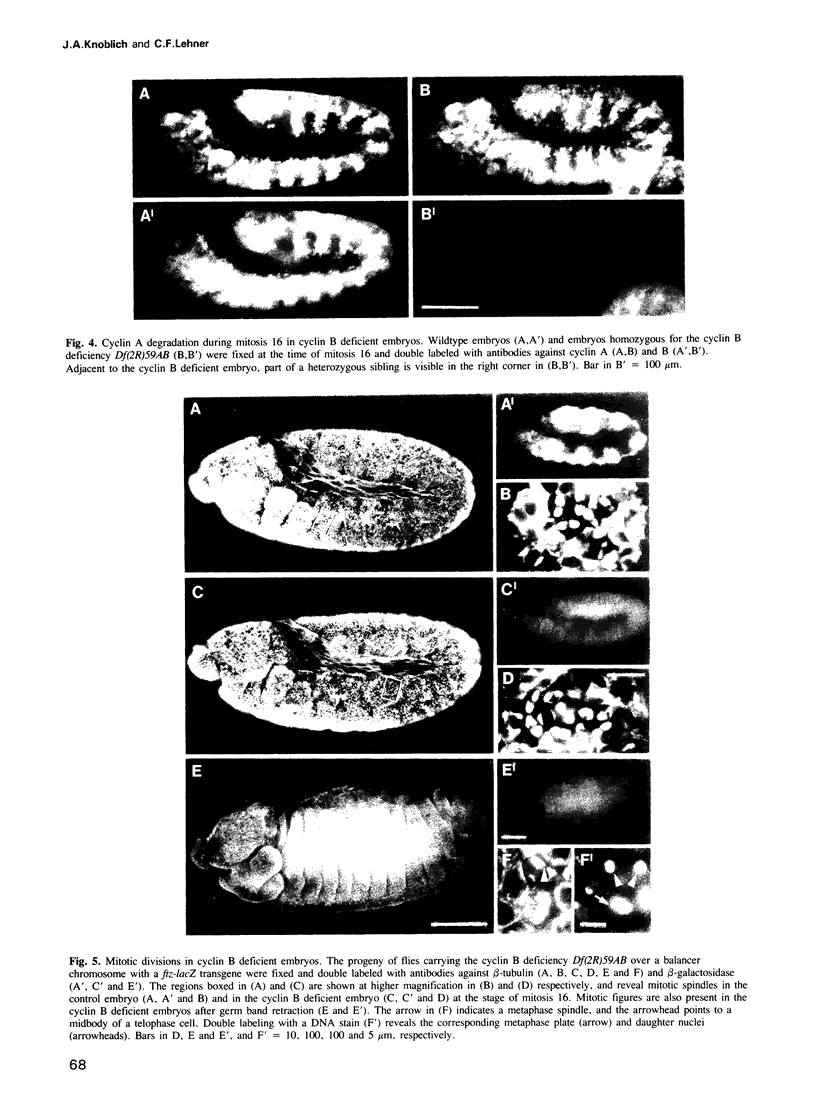
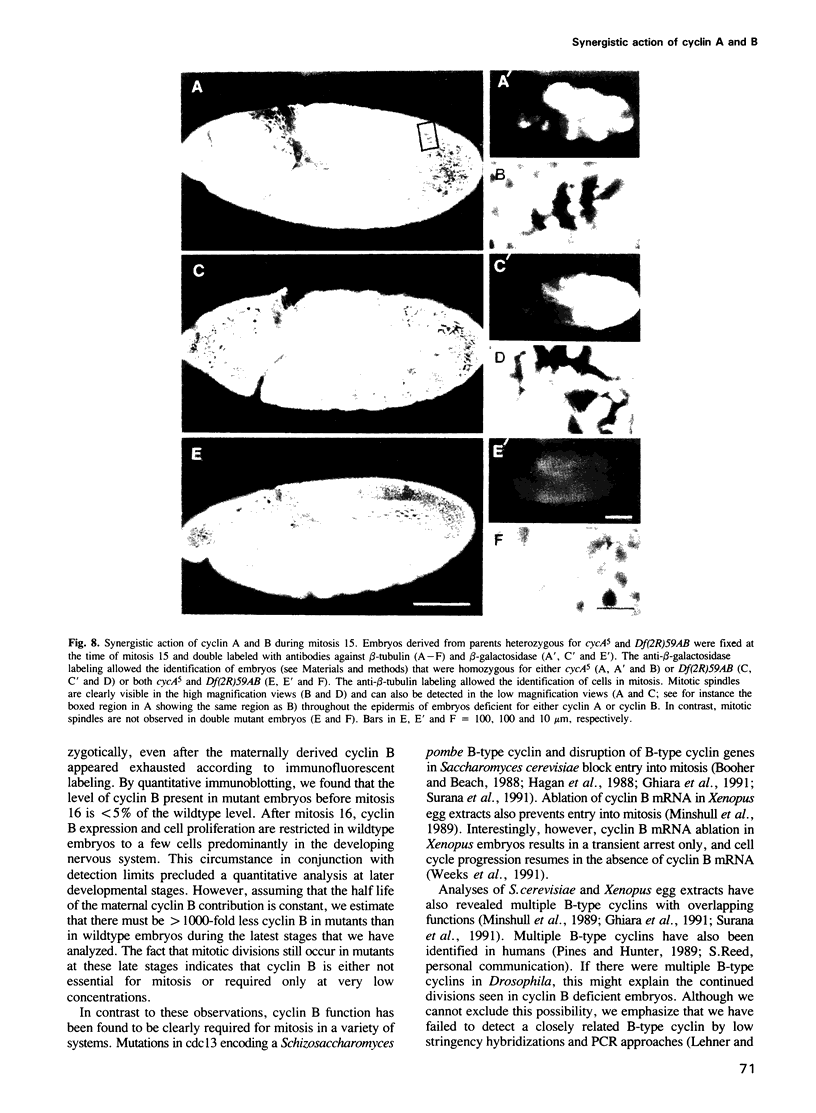
A variety of different cyclin proteins have been identified in higher eukaryotes. In the case of cyclin B, functional analyses have clearly demonstrated an important role in the control of entry into mitosis. The function of cyclin A is more complex. It appears to function in the control of both S- and M-phase. The results of our genetic analyses in Drosophila demonstrate that cyclin A has a mitotic function and that it acts synergistically with cyclin B during the G2-M transition. In double mutant embryos that express neither cyclin A nor cyclin B zygotically, cell cycle progression is blocked just before the exhaustion of the maternally contributed cyclin A and B stores. BrdU-labeling experiments indicate that cell cycle progression is blocked in G2 before entry into the fifteenth round of mitosis. Expression of either cyclin A or B from heat-inducible transgenes is sufficient to overcome this cell cycle block. This block is also not observed in single mutant embryos deficient for either cyclin A or B. In cyclin B deficient embryos, cell cycle progression continues after the apparent exhaustion of the maternal contribution, suggesting that cyclin B might not be essential for mitosis. However, mitotic spindles are clearly abnormal and progression through mitosis is delayed in these cyclin B deficient embryos.
