Abstract
Pituitary-secreted luteinizing hormone (LH) induces ovulation by activating an extracellular regulated kinase 1/2 (ERK1/2) cascade. However, little is known regarding the ERK1/2 downstream effectors that are involved in regulating rapid, transient expression of LH-target gene in ovulatory follicles. By comparing the gene expression profiles of LH-stimulated wild type with ERK1/2-deleted ovarian granulosa cells (GCs), we identified Cited4 as a previously unknown LH target gene during ovulation. LH induced Cited4 expression in pre-ovulatory follicles in an ERK1/2-dependent manner. CITED4 formed an endogenous protein complex and docked on the promoters of LH and ERK1/2 target genes. Both CITED4 expression and CBP acetyltransferase activity leading to histone acetylation were indispensable for LH-induced ovulation-related events. LH induced dynamic histone acetylation changes in pre-ovulatory GCs, including the acetylation of histone H2B (Lys5) and H3 (Lys9). This was essential for the rapid responses and dramatic increases of LH target gene expressions by the ordered activation of ERK1/2 and CITED4-CBP. In addition, histone deacetylases (HDACs) antagonized CITED4-CBP to turn off expression of these genes after exposure to LH. Thus, we determined that CITED4 was a novel LH and ERK1/2 target for triggering ovulation. These results support the proposition that LH induces rapid, significant gene expression in pre-ovulatory follicles by modulating histone acetylation status.
Keywords: follicle, ovulation, CITED4, MAPK signaling pathway, granulosa cell
Introduction
Ovulation is the culmination of female reproductive activity and is essential for successful pregnancy. Most infertility in women is caused by ovulation defects. In mammals, ovulation is triggered by the pituitary gonadotrophin, luteinizing hormone (LH). In response to LH stimulation, the oocytes within fully-grown ovarian follicles resume meiotic maturation. Simultaneously, the cumulus cell masses that surround such oocytes accumulate an extracellular matrix and swell. This is known as cumulus-oocyte complex (COC) expansion. After follicle rupture, fully expanded COCs are released into the oviduct where they await fertilization. The remaining granulosa cells (GCs) of ruptured follicles terminally differentiate into luteal cells and produce progesterone to prepare for establishing pregnancy. Although LH induces all of these significant ovarian histological changes, the underlying molecular mechanisms are only now beginning to be understood.
LH induces the mural GCs of pre-ovulatory follicles to secret three epidermal growth factor (EGF) family proteins, amphiregulin (AREG), epiregulin (EREG) and beta-cellulin (BTC), which are intra-follicular mediators of LH actions (Park et al., 2004). Using GC-specific gene knock-out and knock-in mouse models, we demonstrated that, under physiological conditions, the extracellular regulated kinase (ERK)-1/2 cascade mediated LH and EGF-like factors' actions during ovulation (Shimada et al., 2006; Fan et al., 2009). GC-specific ERK1/2 knockout completely blocked LH-triggered oocyte maturation, COC expansion, follicle rupture and luteinization (Fan et al., 2009). Furthermore, we identified the transcription factors CCAAT/enhancer binding protein C/EBPα and β as essential downstream targets of the RAS-ERK1/2 signaling pathway during the triggering of ovulation and luteinization (Sirois and Richards, 1993; Fan et al., 2009, 2011). GC-specific Cebpa/b knockout female mice did not ovulate and were completely infertile. However, LH could still trigger some early ovulation-related events in Cebpa/b deleted follicles, including delayed oocyte germinal vesicle breakdown and compromised the extent of COC expansion (Fan et al., 2011). These findings indicated that C/EBPα and β were not the only mediators of ERK1/2 actions in pre-ovulatory follicles.
In addition, it remains unknown how ERK1/2 rapidly induces ovulation-related gene expression. Previous studies showed that, within 4 h, >300 genes were up-regulated by more than 4-fold in pre-ovulatory follicles due to LH (Hernandez-Gonzalez et al., 2006; Fan et al., 2009). For some genes that played very important roles during ovulation, their mRNA expression levels increased by several hundred or even a 1000-fold during this short period of time. We hypothesized that, in addition to activating several transcription factors including C/EBPα/β, ERK1/2 must rely on a more robust transcription regulating machinery to trigger LH target gene expression in pre-ovulatory follicles.
In many cell types, the transcription co-activators and histone acetyltransferases p300 and CREB-binding protein (CBP) are recruited to gene promoters by various DNA-bound transcription factors, including C/EBPβ, which results in gene transcription activation (Holmqvist and Mannervik, 2013). Previous studies showed that a CBP/p300-interacting trans-activator with ED-rich tail (CITED) proteins and CBP were involved in endogenous naturally occurring complexes (Braganca et al., 2002). Members of the CITED family bound to CBP/p300 with high affinity and, as co-activators, regulated gene transcription and function (Yahata et al., 2002).
The nucleosome comprises four core histone proteins, H2A, H2B, H3 and H4, and is the primary chromatin building block and genuine CBP/p300 target (LaVoie, 2005). CBP/p300 histone acetyltransferases acetylate multiple lysine residues in the amino terminal tail of histone H2B (Lys5, 12, 15 and 20) at gene promoters during transcription activation (Peterson and Laniel, 2004). Hyper-acetylation of these histone tails neutralizes the positive charge of these domains and is believed to weaken histone-DNA and nucleosome–nucleosome interactions, there by destabilizing chromatin structure and increasing DNA access to various DNA-binding proteins (Workman and Kingston, 1998). However, it remains uncertain if CBP/p300-mediated histone acetylation plays a key role in ovulating follicles.
In this study, we found that LH induced Cited4 expression in pre-ovulatory follicles in an ERK1/2-dependent manner. CITED4 formed an endogenous protein complex with CBP and C/EBP/β, which docked on the promoters of LH and ERK1/2 target genes. Both CITED4 expression and CBP acetyltransferase activity were indispensable for ovulation-related molecular and histological events. We also detected dynamic histone acetylation changes (histone H2B-Lys5 and H3-Lys9) in GCs that were regulated by LH, CBP and histone deacetylases (HDACs) during ovulation. Thus, we identified CITED4 as a novel LH and ERK1/2 target for triggering ovulation. Therefore, we propose that LH induces rapid, significant gene expression in pre-ovulatory follicles by modulating histone acetylation status.
