Abstract
In 2006, the US FDA issued a ‘Guidance for Industry’ regarding submission of New Drug Applications for pancreatic enzyme replacement therapy (PERT) products. Five oral delayed-release PERT products have been approved by the FDA, and several others are under development and/ or evaluation for New Drug Application submission. We present in this paper recommendations of the Cystic Fibrosis Foundation's Cystic Fibrosis (CF) Therapeutics Development Network and Data Safety Monitoring Board regarding study design considerations for evaluating PERT products in patients with CF. Careful attention to study design and accuracy of the outcome measures has confirmed our understanding of the efficacy and safety of PERT for the treatment of exocrine pancreatic insufficiency of CF.
Keywords: coefficient of fat absorption, cystic fibrosis, pancreatic enzyme replacement therapy, pancreatic insufficiency, study design
Background
Pancreatic enzyme preparations of porcine or bovine origin were available in the USA for treatment of exocrine pancreatic insufficiency prior to the Federal Food, Drug, and Cosmetic Act of 1938. As such, these products were not subject to the New Drug Application (NDA) process described in the Code of Federal Regulations (21 CFR Part 314). However, in 2004, the US FDA, after determining that pancreatic enzyme replacement therapy (PERT) products did not meet the requirements of an over the counter preparation, announced that all PERT products must go through an NDA process (Section 505[b][2] applications) as described in the Code of Federal Regulations. As a result, every company marketing PERT products in the USA before April 2004, as well as those seeking marketing authorization for new PERT products, were required to conduct clinical trials demonstrating efficacy and safety, and obtain FDA approval. Since 2009, five oral delayed-release pancreatic enzyme (pancrelipase) products, CREON®, PANCREAZE®, PERTZYE®, ULTRESATM and ZENPEP® [1,101–105], have received FDA approval for replacement therapy of exocrine pancreatic insufficiency due to CF or other conditions. Other porcine-based PERT products are under development and/or evaluation for NDA submission.
The FDA published a draft guidance entitled ‘Guidance for Industry Exocrine Pancreatic Insufficiency Drug Products – Submitting NDAs’, which was finalized in 2006 [2,106]. Section VI of that document outlined considerations for clinical trial development including study designs and outcome measures for efficacy and safety.
Within this document, the FDA recommended three possible study designs:
■ Parallel;
■ Randomized withdrawal;
■ Crossover designs.
It also stated that ‘placebo may be appropriate’ as long as a rescue protocol was established to protect the subjects.
It is understandable that the FDA has recommended that PERT products be closely regulated with reference to chemistry, manufacturing and controls, and be required to demonstrate both efficacy and safety. However, the burden of proving efficacy and safety falls upon patients with CF, since they comprise the largest group of users of PERT products. Several principles should be emphasized:
■ Study designs and outcome measures must be optimized to ensure validity of the study results so that efficacious and safe products will be approved quickly, and products lacking efficacy or safety will be identified;
■ Study designs using placebo should minimize the number of subjects receiving placebo and its duration in order to lessen the risk to this vulnerable population;
■ Special considerations should be given to studying PERT in children who are too young to consent, or even assent, to studies.
This paper outlines recommendations of the Cystic Fibrosis Foundation's CF Therapeutics Development Network and Data Safety Monitoring Board regarding studies of PERT products. Careful attention to study design and accuracy of the outcome measures has confirmed our understanding of the efficacy and safety of PERT for the treatment of CF-associated exocrine pancreatic insufficiency.