Full text
PDF
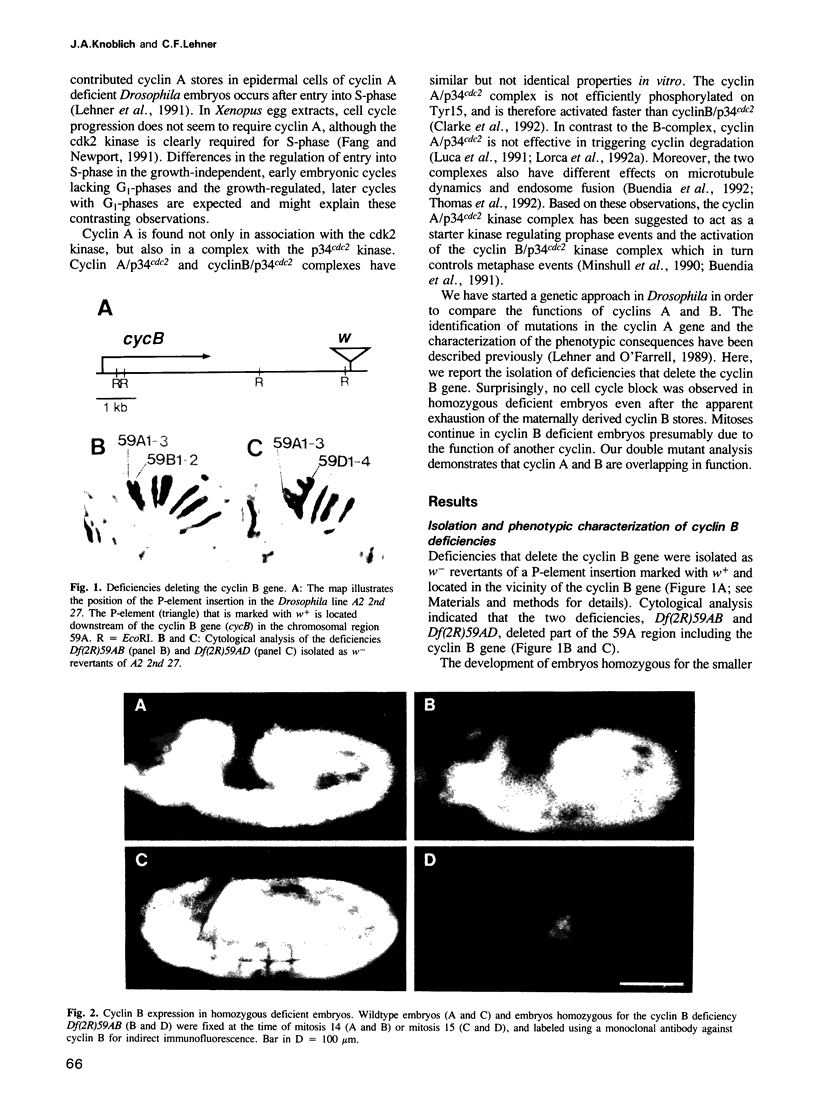
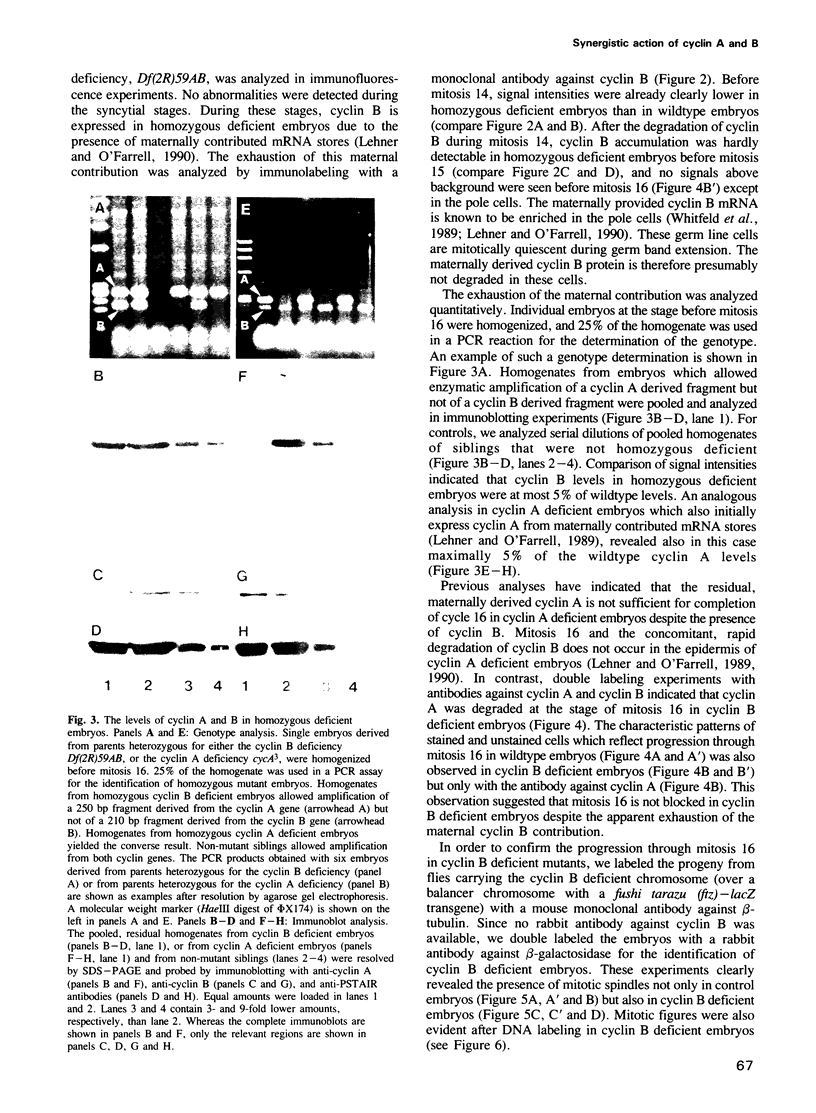





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booher R., Beach D. Involvement of cdc13+ in mitotic control in Schizosaccharomyces pombe: possible interaction of the gene product with microtubules. EMBO J. 1988 Aug;7(8):2321–2327. doi: 10.1002/j.1460-2075.1988.tb03075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buendia B., Clarke P. R., Félix M. A., Karsenti E., Leiss D., Verde F. Regulation of protein kinases associated with cyclin A and cyclin B and their effect on microtubule dynamics and nucleation in Xenopus egg extracts. Cold Spring Harb Symp Quant Biol. 1991;56:523–532. doi: 10.1101/sqb.1991.056.01.059. [DOI] [PubMed] [Google Scholar]
- Buendia B., Draetta G., Karsenti E. Regulation of the microtubule nucleating activity of centrosomes in Xenopus egg extracts: role of cyclin A-associated protein kinase. J Cell Biol. 1992 Mar;116(6):1431–1442. doi: 10.1083/jcb.116.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. R., Leiss D., Pagano M., Karsenti E. Cyclin A- and cyclin B-dependent protein kinases are regulated by different mechanisms in Xenopus egg extracts. EMBO J. 1992 May;11(5):1751–1761. doi: 10.1002/j.1460-2075.1992.tb05227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto S. H., Mudryj M., Pines J., Hunter T., Nevins J. R. A cyclin A-protein kinase complex possesses sequence-specific DNA binding activity: p33cdk2 is a component of the E2F-cyclin A complex. Cell. 1992 Jan 10;68(1):167–176. doi: 10.1016/0092-8674(92)90215-x. [DOI] [PubMed] [Google Scholar]
- Ducommun B., Brambilla P., Félix M. A., Franza B. R., Jr, Karsenti E., Draetta G. cdc2 phosphorylation is required for its interaction with cyclin. EMBO J. 1991 Nov;10(11):3311–3319. doi: 10.1002/j.1460-2075.1991.tb04895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991 Oct 4;67(1):189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Edgar B. A., O'Farrell P. H. The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string. Cell. 1990 Aug 10;62(3):469–480. doi: 10.1016/0092-8674(90)90012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Newport J. W. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991 Aug 23;66(4):731–742. doi: 10.1016/0092-8674(91)90117-h. [DOI] [PubMed] [Google Scholar]
- Featherstone C., Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991 Feb 28;349(6312):808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Gautier J., Solomon M. J., Booher R. N., Bazan J. F., Kirschner M. W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991 Oct 4;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Ghiara J. B., Richardson H. E., Sugimoto K., Henze M., Lew D. J., Wittenberg C., Reed S. I. A cyclin B homolog in S. cerevisiae: chronic activation of the Cdc28 protein kinase by cyclin prevents exit from mitosis. Cell. 1991 Apr 5;65(1):163–174. doi: 10.1016/0092-8674(91)90417-w. [DOI] [PubMed] [Google Scholar]