Materials and Methods
Mice
Wild-type C57/BL6 mice were obtained from the Center of Experimental Animals, Zhejiang University. Mice with GC-specific knockout of Erk1/2 (Erk1/2gc−/−) were previously reported (Fan et al., 2009). Animals were housed under a 14:10 h, light: dark schedule, provided food and water ad libitum, and were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
To study ovarian responses to exogenous gonadotrophins, female mice at post-natal day (PD) 23 were analyzed to avoid the complexity of ovarian functions associated with estrous cycles and endogenous surges of gonadotrophins. Immature mice were injected intraperitoneally with 5 IU pregnant mare serum gonadotrophin (PMSG; Merck, Billerica, MA, USA) to stimulate pre-ovulatory follicle development followed 48 h later with 5 IU human chorionic gonadotrophin (hCG, American Pharmaceutical Partners, Schaumburg, IL, USA) to stimulate ovulation.
For C646 treatments, female mice at PD23 were injected intraperitoneally with 5 IU PMSG followed 48 h later with 5 IU hCG alone (as control) or 5 IU hCG plus 0.05 µmol C646/mouse, which was dissolved in 0.1 ml saline. The dosage used was based on our experiment results in cultured GCs. Mice were sacrificed at 8 and 16 h post-hCG (n = 6 for each time point), and ovaries were isolated for histological analyses.
Granulosa cell culture
GCs were harvested from PMSG-primed (24 h), PD23 mice as described previously (Fan et al., 2010). Briefly, GCs were released from antral follicles by puncturing with a 26.5 gauge needle. Cells were cultured at a density of 1 × 106 cells in Dulbecco modified Eagle's Medium (DMEM)/F12 medium (Invitrogen) containing 5% fetal bovine serum (FBS) (Invitrogen), 100 U/ml penicillin and 100 µg/ml streptomycin in 24-well culture dishes. After overnight culture, cells were washed and cultured in serum-free medium before any further treatments.
To induce the expression of LH target genes in vitro, granulosa cells were treated with forskolin (10 µM) plus phorbol 12-myristate 13-acetate (PMA, 20 nM) for 4–24 h (Doyle et al., 2004).
RNA isolation and real-time RT–PCR
Total RNA was extracted using Trizol (Invitrogen) according to the manufacturer's instructions. Real-time RT–PCR analysis used Q Tag SYBR Green Master Mix (Becton Dickinson Medical Devices Co., Ltd, USA) and an Applied Biosystems 7500 Real-Time PCR System. Relative mRNA levels were calculated by normalizing to the levels of endogenous β-actin mRNA (used as an internal control) using Microsoft EXCEL®. For each indicated gene, the relative transcript level of the control sample (left-hand bar of each graph) was set as 1. The relative transcript levels of other samples were compared with the control, and the fold-changes shown in the graph. For each experiment, qPCR reactions were done in triplicate. Primer sequences are available upon request to the authors.
Western blot analysis
Protein extracts were dissolved in SDS sample buffer. Protein lysates (30 µg total protein per lane) were separated by SDS–PAGE and electrophoretically transferred to PVDF membranes (Millipore Corp., Bedford, MA, USA). After probing with primary antibodies overnight at 4°C, the membranes were washed in TBST and incubated with an HRP-linked secondary antibody. Finally, bands on the membranes were detected using an Enhanced Chemiluminescence Detection Kit (Amersham). The primary antibodies used were: anti-CBP (SC-583), anti-C/EBPα (SC-61), anti-C/EBPβ (SC-150) and anti-actin (SC-1616) from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The C/EBPβ antibody used in this study is a polyclonal antibody that recognized both long and short isoforms of C/EBPβ. Anti-acetylated-H2B-Lys5 (#2799) and -H3-Lys9 (#4353) antibodies were purchased from Cell Signaling. Anti-CITED4 antibody was gifted by T. Shioda. All antibodies were used in western blotting at 1:1000 dilutions in blocking buffer. The same antibodies were also used for immunohistochemistry (IHC) and immunofluorescence.
Histological analysis and immunohistochemistry
Ovaries were fixed overnight in 10% PBS buffered formalin and then embedded in paraffin. Ovary samples were serially sectioned at 5 µm thicknesses and were stained with hematoxylin and eosin. For IHC, sections were deparaffinized and rehydrated, and were incubated with rabbit anti-CBP antibody (SC-583 from Santa Cruz, 1:200) for 1 h at room temperature, followed by biotin-labeled secondary antibodies for 30 min. Staining procedure was performed using the Vectastain ABC kit and DAB peroxidase substrate kit (Vector Laboratories, Burlingame, CA, USA).
Plasmid transfections and luciferase assay
Expression plasmid of HA-Cited4 was gifted by Dr T. Shioda. The CBP expression plasmid was gifted by Dr X. Lin. The plasmids for C/EBP luciferase assay were gifted by Dr P.F. Johnson, and were described previously. Cultured GCs were transfected with these plasmids (500 ng/well) using lipofectamine (Invitrogen) according to manufacturer's instructions. In luciferase assay, Firefly luciferase activity was normalized to Renilla luciferase activity (co-transfected as an internal control).
Immunofluorescence
Ovarian tissues were fixed in 4% paraformaldehyde, embedded in O.C.T. compound (Sakura Finetek USA, Inc.), and stored at −80°C before preparing 7 µm sections using a Leica CM1950 cryomicrotome (Leica Microsystems, Wetzlar, Germany). Cultured GCs were seeded on cover slips. Twenty-four hours later, cells were washed with PBS, fixed with 4% paraformaldehyde, permeabilized with PBS containing 0.3% Triton X-100 (PBST) and incubated with blocking buffer (PBST containing 5% bovine serum albumin). Sections or cells were sequentially probed with primary antibodies as indicated in Results and Alexa Fluor 594- or 488-conjugated secondary antibodies (Molecular Probes). Slides were mounted using VectaShield with 4′, 6-diamidino-2-phenylindole (DAPI, Vector Laboratories). Digital images were acquired using an epifluorescence microscope (Nikon Eclipse 80i) with 4-100× objectives.
Immunoprecipitation and ChIP assay
To detect protein–protein interactions, we collected ovaries from mice treated with PMSG alone (44 h) or PMSG (44 h) plus hCG (4 h). For each experimental group, ovaries from four mice were lysed in NP-40 buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl 10% glycerol and 0.5% NP-40; protease and phosphatase inhibitors were added before use.). The lysates were centrifuged at 12 000g for 30 min, and the supernatant was used for immunoprecipitation with anti-CBA and protein A affinity beads (Sigma). After incubation at 4°C for 4 h, beads were washed three times with NP-40 buffer. SDS sample buffer was added to the beads and the immunoprecipitates were used for western blot analysis.