Efficacy outcome measures
Coefficient of fat absorption
The coefficient of fat absorption (CFA) is the current standard for evaluating the efficacy of PERT products, although there is a dearth of evidence demonstrating that CFA correlates with clinically relevant outcomes. The CFA is determined by a fat balance study employing a 72 h controlled high-fat diet and quantitative stool collection. The CFA is defined as fat intake (g) minus fat excreted (g)/fat intake (g), and, after multiplying by 100, is expressed as a percentage. The CFA is a composite measure of all of the mechanisms that can lead to fat malabsorption, and does not directly measure fat maldigestion caused by pancreatic insufficiency. So, what is a clinically relevant change in CFA? Absorption of an additional 10% (10 g of a 100 g fat/day diet at 9 calories/g of fat) could lead to an additional 90 calories/day, or 32,850 calories/ year, equivalent to slightly more than a 9lb weight gain/year, which is a clinically meaningful weight gain in a patient with CF. Others have argued that a 5% increase in CFA is clinically relevant [3]. It should be pointed out that here, and throughout the rest of this paper, the percent change indicates an absolute difference rather than a relative one (e.g., a change in CFA from 60 to 70% may be referred to as a 10% increase, but represents a 16.7% relative improvement). Since most of the PERT products studied in patients with CF resulted in a 30% or greater increase in CFA compared with placebo in a substantial number of studies [4–9], this effect size could be the benchmark for short-term efficacy studies; however, PERT products demonstrating a <30% increase in CFA on treatment compared with placebo have also been approved by the FDA [10]. Of note, a systematic review of the literature was unable to find sufficient evidence to establish a dose-response association between PERT products and CFA or growth [11]. Potential confounders of studies of PERT products and possible new outcome measures in patients with CF have also been reviewed [12]. Other factors besides malabsorption contribute to fat excretion; however, any impact of PERT on absorption would indicate efficacy. Although several efficacy measures have been used, at the time this paper has been written, the primary outcome measure for short-term studies of the efficacy of PERT products should be the CFA. Factors for performance of research-quality fat balance studies are listed in Box 1 [13–16].
Controlled high-fat diet
The high-fat diet used for measurement of CFA should be supervised by a dietitian and optimally contain 2 g of fat/kg of body weight/day or 60 g of fat/m2/day, although an arbitrary dose of approximately 100 g of fat/day is typically used. Of note, 100 g of fat/day may be a reduction in caloric intake for many patients with CF. For studies involving a wide range of ages (and thus body size), fat intake based on the per m2 or per kg of body weight of the subject is preferable. Investigators designing studies in infants should be aware that the recommended dietary fat intake/kg of body weight for healthy infants is much higher than in older individuals. Infants with CF and exocrine pancreatic insufficiency will need an even greater fat intake because of increased malabsorption. Investigators also need to be aware that the source and composition of various milks and formulas can markedly affect the amount of fat excreted, even for a similar fat intake. In Fomon et al.'s classic studies, for a similar fat intake, infants fed homogenized cow's milk had greater fat excretion than those fed processed human milk [17]. It is also difficult to adequately control the dietary fat intake of infants, as infants cannot be forced to feed. Thus, the increased dietary fat requirement, the increased degree of malabsorption in infants with CF, different dietary sources of fat and the variability of daily fat intake need to be taken into consideration in the design of PERT studies in the youngest patients.
Stool collection
Ideally, the beginning and end of the stool collection is determined through the use of dye markers that are taken orally 72 h apart from each other after the start of the controlled high-fat diet. Although Carmine Red has been used in the past, most recent studies utilized FD&C Blue Dye #2. The blue color is easier to see in stool. Of note, studies to determine the correct dosing of dye markers in infants and young children have not been published. The colloquially termed ‘72-h stool collection’ takes longer than 72 h, since the appearance of the both the first and second dye markers require a transit time through the digestive tract. Validity of the fat balance study is highly dependent on the proper collection of the number of stools defined by the markers. It is most accurate when the stools are collected and observed for markers by trained research staff, and therefore should preferably be done in a research setting. The lack of dose information on oral dye markers, and the inadvisability of inpatient stays, make this ideal methodology difficult to achieve in infants and young children.
Stool fat measurement
Methodology for measuring the fat content of stool has been available for many decades, with the gravimetric assay of van de Kamer or modifications of this assay being used most often. The van de Kamer method is limited by inability to detect medium chain length fats and fatty acids [13]. These can be determined by the less widely used Jeejeebhoy gravimetric method [14]. Recently, measurement of stool fat by NMR spectroscopy has gained acceptance as an alternative to the standard gravimetric method. NMR fat determination strongly correlates with the gravimetric method, and has good inter- and intra-assay precision and linearity, [15]. Fat content is generally based on obtaining and analyzing stool collected over several days, not on single stools. NMR spectroscopy determines the percentage of fat in the stool collection, and by knowing the weight of the entire stool collection, the results can be reported as grams of fat per 24 h. It is recommended that stool fat analyses be performed at a central research laboratory to decrease the variability of the results.