- Girard F., Strausfeld U., Fernandez A., Lamb N. J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991 Dec 20;67(6):1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Moreno S., Owen D. J., Sazer S., Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991 Nov;10(11):3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Hagan I., Hayles J., Nurse P. Cloning and sequencing of the cyclin-related cdc13+ gene and a cytological study of its role in fission yeast mitosis. J Cell Sci. 1988 Dec;91(Pt 4):587–595. doi: 10.1242/jcs.91.4.587. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hoogwerf A. M., Akam M., Roberts D. A genetic analysis of the rose-gespleten region (68C8-69B5) of Drosophila melanogaster. Genetics. 1988 Apr;118(4):665–670. doi: 10.1093/genetics/118.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongens T. A., Hay B., Jan L. Y., Jan Y. N. The germ cell-less gene product: a posteriorly localized component necessary for germ cell development in Drosophila. Cell. 1992 Aug 21;70(4):569–584. doi: 10.1016/0092-8674(92)90427-e. [DOI] [PubMed] [Google Scholar]
- Koff A., Cross F., Fisher A., Schumacher J., Leguellec K., Philippe M., Roberts J. M. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991 Sep 20;66(6):1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Differential phosphorylation of vertebrate p34cdc2 kinase at the G1/S and G2/M transitions of the cell cycle: identification of major phosphorylation sites. EMBO J. 1991 Feb;10(2):305–316. doi: 10.1002/j.1460-2075.1991.tb07951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Mutations of p34cdc2 phosphorylation sites induce premature mitotic events in HeLa cells: evidence for a double block to p34cdc2 kinase activation in vertebrates. EMBO J. 1991 Nov;10(11):3331–3341. doi: 10.1002/j.1460-2075.1991.tb04897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W. G. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992 Jul 10;70(1):139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Lahue E. E., Smith A. V., Orr-Weaver T. L. A novel cyclin gene from Drosophila complements CLN function in yeast. Genes Dev. 1991 Dec;5(12A):2166–2175. doi: 10.1101/gad.5.12a.2166. [DOI] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989 Mar 24;56(6):957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990 May 4;61(3):535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., Yakubovich N., O'Farrell P. H. Exploring the role of Drosophila cyclin A in the regulation of S phase. Cold Spring Harb Symp Quant Biol. 1991;56:465–475. doi: 10.1101/sqb.1991.056.01.053. [DOI] [PubMed] [Google Scholar]