ChIP on ovaries using the CBP antibody (SC-583 from Santa Cruz) was performed as previously described (Duggavathi et al., 2008). GCs were collected from mice treated with PMSG alone (44 h) or PMSG (44 h) plus hCG (4 h). For each sample used for ChIP assay, GCs from four mice were pooled together and lysed. The Areg and Ptgs2 proximal promoters, which contains the putative cAMP responsive elements, were amplified by PCR. Enrichment of the amplicon normalized to 10-fold-diluted input from immunoprecipitation with CBP1 antibody and normal rabbit IgG was compared semi-quantitatively.
Statistical analysis
Results are given as means ± SDs; each experiment included at least three independent samples and was repeated at least three times. Group comparisons were made by unpaired Student's two-tailed t-tests. P-values of <0.05 were considered significant.
Results
The pre-ovulatory LH surge induces Cited4 expression in cumulus and granulosa cells
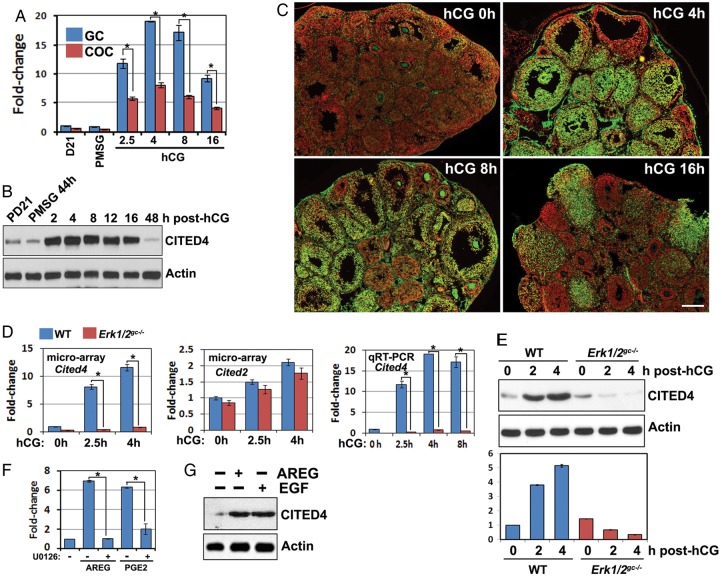
In previous studies, the gene expression profiles in pre-ovulatory follicles in response to exogenous gonadotrophin stimulation were determined by micro array analyses (Hernandez-Gonzalez et al., 2006). These results indicated that Cited4 mRNA levels were significantly up-regulated in cumulus cells within 8 h after hCG treatment. In this study, we further characterized Cited4 expression dynamics in mural GCs and the cumulus cells of pre-ovulatory follicles. As shown in Fig. 1A, Cited4 mRNA levels were dramatically induced by hCG in GCs and COCs within 2.5 h, reached a plateau at 4–8 h after hCG injection and then gradually declined. At all tested time points, the relative Cited4 levels in GCs were ∼2-fold higher than in COCs. Western blotting of ovary lysates and immunofluorescent staining for ovary sections showed that CITED4 protein levels were also remarkably increased after hCG treatment (Fig. 1B and C).
Figure 1.
Pre-ovulatory LH surge induces Cited4 expression in ovarian granulosa cells (GCs) through the ERK1/2 signaling pathway. (A) Quantitative RT–PCR results for relative in vivo Cited4 mRNA expression levels in mouse GCs and cumulus-oocyte complexes (COCs) isolated at the indicated time points after pregnant mare's serum gonadotrophin (PMSG)/hCG treatment. *P < 0.001. (B) Western blotting results for in vivo CITED4 protein levels in mouse ovaries isolated at the indicated time points after PMSG/hCG treatment. (C) Immunofluorescence results for CITED4 expression in ovaries before and after hCG treatment. Scale bar = 200 µM. (D) Microarray and qRT–PCR results for relative Cited2/4 mRNA expression levels in GCs isolated from ovaries of WT and Erk1/2gc−/−mice at 0, 2.5 and 4 h after hCG treatment. *P < 0.001. (E) Western blotting results for CITED4 protein levels in ovaries of WT and Erk1/2gc−/−mice after hCG treatment. The lower panel showed the quantification of the intensity of the CITED4 immunoreactive band from three different experiments. (F) Quantitative RT–PCR results for relative Cited4 mRNA expression levels in cultured primary GCs with and without treatment with amphiregulin (AREG, 100 ng/ml), prostaglandin E2 (PGE2, 500 ng/ml) or U0126 (10 µM), for 4 h.*P < 0.001. (G) Western blotting results for CITED4 expression levels in cultured GCs with and without treatment with AREG (100 ng/ml) or epidermal growth factor (EGF, 10 ng/ml) for 4 h.
RAS-MAPK signaling is crucial for LH-stimulated Cited4 expression in granulosa/cumulus cells
We previously reported that the RAS-MAPK signaling pathway was crucial for LH-induced ovulation (Fan et al., 2008, 2009; Fan and Richards, 2010). To evaluate the effects of ERK1/2 deletion on LH-target gene expression in the ovary, we used microarray analyses for GCs isolated from WT and Erk1/2gc−/− mouse ovaries at 0, 2.5 and 4 h after hCG treatment. These results indicated that ERK1/2 deletion nearly completely blocked hCG-induced Cited4 expression in GCs (Fig. 1D, top panel). We further confirmed these microarray results using quantitative RT–PCR for both mural GCs and COCs isolated from Erk1/2gc−/− mouse ovaries; this showed that hCG-stimulated Cited4 expression was abolished (Fig. 1D, bottom panel).
However, the mRNA levels of Cited2, the best studied of the Cited family, were not regulated by hCG and ERK1/2 in GCs (Fig. 1D, middle panel). Western blotting results also showed that the CITED4 protein levels in Erk1/2gc−/− mouse GCs were significantly lower than those in control ovaries at 2–4 h after hCG treatment (Fig. 1E). We also investigated if direct ERK1/2 inhibition could block Cited4 expression in cultured primary GCs. Indeed, amphiregulin (AREG) and prostaglandin E2 (PGE2), the physiological intra-follicular mediators of LH actions, induced Cited4 expression in cultured GCs, but their effects were blocked by the MEK1/2 inhibitor U0126 (Fig. 1F). The CITED4 protein levels were also increased in cultured GCs at 4 h after AREG and EGF treatment (Fig. 1G).