The steatocrit method for determining stool fat is based on a capillary tube filled with stool that is spun to separate solids from the fat layer, and by determining the ratio of the two. Although it is a simple method in part because the test is generally performed on a single stool specimen, results are not very reproducible. A modification of the steatocrit is based on pretreating the sample with acid for more effective separation into lipid and water phases [18]. However, this technique showed correlation with CFA in only one of three arms of a crossover trial [19]. Nevertheless, its use has been accepted by the FDA for evaluation of a PERT product in young children with CF [7].
Another method for determining fat excretion on a spot stool specimen relies on the co-administration of a 13C-labeled triglyceride, dysprosium chloride (a nonabsorbable marker) and a stool dye marker [20]. Assuming that the three substances transit the intestine at the same rate, the ratio of fecal 13C-labeled trigyceride and dysprosium is determined in a stool specimen (that is colored by the dye marker), permitting quantitation of triglyceride excretion. Studies in patients with CF have shown good correlation between this method and the CFA obtained from a 72-h stool collection [21]. This method has not yet been employed in a clinical trial of PERT.
Other measures of fat absorption
Methods to evaluate fat absorption that do not rely on stool collections have been proposed, such as the 13C breath test. This test has the advantage of integrating the effects of gastric emptying on fat absorption. 13C breath tests have been used in several small series of subjects with CF, and may be useful to determine PERT efficacy [22–24]. There is scant information in the literature correlating these tests with CFA, thus, this test cannot be recommended at this time as a replacement for CFA determinations.
Pharmacokinetic-type tests have also been proposed to study fat absorption. These involve the ingestion of a marker nutrient that is then measured in a blood sample taken at baseline and at intervals after the test meal [25,26]. These tests could have the advantage of being more precise measures, and possibly require a shorter time period of study, compared with collecting stool for determining CFA. These tests may also yield information about the timing of fat absorption. Ideally, fat would be digested and absorbed fairly proximally in the gastrointestinal tract. Disadvantages include the variability of gastric emptying and the need for indwelling catheters or repeated venipuncture, which could be problematic in infants and young children. Given that these tests are still being developed, they are not yet ready to be recommended as alternatives to the CFA measure.
Measures of protein & carbohydrate
As PERT also impacts protein digestion, the coefficient of nitrogen absorption as a measure of protein digestion should optimally be determined in addition to the CFA. The coefficient of nitrogen absorption would be considered a secondary outcome measure. There is presently no validated measure for carbohydrate absorption and therefore a standard measurement cannot be recommended at this time. This makes the study of PERT efficacy difficult, especially since comparator studies often standardize the treatment arms based on the lipase dose but not the protease or amylase dose [27,28], which could affect reports of subjective symptoms.
Clinical & patient reported outcomes
Weight gain
Weight gain or growth in children, and maintenance of weight in older individuals, can be used as supporting evidence for the efficacy of PERT in individuals with exocrine pancreatic insufficiency. However, use of this outcome requires long-term studies and is not at all appropriate for placebo-controlled trials of PERT products. Weight loss would be an adverse event in a short-term study that employs a placebo, or could be an outcome in a long-term comparator study, and should be closely monitored.
Patient-reported outcomes
Patient-reported outcomes are being developed to study pulmonary outcomes in patients with CF, but little work has focused on standardizing these for gastrointestinal outcomes. Despite common clinical practice to adapt the PERT dose to symptoms, one large cross-sectional study did not support that PERT dose correlates with symptoms, weight or CFA in clinically stable patients [29]. CFA correlates with number and weight of stools in some, but not all studies [28,30]. The Cystic Fibrosis Questionnaire-Revised includes questions about gastrointestinal symptoms and so could be employed [31]; however, one study of a PERT product showed positive improvements in CFA versus placebo but no change in Cystic Fibrosis Questionnaire-Revised scores [32]. Recently published studies of PERT products have employed a variety of scores of symptoms and signs [6,7,9,10]. Stool frequency (number of bowel movements) and stool characteristics are commonly reported as well. These data can provide valuable supporting evidence for PERT efficacy.