- Leptin M., Grunewald B. Cell shape changes during gastrulation in Drosophila. Development. 1990 Sep;110(1):73–84. doi: 10.1242/dev.110.1.73. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Lorca T., Labbé J. C., Devault A., Fesquet D., Capony J. P., Cavadore J. C., Le Bouffant F., Dorée M. Dephosphorylation of cdc2 on threonine 161 is required for cdc2 kinase inactivation and normal anaphase. EMBO J. 1992 Jul;11(7):2381–2390. doi: 10.1002/j.1460-2075.1992.tb05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T., Labbé J. C., Devault A., Fesquet D., Strausfeld U., Nilsson J., Nygren P. A., Uhlen M., Cavadore J. C., Dorée M. Cyclin A-cdc2 kinase does not trigger but delays cyclin degradation in interphase extracts of amphibian eggs. J Cell Sci. 1992 May;102(Pt 1):55–62. doi: 10.1242/jcs.102.1.55. [DOI] [PubMed] [Google Scholar]
- Luca F. C., Shibuya E. K., Dohrmann C. E., Ruderman J. V. Both cyclin A delta 60 and B delta 97 are stable and arrest cells in M-phase, but only cyclin B delta 97 turns on cyclin destruction. EMBO J. 1991 Dec;10(13):4311–4320. doi: 10.1002/j.1460-2075.1991.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren K., Walworth N., Booher R., Dembski M., Kirschner M., Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991 Mar 22;64(6):1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- Léopold P., O'Farrell P. H. An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient in G1 cyclins. Cell. 1991 Sep 20;66(6):1207–1216. doi: 10.1016/0092-8674(91)90043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. B., McGowan C. H., Lenaers G., Jones R., Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991 Dec;10(13):4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Blow J. J., Hunt T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell. 1989 Mar 24;56(6):947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Minshull J., Golsteyn R., Hill C. S., Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990 Sep;9(9):2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Norbury C., Blow J., Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991 Nov;10(11):3321–3329. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992 Mar;11(3):961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L. L., Atherton-Fessler S., Lee M. S., Ogg S., Falk J. L., Swenson K. I., Piwnica-Worms H. Cyclin promotes the tyrosine phosphorylation of p34cdc2 in a wee1+ dependent manner. EMBO J. 1991 May;10(5):1255–1263. doi: 10.1002/j.1460-2075.1991.tb08067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991 Oct;115(1):1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J., Gu Y., Morgan D. O. Human cyclin-dependent kinase 2 is activated during the S and G2 phases of the cell cycle and associates with cyclin A. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2824–2828. doi: 10.1073/pnas.89.7.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy L. M., Swenson K. I., Walker D. H., Gabrielli B. G., Li R. S., Piwnica-Worms H., Maller J. L. Activation of p34cdc2 kinase by cyclin A. J Cell Biol. 1991 May;113(3):507–514. doi: 10.1083/jcb.113.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987 May 22;49(4):559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Shirodkar S., Ewen M., DeCaprio J. A., Morgan J., Livingston D. M., Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992 Jan 10;68(1):157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. Cyclin activation of p34cdc2. Cell. 1990 Nov 30;63(5):1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Lee T., Kirschner M. W. Role of phosphorylation in p34cdc2 activation: identification of an activating kinase. Mol Biol Cell. 1992 Jan;3(1):13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld U., Labbé J. C., Fesquet D., Cavadore J. C., Picard A., Sadhu K., Russell P., Dorée M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature. 1991 May 16;351(6323):242–245. doi: 10.1038/351242a0. [DOI] [PubMed] [Google Scholar]
- Surana U., Robitsch H., Price C., Schuster T., Fitch I., Futcher A. B., Nasmyth K. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell. 1991 Apr 5;65(1):145–161. doi: 10.1016/0092-8674(91)90416-v. [DOI] [PubMed] [Google Scholar]
- Thomas L., Clarke P. R., Pagano M., Gruenberg J. Inhibition of membrane fusion in vitro via cyclin B but not cyclin A. J Biol Chem. 1992 Mar 25;267(9):6183–6187. [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Walder J. A., Dagle J. M. Cyclin B mRNA depletion only transiently inhibits the Xenopus embryonic cell cycle. Development. 1991 Apr;111(4):1173–1178. doi: 10.1242/dev.111.4.1173. [DOI] [PubMed] [Google Scholar]
- Whitfield W. G., González C., Sánchez-Herrero E., Glover D. M. Transcripts of one of two Drosophila cyclin genes become localized in pole cells during embryogenesis. Nature. 1989 Mar 23;338(6213):337–340. doi: 10.1038/338337a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- Zindy F., Lamas E., Chenivesse X., Sobczak J., Wang J., Fesquet D., Henglein B., Bréchot C. Cyclin A is required in S phase in normal epithelial cells. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1144–1154. doi: 10.1016/0006-291x(92)91851-g. [DOI] [PubMed] [Google Scholar]