CITED4 and CBP are required for LH-target gene expressions in ovarian granulosa cells
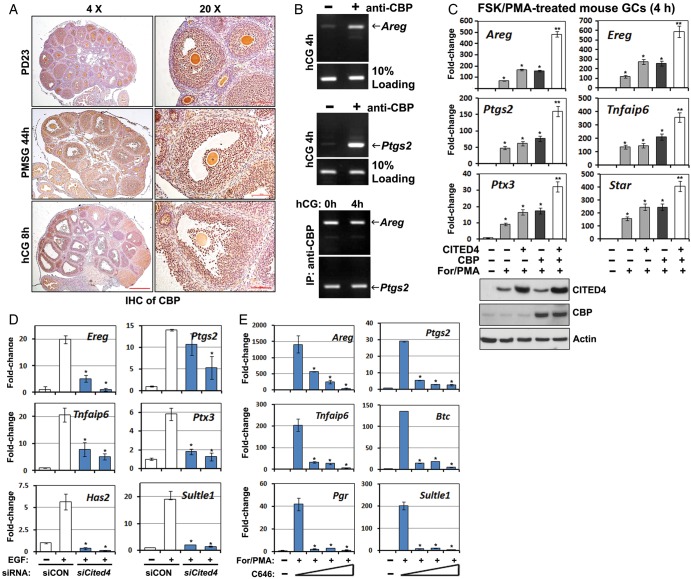
Because CITED family proteins reportedly functioned as co-factors of CBP/P300 (Yahata et al., 2002), we also investigated CBP expression patterns in mouse ovaries. Different from the transient expression pattern of CITED4, the CBP protein was constitutively expressed in the GCs of growing and ovulatory follicles in a gonadotrophin-independent manner (Fig. 2A). Chromatin immunoprecipitation using a CBP antibody was done for ovary lysates prepared at 4 h after hCG treatment. These results indicated that endogenous CBP physically interacted with the promoter regions of Areg and Ptgs2, which are well-established LH-target genes during ovulation (Fig. 2B, top and middle panels). However, these interactions were unlikely to be regulated by LH/hCG, as CBP efficiently bound with Areg and Ptgs2 promoters even before hCG treatment (Fig. 2B, bottom panel). These results suggested that a pre-ovulatory LH surge activated a CBP transcription complex by inducing CITED4 expression rather than regulating CBP itself.
Figure 2.
CBP and CITED4 are required for LH-target gene expression in granulosa cells. (A) Immunohistochemistry results for CBP expression in mouse ovaries at PD23 with and without PMSG/hCG treatment. Scale bar = 500 µM for ×4 images; Scale bar = 100 µM for ×20 images. (B) Chromatin immunoprecipitation (ChIP) results for CBP binding to Areg and Ptgs2 promoter regions. GCs were collected from PMSG-primed mice before and after hCG treatment for 4 h. For each sample used for ChIP assay, GCs from four mice were pooled together and lysed. (C) qRT–PCR results for relative mRNA levels of the indicated genes in cultured GCs stimulated by forskolin (10 µM)/PMA (20 nM) and with or without CITED4 and CBP overexpression. *P < 0.001 when compared with sample 1 from left. **P < 0.001 when compared with sample 2 from left. (D and E) qRT–PCR results for relative mRNA levels of the indicated genes in cultured GCs stimulated by EGF and forskolin/PMA and with or without RNAi Cited4 depletion (D) or CBP inhibitor C646 treatment (E). C646 was added to the culture medium at 30 min before For/PMA stimulation, with the final concentrations of 1, 2 or 5 µM. *P < 0.001 when compared with sample 2 from left.
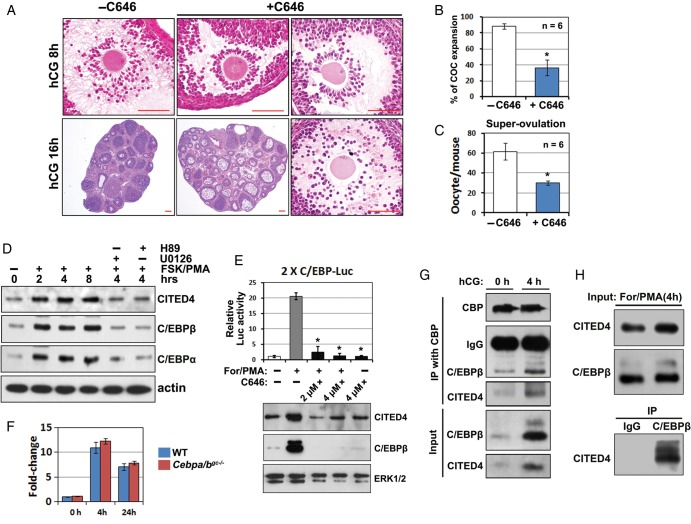
We then investigated if a CBP-CITED4 complex was required for LH-triggered target gene expression in primary GCs. CITED4 or CBP overexpression moderately increased GC responses to forskolin and PMA, which are known to mimic pharmacologically LH actions in vitro. However, CITED4/CBP co-transfection significantly sensitized GC responses to forskolin and PMA (Fig. 2C). In contrast, RNAi depletion of Cited4 using two independent siRNAs in GCs decreased the levels of EGF-induced LH-target gene expression (Fig. 2D). C646, a specific CBP/P300 inhibitor, also blocked these genes' expression at the concentrations of 1, 2 and 5 µM in culture medium (Fig. 2E). More importantly, when C646 (5 µmol/mouse) was co-injected with hCG in a super-ovulation experiment, it impaired COC expansion in ∼40% of the pre-ovulatory follicles at 8 h post-hCG, and significantly reduced the numbers of oocytes that were ovulated (Fig. 3A–C). Some COCs were trapped in the unruptured follicles, but they are fully expanded (Fig. 3A). These observations suggested that with the dosage of C646 used, COC expansion was delayed, but not fully blocked in vivo.
Figure 3.