Study design
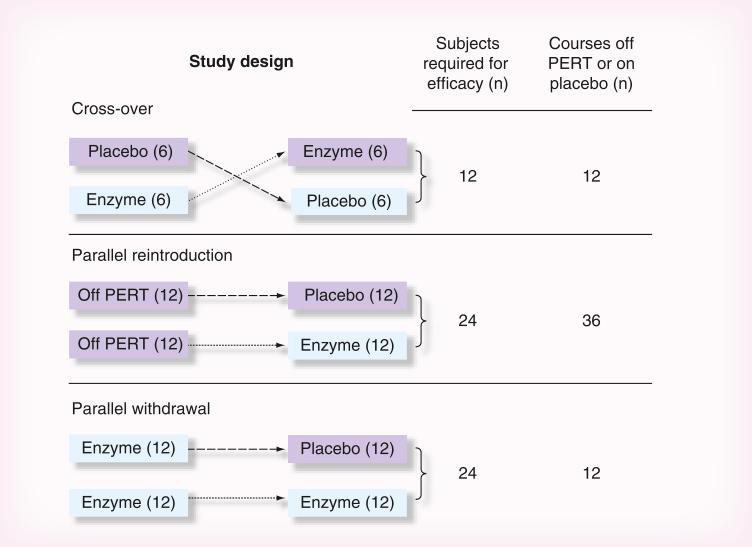
There are three main study designs recommended in the FDA guidance that could utilize placebo as a comparator: parallel design (referred to as parallel reintroduction design in this paper), randomized withdrawal design (referred to as parallel withdrawal design in this paper) and crossover design. These designs are discussed below and presented in Figure 1, within the context of using CFA as a primary end point in these studies.
Figure 1.
Comparison of estimated sample sizes and exposure to placebo using different study designs. Sample size calculations assume a two-sided type I error of 5%. Crossover design: A sample size of 12 subjects would provide 92% power to detect a 30% increase (absolute difference) in coefficient of fat absorption (CFA) between the enzyme and placebo periods assuming a standard deviation of 28% for the within-patient difference in CFA measurements. Parallel reintroduction and parallel withdrawal design: A sample size of approximately 12 per treatment group (24 total) would provide 90% power to detect at least a 30% absolute difference in CFA between the enzyme and placebo groups, assuming a standard deviation of 10% in the enzyme group and 28% in the placebo group. Although the reintroduction and withdrawal designs have similar sample size requirements, the reintroduction design requires three-times as many courses off PERT or on placebo. Each of the periods where subjects are off PERT or on placebo, or are receiving enzymes, regardless of study design, lasts 6–8 days. Not depicted in this figure are run-in periods on enzymes before each of the evaluation periods shown above, which may vary among specific studies, but are not dependent on study design.
PERT: Pancreatic enzyme replacement therapy.
Parallel reintroduction design
In a parallel reintroduction design, patients would be randomized in a 1:1 fashion to receive either a PERT product or placebo. Prior to randomization, a baseline CFA would be obtained from each patient starting after the patient has been off enzymes for at least 48 h. A second CFA measurement would be obtained while the patient was on the study drug (PERT product or placebo). The primary efficacy measure would be the change in CFA from baseline to end-of-study drug exposure. A major disadvantage of the parallel reintroduction design is the greater number of total periods (and thus days) for which patients need to either be off of enzymes or on placebo as compared with either a parallel withdrawal or crossover design. Each period on placebo (or active enzyme) lasts 6–8 days to allow sufficient time to complete the stool collection for CFA assessment, which is not a trivial amount of time off PERT. Patients randomized to placebo at the outset will have two consecutive periods for which they are not receiving PERT, which puts these patients at more risk of having adverse events attributed not receiving a PERT product. None of the eight clinical trials assessing PERT products cited in this paper utilized the parallel reintroduction design, presumably because of these design disadvantages.
If CFA is used as a measure of pancreatic mal digestion, the sample size requirements for the parallel reintroduction design comparing placebo and PERT groups can be based on a two-sample t-test comparing the difference in average CFA from baseline to end of study drug exposure. Based on estimates from the literature, and assuming conservative estimates for the standard deviations, a sample size of approximately 12 per treatment group (24 total) would provide 90% power to detect at least a 30% increase in CFA in the PERT group as compared with placebo (assuming a type I error of 5%, and a standard deviation of 10% in the active group and 28% in the placebo group). This study design requires twice as many subjects as a crossover design.
Parallel withdrawal design
To minimize the time during which patients are not taking enzymes with their meals, a parallel withdrawal design could be used. This design includes a run-in phase in which the PERT product is administered to all patients. The baseline CFA measurement is taken while patients are on PERT, rather than when they are off PERT (as in the parallel reintroduction design). The second CFA measure is then determined on the randomized subjects at the end of study drug administration (placebo or PERT product), and the difference between the end of study drug and baseline CFA is used as the primary outcome measure. In some parallel withdrawal studies, only patients who respond and achieve stabilization with a predetermined level of CFA on an ‘optimal’ dose of the PERT product are randomized [3,10]. However, a significant number of patients with CF do not achieve CFA on treatment that is thought to be ‘optimal’ [33], thus bringing into question whether the results of this latter type of stabilization study are generalizable to the CF population as a whole. Moreover, it is also possible that a ‘responder’ (achieving a predetermined high level for CFA) to the PERT product under study is not necessarily a responder, but rather has only mild malabsorption. In such patients, demonstrating a difference in malabsorption between the active drug and placebo becomes more difficult. In other parallel withdrawal studies, all patients are randomized to either placebo or PERT, irrespective of their baseline CFA. This approach is less biased.
The sample size recommendations for this design are similar to those for the parallel reintroduction design, although the assumption for this design is that those randomized to PERT will maintain CFA stabilization, while patients randomized to placebo would experience a 30% or greater decrease in CFA, indicating worsening fat absorption. Thus, the overall treatment effect and variability assumptions are the same between a parallel reintroduction design and a parallel withdrawal design, but there are fewer courses off enzyme or on placebo in a parallel withdrawal design. Two of the eight clinical trials assessing PERT products cited in this paper utilized the parallel withdrawal design [4,10].
Crossover design
The most frequently used schema for studies of PERT products is the crossover design, as evidenced by five of the eight PERT efficacy and safety clinical trials cited in this paper utilizing this design [5–9]. The results of a sixth previously unpublished trial using the crossover design is presented in this issue of Clinical Investigation as an example of the most frequently used study design for assessing PERT products [34].
In these studies, patients are randomized 1:1 in a crossover fashion to enzyme–placebo or placebo– enzyme. The CFA is determined during both periods (once while on enzyme and once while on placebo). An important assumption of this design is that the CFA at the beginning of each of the two treatment periods is equivalent, which eliminates the need to determine CFA measurements immediately prior to the administration of each study drug. This design permits a statistical analysis that utilizes a paired t-test whereby each patient serves as his or her own control. Compared with traditional parallel reintroduction designs, crossover designs often reduce between-subject variability and provide more statistical power at a given sample size. However, this advantage could be negated by the observation that within-subject variation of CFA can be quite large [35,36]. Assuming that the un measured within-subject variation CFA at the beginning of each of the two treatment periods are equivalent, and assuming a conservative estimate for the standard deviation of 28% for the difference between CFA measurements and type I error of 5%, a sample size of 12 patients would provide approximately 92% power to detect at least a 30% increase in CFA on PERT as compared with placebo. This is half the number of subjects required compared with parallel reintroduction or withdrawal designs. However, crossover studies comparing PERT with placebo have included approximately the same number of subjects as other study designs in order to collect safety data [5–9]. Another advantage of the crossover design is that for a given effect size, the number of courses off enzyme or on placebo is half of that required for a parallel reintroduction or withdrawal design, reducing the risk for adverse events.