CBP-CITED4 plays a role upstream of C/EBPβ during ovulation. (A) CBP inhibition impairs ovulation in vivo. WT mice (PD23) were super-ovulated by PMSG and hCG treatment. In the experimental group (n = 6), C646 was co-injected with hCG. Ovaries were paraffin-embedded and HE stained at 8 and 16 h post-hCG. Scale bar = 100 µM. (B) Rates of COC expansion in pre-ovulatory follicles at 8 h after hCG or hCG/C646 treatment. *P < 0.001. (C) Numbers of ovulated oocytes harvested from oviducts were counted at 16 h after hCG and C646 treatment. *P < 0.001. (D) Forskolin/PMA-induced CITED4 and C/EBPα/β expression in cultured GCs. Primary GCs were harvested from mice at PD23, cultured overnight and then treated with FSK (10 µM)/PMA (20 nM), U0126 (10 µM) or H89 (10 µM) for the indicated periods of time. Endogenous CITED4, C/EBPα and β expressions after the indicated treatments were detected by western blotting. (E) The upper panel showed the Luciferase assay results for the effects of forskolin/PMA and C646 (4 µM) on C/EBPβ activity in cultured GCs. GCs were transfected with a 2xC/EBP Luciferase reporter plasmid at 24 h before forskolin/PMA treatment, and Luciferase activity was assessed at 4 h after forskolin/PMA treatment. The lower panels showed the expression levels of endogenous CITED4 and C/EBPβ in the same GCs used for Luciferase assay. ERK1/2 was blotted as a protein loading control. *P < 0.001 when compared with sample 2 from left. (F) qRT–PCR results for relative Cited4 mRNA expression levels in GCs isolated from ovaries of WT and Cebpa/bgc−/−mice at 0, 4 and 24 h after hCG treatment. (G) Interaction of CBP-CITED4 with C/EBPβ in vivo. Lysates were prepared for the ovaries from mice at PD23 with or without hCG treatment for 4 h. Endogenous CBP was immunoprecipitated using an anti-CBP antibody. Co-precipitation of CITED4 and C/EBPβ was detected by western blotting. (H) Interaction of C/EBPβ with CITED4 in vivo. Lysates were prepared for the ovaries from PD23 mice at 4 h after hCG treatment. Endogenous C/EBPβ was immunoprecipitated using an anti-C/EBPβ antibody. Co-precipitation of CITED4 was detected by western blotting. As a negative control, the anti-C/EBPβ antibody was replaced with normal rabbit IgG in the parallel IP experiment.
CITED4-CBP regulates C/EBPβ expression and activity in mouse granulosa cells
Our previous study has shown that C/EBPα and C/EBPβ are required for follicle rupture and ovulation. Therefore, we investigated if C/EBPα and C/EBPβ are downstream molecules of CBP and CITED4.
In primary GCs, we confirmed that forskolin and PMA induced the expressions of CITED4, C/EBPα and C/EBPβ between 2 and 8 h of treatment, and that this could be blocked by the PKA inhibitor H89 and the MEK1/2 inhibitor U0126 (Fig. 3D). These results suggested that our primary GC culture system mimicked the previously reported in vivo scenarios and could be used as a model to study physiological C/EBPβ activity regulation. Thus, we investigated the role of CBP in C/EBP expression and activity. In GCs transfected with a Luciferase reporter that contained two C/EBP responsive elements (2xC/EBP-Luc), forskolin/PMA significantly increased C/EBP transcription activity, as well as C/EBPβ expression (Fig. 3E). However, the CBP inhibitor C646 abolished forskolin/PMA-induced C/EBPβ expression and activity (Fig. 3E). In contrast, the hCG-induced Cited4 mRNA expression was not affected in Cebpa/b-deleted GCs (isolated from Cebpafl/fl; Cebpbfl/fl; Cyp19-Cre mice), as detected by real-time RT–PCR (Fig. 3F). Collectively, these results suggested that CBP played a role upstream of C/EBPβ and was required for its expression.
For human cell lines, it was reported that CITED4-CBP bound to C/EBPβ and promoted its transcription activity (Lee et al., 2010a, b). However, a few reports showed their in vivo interaction and the physiological importance of this interaction. We did co-immunoprecipitation experiments with GC lysates using a CBP antibody. These results showed that CBP physically interacted with endogenous CITED4 and C/EBPβ at 4 h post-hCG treatment at which time these proteins were abundantly expressed in mouse ovaries (Fig. 3G). Only the C/EBPβ lone isoform was detected in the immunoprecipitates. This is understandable because only the lone isoform contains the DNA-binding domain and is the functional C/EBPβ. Furthermore, in GC lysates made at 4 h post-hCG, endogenous CITED4 was able to be specifically pulled-down by a C/EBPβ antibody (Fig. 3H, right lane), but not a normal rabbit IgG as negative control (Fig. 3H, left lane). These results suggested that in addition to induce C/EBPβ expression, CITED4-CBP and C/EBPβ formed a triple complex to regulate C/EBPβ target gene expression during ovulation.
Histone deacetylase activity turns off LH target gene expression
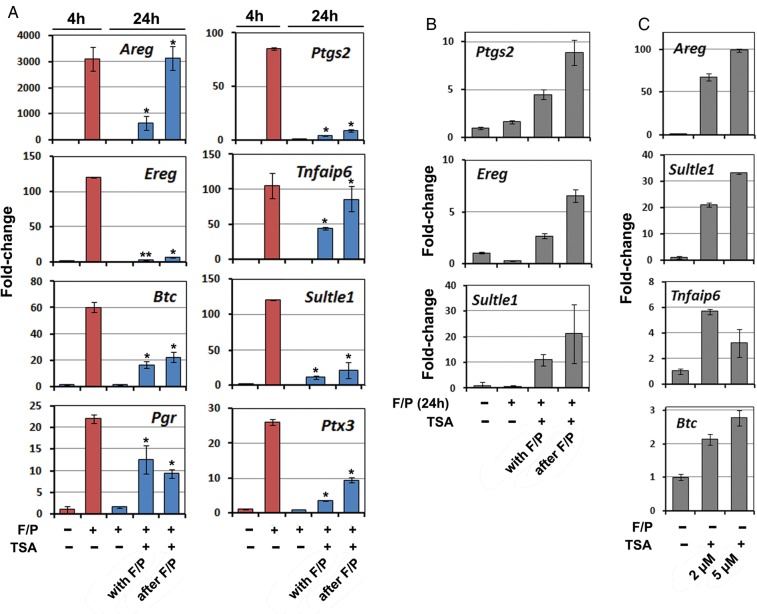
It has been noted for a long time that induced LH-target gene expression in GCs was transient. As shown in Fig. 4A, the indicated genes were remarkably induced by forskolin/PMA within 4 h, but their expression levels returned to basal levels 20 h later. How ovulation-related LH-target genes are silenced in GCs has not been thoroughly studied. Histone deacetylases (HDACs) counteract CBP and function as transcription repressors in many biological events (Graff and Tsai, 2013). Interestingly, when GCs were treated with forskolin/PMA plus the HDAC inhibitor trichostatin A (TSA), the expression levels of some LH-target genes (Areg, Tifaip6, Btc, Pgr and Ptx3) remained high at 24 h after stimulation (Fig. 4A). For some other genes, including Ptgs2, Ereg and Sult1e1, although TSA treatment did not maintain their high expression levels when compared with their maximal levels at 4 h of stimulation, their mRNA levels in TSA-treated GCs were still higher than those in control GCs, at 24 h after Forskolin/PMA stimulation (Fig. 4A and B). More surprisingly, TSA treatment alone resulted in expression of some LH-target genes without stimulation by ovulation signals (Fig. 4C). These results suggested that while CITED4-CBP-mediated transcription activation played an essential role in inducing gene expression during ovulation, HDAC might be a key factor for gene silencing after ovulation.