Crossover designs require the patient to return to baseline conditions between the two treatment periods to minimize carryover effects, as this is a major assumption that eliminates the need for baseline CFA measurements at the beginning of each study drug period. Many crossover designs include run-in and/or ‘stabilization’ periods with the new PERT, both before randomization and in place of a traditional washout period between the crossover periods. Thus, patients who are randomized to active drug first will have three concurrent sessions of enzymes (run-in, active phase and run-in) prior to receiving placebo, which raises the question of the comparability of data with patients who are randomized to placebo first. This can impose a bias in the data that may not be able to be accounted for in the analysis.
Use of placebo & challenges of study design in young children
The FDA guidance clearly states that PERT product approval must also include pediatric patients, because children with CF make up a significant portion of the target population. Sponsors are also encouraged to develop age appropriate formulations for children. At present, capsules are opened, or in the case of one new formulation of pancrealipase, dosed using a standardized scoop [27], the enteric-coated beads are mixed with soft food and are given to the patient with feedings. These beads cannot be crushed or chewed as this will destroy the enteric coating and inactivate the enzymes within. Of note, this method of administration of PERT products has been used for decades with clinical success, and thus might be considered as an age-appropriate formulation for young children. However, development of formulations specifically for infants and young children is encouraged. Studies for improving our understanding of when to initiate and how to dose PERT products in infants and very young children has become an ever important issue as newborn screening has become more widespread in the USA and elsewhere in the world. These questions are more critical than the establishment of efficacy versus placebo in infants and young children who have been proven to be pancreatic insufficient.
Growth is critical for infants and toddlers, so even several days off enzymes to obtain a CFA poses a more than minimal risk. Infants and toddlers cannot easily express abdominal pain, and toddlers are prone to stool withholding. Exposure to placebo would not offer a compensating prospect for direct benefit, and because of the possibility for both weight loss and bowel obstruction, the increased risk of placebo exposure is more than a minor increase over conventional risk [37]. Thus, the Cystic Fibrosis Foundation Data Safety Monitoring Board and the Therapeutics Development Network recommend that children below the age of assent and consent (younger than 7 years of age) should not be subjected to a period of placebo or off enzymes.
CFA or other outcomes based on structured diets and timed stool collections can be problematic in toddlers. Toddlers may have very erratic dietary patterns, making it difficult to assure a standardized daily intake of fat. Interference with toileting can cause behavioral problems, and stool withholding can make it extremely difficult to assure that a representative or complete stool collection has been obtained. Although marker-to-marker stool collections for the determination of CFA are best achieved in an inpatient research setting, concerns regarding unnecessary exposure of a young child to infection in a hospital setting suggest that these studies should be performed at home, recognizing that accuracy of the CFA will probably be compromised. Thus, alternatives to the classic CFA have been proposed. Samples from individual stools are easier to obtain than a timed collection making this an appealing option, but the reason for a timed collection is that stool fat losses are not constant from stool to stool, partly because fat intake varies from meal to meal, and partly due to variable absorption of fat from the intestinal tract. For these reasons development of a non-stool based measure of PERT efficacy is sorely needed, especially one that can be used in very young children.
One potential solution to the dilemma of avoiding placebo but needing an evidence base for dosing would be to conduct a dose-comparison study and follow a clinically relevant and objective outcome, such as rate of weight gain to prove PERT efficacy. This would be easier to accomplish expediently in young infants who have a very rapid rate of weight gain [38]; using weight gain as an outcome would require longer study duration in preschool age children (many months, possibly a year). Moreover, a study design that has weight gain as the primary end point would need to incorporate close follow-up visits and a rescue plan for lack of appropriate weight gain, particularly for infants. One might consider either an adaptation of the PERT (e.g., higher dose), or if fat excretion does not appear to be excessive, nutrition intervention should be considered (e.g., increased caloric-density of infant formula). In the latter case, the primary end point could be ‘time to nutritional intervention’ based on a predetermined level of insufficient weight gain. The benefits of this end point are that it allows physicians to intervene when needed without compromising the primary outcome of the trial when such interventional changes may occur. A potential drawback is that a range of factors can contribute to poor weight gain in infants [39], these potential confounders would need to be addressed.