Figure 4.
Effect of histone deacetylases (HDAC) inhibitor trichostatin A (TSA) on LH-target expressions in cultured granulosa cells. (A and B) Quantitative RT–PCR results showing that forskolin (10 µM)/PMA (20 nM) induced transient expressions of ovulation-related genes, which were turned off at 24 h after stimulation. However, the HDAC inhibitor TSA (2 µM) blocked genes from being turned off when added to cells along with Forskolin/PMA or at 4 h after forskolin/PMA treatment. *P < 0.001 when compared with sample 3 from left. **P < 0.05 when compared with sample 3 from left. (C) Quantitative RT–PCR results showing that TSA (2 and 5 µM) increased expression of LH-target genes in GCs without forskolin/PMA stimulation.
Ovulation signals stimulate histone acetylation in ovarian granulosa cells through ERK1/2 and CBP
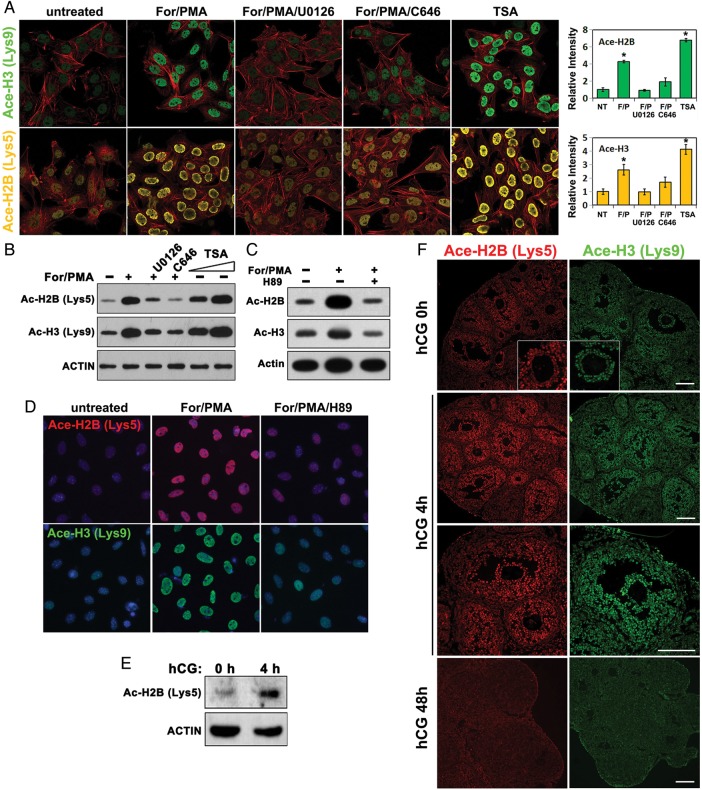
Our results thus far suggested that CBP and HDAC-regulated histone acetylation/de-acetylation played a key role in turning LH-target genes on and off during ovulation. Thus, we investigated ovarian histone acetylation status both in vitro and in vivo. In cultured CGs, forskolin/PMA increased the acetyl-histone H2B (Lys5)/H3 (Lys9) levels, but this effect was blocked by either the MEK1/2 inhibitor U0126 or the CBP inhibitor C646 (Fig. 5A and B). In addition, a dramatic increase in acetyl-H2B/H3 levels was detected in GCs that were treated with the HDAC inhibitor TSA (Fig. 5A and B). In a separate experiment, H89, which inhibited cAMP/PKA signaling pathway, also blocked forskolin/PMA induced histone acetylation (Fig. 5C and D). Western blotting results showed that the overall levels of acetylated-histone H2B increased after hCG treatment in vivo (Fig. 5E and F). High levels of acetylated-histone H2B (Lys5) and H3 (Lys9) were detected by immunofluorescent staining in GCs of pre-ovulatory follicles at 4 h after hCG treatment (Fig. 5F). At 48 h after hCG treatment, the terminally luteinized GCs lost their significant nuclear staining of acetylated histones (Fig. 5F). Collectively, these results indicated that a pre-ovulatory LH surge stimulated histone acetylation in GCs through the ERK1/2 pathway and cAMP/PKA pathway, and depended on CBP activity, whereas HDACs antagonized this effect.
Figure 5.
Pre-ovulatory LH surge induces histone acetylation in granulosa cells through ERK1/2 and CBP activity. (A–D) Immunofluorescence (A and D) and western blotting (B and C) results for acetylated histone H2B (Lys5) and H3 (Lys9) levels in cultured GCs treated with the indicated reagents. The concentrations used were: forskolin, 10 µM; PMA, 20 nM; U0126, 10 µM; TSA, 2 µM; and H89, 10 µM. Immunofluorescent signal intensities were quantified using Image J software. *P < 0.001 when compared with sample 1 from left. (E) Western blotting results for acetylated histone H2B (Lys5) levels in mouse ovaries before and after hCG treatment. (F) Immunofluorescence results for acetylated histone H2B (Lys5) and H3 (Lys9) levels in mouse ovaries before and after hCG treatment. Scale bar = 100 µM.
Discussion
Members of the CITED family bind directly to CBP/p300 through their conserved C-terminal acidic domain. Because no CITED family proteins have any significant DNA-binding motifs or detectable affinity for DNA, it has been proposed that CITED proteins may interact with sequence-specific DNA-binding proteins and function as CBP/p300-dependent transcription co-activators (Yahata et al., 2002). In support of this hypothesis, we showed that endogenous CITED4-CBP bound to C/EBPβ, a key transcription factor involved in ovulation and luteinization. Previous studies have shown that CITED4 shows strong milk cycle-dependent induction in pregnant and lactating mammary epithelial cells (Yahata et al., 2002). Prolactin, another peptide hormone secreted by the pituitary, strongly induces Cited4 expression in SCp2 mouse mammary epithelial cells (Yahata et al., 2002). Interestingly, similar to its role in the ovary, C/EBPβ is important for mammary gland development, especially for expression of differentiation-related genes (LaMarca et al., 2010). Therefore, CITED4-CBP may also form a complex with C/EBPβ to regulate hormone-target gene expressions in mammary gland. These observations and our results implied possible roles for Cited4 in regulating gene expression during development and differentiation.