Safety outcome measures
The following parameters should be assessed for all PERT studies. All animal-based enzyme products contain significant amounts of purines, which are metabolized to uric acid. Hyperuricemia and uricosuria have been reported [5,40]. Thus, blood levels of uric acid and a single spot urine analysis for the uric acid to creatinine ratio [41] should be measured in studies of porcine PERT products. Other blood studies should be included for standard safety measures, including alanine aminotranferase, aspartate aminotransferase, gamma-glutamyl transferase, chemistry panel and complete blood count. It should be noted that these laboratory values can vary greatly over time in patients with CF [42], and that the normal ranges for CF patients may be different than for healthy volunteers. The exact pathogenesis of fibrosing colon-opathy, a complication of high doses of PERT products [43–45], remains unclear; but a measure of direct toxicity of PERT on intestinal mucosa would be an important safety parameter, especially in infants. Stool analysis including detection of stool heme and stool white blood cell counts is likely to be of limited value because swallowed puru-lent sputum that could contain blood may confound the results, and because the incidence and prevalence of these abnormalities is unknown.
Malabsorption of fat can lead to low levels of fat soluble vitamins. Pancreatic enzymes improve fat absorption, and treatment with PERT leads to increases in alpha-tocopherol (vitamin E) levels, although other factors can also affect serum vitamin levels. Maintenance of normal micronutrient status over time should be documented in long-term safety studies of PERT. One can see changes in serum levels of alpha-tocopherol approximately 28 days after supplementation has been initiated using d-alpha-tocopheryl succinate (the common form in tablets and multivitamins), and after 3 weeks when using d-alpha tocopherol and its acetate ester (found in softgels) [46,47]. Changes in alpha tocopherols levels should not be measured as a safety parameter for any period of time shorter than 4 weeks.
An important safety consideration for any of the placebo-controlled study designs discussed above would be the need for rescue and a return to the PERT product that the patient was using prior to the trial. The most concerning and serious acute gastrointestinal adverse event is intestinal obstruction. Patients with CF may develop distal intestinal obstructive syndrome, which has occurred in published PERT trials, primarily with exposure to placebo [4,5,48]. Placebo exposure has also been associated with an increased incidence of abdominal pain, diarrhea, cramping and flatulence. Thus patients must be closely monitored and evaluated for these symptoms. In order to ensure consistency across studies for classification of distal intestinal obstructive syndrome, a standard definition should be used, such as that proposed by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition Cystic Fibrosis Working Group [49].
Concomitant medications
Some CF patients are prescribed acid-reducing medications (antacids, histamine-2 blockers and/or proton pump inhibitors) for optimizing the efficacy of PERT, while some patients are prescribed these medications for the treatment or prevention of gastrointestinal symptoms. Some PERT studies have required that patients refrain from taking these medications for the duration of the study [10]. The rationale for excluding the use of these medications is to reduce any confounding effect they may have on the efficacy of the PERT product [12,35]. There is no clear consensus as to whether these concomitant medications should be eliminated. Exclusion of these medications could be considered important in order to have an accurate measurement of change in CFA. However, for those patients taking these medications, discontinuation may be a deterrent to enrollment and may also delay enrollment, as well as have an adverse outcome in those patients taking these medications for the treatment of gastrointestinal symptoms. Since the use of acid-reducing medicines are commonly used in the management of CF patients, there is a strong rationale for studying PERT efficacy in the presence of these medications, especially if the patients are already taking them. Since these medications can affect CFA, a strategy for balanced allocation of subjects to each dosing arm should be considered. Other medications are often excluded during PERT trials for their potential effect on gastrointestinal motility, which may affect the frequency of bowel movements and characteristics of stool. Among these are certain antibiotics known to affect motility, and laxatives (e.g., macrolides).
Box 1. Factors for performance of research-quality stool fat balance studies for calculation of coefficient of fat and coefficient of nitrogen absorption.