CBP and its homolog p300 play pivotal roles in the transcriptional regulation of genes that are involved in many aspects of biological phenomena (Holmqvist and Mannervik, 2013). For the first time, we have demonstrated that CBP activity was required for LH-induced target gene expression and ovulation. Furthermore, our results suggest that CITED4-CBP function as a downstream effector of ERK1/2 in pre-ovulatory follicles, stimulated histone acetylation (histone H2B-Lys5 and H3-Lys9) at multiple gene promoters, facilitated the transcription of these genes, and thereby efficiently propagated the ovulation signals. Previous studies have shown that CBP was also activated by cAMP-dependent phosphorylation—this may contribute to the activation of LH-target genes (Constantinescu et al., 2004). In addition, the balanced histone acetylation/deacetylation processes that are regulated by CBP/HDAC determined the transient expression pattern of LH-target genes during ovulation. These new findings are summarized in Fig. 6.
Figure 6.
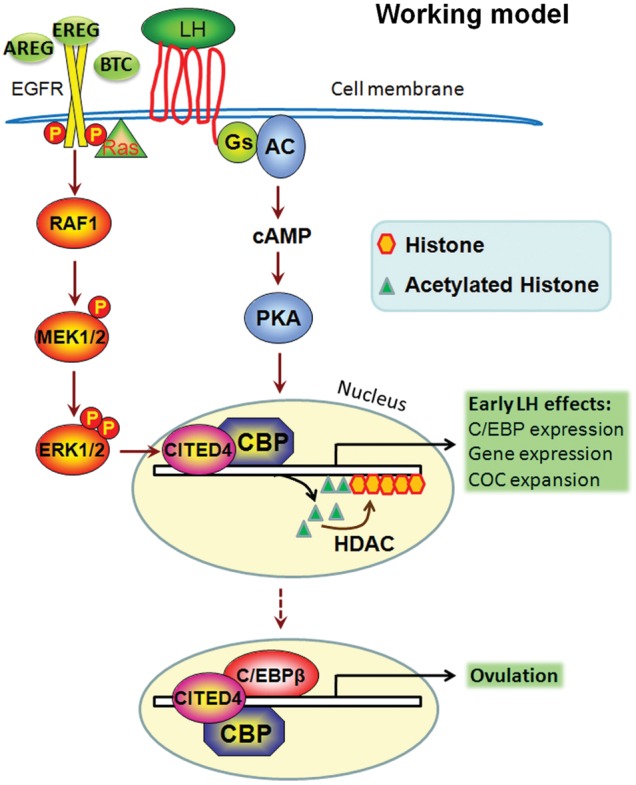
Schematic diagram of CBP-CITED4 functions in GCs during ovulation. A pre-ovulatory LH surge induces the activation of the cAMP/PKA/CREB pathway and the rapid expression of EGF-like factors, which function as intra-follicular LH effectors and further activate the ERK1/2-C/EBPβ cascade. ERK1/2 induces the expression of CITED4, which then forms a transcription initiating complex with CBP. This complex acetylates histones at the promoter regions of LH target genes, and triggers the rapid expression of those early LH-responsive genes, including Cebpb. Histone deacetylases (HDACs) reverse the effects of CBP and turn off these genes' expression. After accumulation of C/EBPβ, CITED4-CBP and C/EBPβ may form a triple complex to regulate further C/EBPβ target gene expression during ovulation, as indicated by the dashed arrow.
In this study, we investigated the regulation of CITED4-CBP/HDAC on multiple LH-target genes. The physiological functions of these genes in ovulation have been well studied. Areg, Ereg and Btc encode for EGF-like factors (Park et al., 2004). Ptgs2 encodes for prostaglandin synthase 2 (Lim et al., 1997). Both EGF-like factors and prostaglandins are intro-follicular mediators of LH signaling (Shimada et al., 2006). Pgr, Sult1e1, Ptx3, Has2 and Tnfaip6 are further downstream genes that required for cumulus expansion and follicle rupture (Fan et al., 2009). As shown in Fig. 2C and D, most of these genes were regulated by CITED4-CBP at the transcription level. Also, as shown in Fig. 4, many of these genes failed to be silenced in differentiated GCs after HDAC inhibition. Therefore, rather than controlling a small number of selected targets, CITED4-CBP/HDAC appear to be general regulators of LH target genes. However, when treated with TSA alone, not all of these genes showed increased expression in GCs (data not shown). This suggested that additional mechanisms are involved in their expression control.
Cited2 is the most characterized gene of Cited-family. It would be reasonable to hypothesize that Cited2 may compensate for the absence of Cited4. Even though Cited2 is not differentially regulated by gonadotrophins in the ovary (Fig. 1D), we cannot rule out the possibility that Cited2 plays a role in regulating basal CBP activity in the ovary. This hypothesis is supported by our observations that basal levels of acetyl-histone H2B/H3 can be detected in GCs before hCG treatment and Cited4 expression.
It is well established that EGF-like factors and PGE2 are involved in a positive feedback loop that amplifies LH-target gene expression. As shown by our previous study (Fan et al., 2009), ERK1/2 deletion did not prevent the initial induction of Areg and Ereg at 2 h post-hCG. Rather, ERK1/2 and its targets were important to maintain the expression of these EGF-like factors (Areg, Ereg and Btc) at 4 h post-hCG. Physiologically, PGE2 may be important for maintaining high Cited4 expression levels at 4–8 h post-hCG. In addition, although Ptgs2 was induced maximally by 4 h post-hCG, significant Ptgs2 expression was also observed at 2 h post-hCG. Therefore, CITED4 may play a more important role for maintaining rather than initiating LH-target gene expression.
Our previous findings indicated that C/EBPα and β were essential ERK1/2 targets for inducing ovulation (Fan, et al., 2011), but they were not the only mediators of ERK1/2 actions in pre-ovulatory follicles. For example, the expression of early LH-responsive genes, including those encode for EGF-like factors and cumulus expansion-related proteins, were not blocked in GC-specific Cebpa/b knockout mice. In this study, we provided evidence that CITED4-CBP activity was required for inductions of these genes in GCs, and CITED4-CBP played a role upstream of C/EBPs. Therefore, the CITED4-CBP and C/EBPs may be involved in different transcription-stimulating complexes that sequentially mediate ovulation-related gene expressions.
To our knowledge, the expression patterns, hormonal regulation and functions of HDACs in mammalian ovary have not been thoroughly investigated. Zhang and Dufau (2001, 2002) reported that HDAC1/2 associated with proximal promoter of LH receptor gene (Lhcgr). In addition, the HDAC inhibitor TSA increased H3/4 acetylation within the proximal promoter and thereby increased transcription of the LH receptor gene. These studies suggest a HDAC-mediated mechanism by which the LH receptor gene might be down-regulated following the LH surge. By looking at our microarray data, we found that several HDAC isoforms were abundantly expressed in the ovary, but their mRNA levels remained stable before and after hCG treatment (data not shown). These observations suggested that the LH-surge activated CITED4-CBP complex was the major regulated step of histone acetylation in GCs, whereas HDACs provided a constitutively active role in acetylated histone turnover.
Authors' roles
Y.-L.Z., Y.X. and C.Y. made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data, J.S.R., J.L. and H.-Y.F. drafted the article or revised it critically for important intellectual content, and H.-Y.F. gave final approval of the version to be published.
Funding
National Basic Research Program of China (2011CB944504, 2012CB944403), National Natural Science Foundation of China (81172473), Natural Science Foundation of Zhejiang Province (R2100145) and Basic Scientific Research Funding of Zhejiang University (2011QN81001) to H.-Y.F. J.S.R. was supported by NIH grant NIH-HD-16229 and NIH through cooperative agreement [U54- HD-007495 (Project2)] as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (SCCPIR).
Conflict of interest
None declared.
Acknowledgements
We thank P.F. Johnson for providing the C/EBPβ expression plasmid and the 2XC/EBP luciferase reporter plasmid. We thank T. Shioda for providing the CITED4 expression plasmid and antibody.
References
- Braganca J, Swingler T, Marques FI, Jones T, Eloranta JJ, Hurst HC, Shioda T, Bhattacharya S. Human CREB-binding protein/p300-interacting transactivator with ED-rich tail (CITED) 4, a new member of the CITED family, functions as a co-activator for transcription factor AP-2. J Biol Chem. 2002;277:8559–8565. doi: 10.1074/jbc.M110850200. [DOI] [PubMed] [Google Scholar]
- Constantinescu A, Wu M, Asher O, Diamond I. cAMP-dependent protein kinase type I regulates ethanol-induced cAMP response element-mediated gene expression via activation of CREB-binding protein and inhibition of MAPK. J Biol Chem. 2004;279:43321–9. doi: 10.1074/jbc.M406994200. [DOI] [PubMed] [Google Scholar]
- Doyle KM, Russell DL, Sriraman V, Richards JS. Coordinate transcription of the ADAMTS-1 gene by luteinizing hormone and progesterone receptor. Mol Endocrinol. 2004;18:2463–2478. doi: 10.1210/me.2003-0380. [DOI] [PubMed] [Google Scholar]
- Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22:1871–1876. doi: 10.1101/gad.472008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Richards JS. Minireview: physiological and pathological actions of RAS in the ovary. Mol Endocrinol. 2010;24:286–298. doi: 10.1210/me.2009-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development. 2008;135:2127–2137. doi: 10.1242/dev.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, O'Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol. 2010;24:1529–1542. doi: 10.1210/me.2010-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Johnson PF, Richards JS. CCAAT/enhancer-binding proteins (C/EBP)-alpha and -beta are essential for ovulation, luteinization, and the expression of key target genes. Mol Endocrinol. 2011;25:253–268. doi: 10.1210/me.2010-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Tsai LH. Histone acetylation: molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS. Gene Expression Profiles of Cumulus Cell Oocyte Complexes during Ovulation Reveal Cumulus Cells Express Neuronal and Immune-Related Genes: Does this Expand Their Role in the Ovulation Process? Mol Endocrinol. 2006;20:1300–1321. doi: 10.1210/me.2005-0420. [DOI] [PubMed] [Google Scholar]
- Holmqvist PH, Mannervik M. Genomic occupancy of the transcriptional co-activators p300 and CBP. Transcription. 2013;4:18–23. doi: 10.4161/trns.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca HL, Visbal AP, Creighton CJ, Liu H, Zhang Y, Behbod F, Rosen JM. CCAAT/enhancer binding protein beta regulates stem cell activity and specifies luminal cell fate in the mammary gland. Stem Cells. 2010;28:535–544. doi: 10.1002/stem.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie HA. Epigenetic control of ovarian function: the emerging role of histone modifications. Mol Cell Endocrinol. 2005;243:12–18. doi: 10.1016/j.mce.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lee S, Miller M, Shuman JD, Johnson PF. CCAAT/Enhancer-binding protein beta DNA binding is auto-inhibited by multiple elements that also mediate association with p300/CREB-binding protein (CBP) J Biol Chem. 2010a;285:21399–21410. doi: 10.1074/jbc.M110.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Shuman JD, Guszczynski T, Sakchaisri K, Sebastian T, Copeland TD, Miller M, Cohen MS, Taunton J, Smart RC, et al. RSK-mediated phosphorylation in the C/EBP{beta} leucine zipper regulates DNA binding, dimerization, and growth arrest activity. Mol Cell Biol. 2010b;30:2621–2635. doi: 10.1128/MCB.00782-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–R551. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- Sirois J, Richards JS. Transcriptional regulation of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Evidence for the role of a cis-acting C/EBP beta promoter element. J Biol Chem. 1993;268:21931–8. [PubMed] [Google Scholar]
- Workman JL, Kingston RE. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- Yahata T, Takedatsu H, Dunwoodie SL, Braganca J, Swingler T, Withington SL, Hur J, Coser KR, Isselbacher KJ, Bhattacharya S, et al. Cloning of mouse Cited4, a member of the CITED family p300/CBP-binding transcriptional coactivators: induced expression in mammary epithelial cells. Genomics. 2002;80:601–613. doi: 10.1006/geno.2002.7005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dufau ML. EAR2 and EAR3/COUP-TFI regulate transcription of the rat LH receptor. Mol Endocrinol. 2001;15:1891–1905. doi: 10.1210/mend.15.11.0720. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dufau ML. Silencing of transcription of the human luteinizing hormone receptor gene by histone deacetylase-mSin3A complex. J Biol Chem. 2002;277:33431–8. doi: 10.1074/jbc.M204417200. [DOI] [PubMed] [Google Scholar]