■ Research-quality stool fat and nitrogen balance studies should ideally be performed in a clinical research unit or other monitored setting. Balance studies should include:
■ A 48-h wash-out period prior to initiation of a 72-h controlled fat and protein diet;
■ A high-fat diet (optimally 2 g/kg/day or 60 g/m2/day, or otherwise 100 g/day) planned by a research dietitian and with intake verified by objective measures;
■ A protein intake of 2 g/kg/day provided in a diet planned by a research dietitian and with intake verified by objective measure;
■ Use of stool dye markers given at the beginning and the end of the 72-h controlled diet. The first stool containing dye should be discarded. The collection should continue up to and including the first stool marked by the second dye marker. The use of FD&C Blue Dye #2 is preferred;
■ Stool collection should be weighed;
■ There should be a prospective plan to record lost stool, with minimums set for completion of collection;
■ Stool fat should be analyzed in a central reference laboratory by a standardized method that should be reported in any published results;
■ Stool nitrogen should be analyzed in a central laboratory by the Dumas combustion method or other equivalent methodology;
■ The coefficient of fat absorption should be calculated as follows and expressed as a percentage:
■ The coefficient of nitrogen absorption is calculated in the same way using nitrogen intake and nitrogen excreted. Note that most nutrient analysis programs report in grams of protein, not nitrogen (1 g of protein divided by 6.2 = 1 g of nitrogen);
■ Fat balance studies should not be performed on subjects who are not in a stable state of health, or who are receiving antibiotics for an acute illness or other medications that affect intestinal motility, maldigestion or malabsorption.
Future perspective.
These recommendations from the Cystic Fibrosis Foundation's CF Therapeutics Development Network and Data Safety Monitoring Board focus on the design of PERT studies for the express purpose of conducting these studies in accordance with the FDA guidance for submission of NDAs for PERT products. Feasibility and safety considerations were included among the recommendations for study design and procedures. There is an ongoing need for developing a specific test of absorption of fat to assess PERT products, rather than relying on measuring fat excretion. There is also an ongoing need for other types of well-designed clinical trials of enzyme therapy in the CF population. Unaddressed issues include the relationship between CFA and long-term clinical efficacy, the relationship between dose and clinical response, the duration of effect of a dose and the optimal timing of dosing, and the comparative effectiveness of various PERT products, among others. The study of the efficacy and safety of PERT products in infants and young children is particularly challenging. Now that CF newborn screening is nearly universal, studies addressing how to determine the starting dose and how to adjust dosing of PERT products are needed. Careful attention to study design and accuracy of outcome measures for all ages will advance our understanding of the efficacy and safety of PERT for the treatment of CF-associated exocrine pancreatic insufficiency.
Executive summary.
Study designs for evaluating pancreatic enzyme replacement therapy products in patients with cycstic fibrosis must be optimized to ensure validity of study results
■ Parallel reintroduction, parallel withdrawal, and crossover designs are the recommended designs for short-term efficacy and safety studies of porcine-based pancreatic enzyme replacement therapy (PERT) products. The crossover design evaluating a PERT product versus placebo is most frequently used.
■ Crossover designs require half the number of subjects to demonstrate efficacy compared with other study designs.
Outcome measures for the evaluation of PERT products in patients with cycstic fibrosis must be robust to assess efficacy & safety
■ The preferred primary short-term efficacy outcome measure is the difference in the coefficient of fat absorption between PERT and placebo, obtained through carefully conducted timed fat-balance studies. Adherence to a controlled high-fat diet and a 72-h stool collection using dye markers in an in-patient clinical research setting is recommended.
■ Specific tests of absorption rather than excretion of fat (and nitrogen) are preferred, but not yet ready for clinical trials seeking marketing approval of PERT products.
■ Common secondary efficacy measures include coefficient of nitrogen absorption, weight gain, symptom frequency and severity, number of bowel movements, weight of stool and other stool characteristics.
■ Monitoring for safety during treatment and placebo periods should include assessments for weight loss, abdominal pain and intestinal obstruction (constipation). Blood and urine should be assessed for uric acid.
Special considerations should be given to studying PERT in children who are unable to consent or assent to studies
■ The Cystic Fibrosis Foundation Data Safety Monitoring Board and the Therapeutics Development Network recommend that children below the age of assent and consent (<7 years of age) should not be subjected to a period of placebo or off enzymes.
■ Coefficient of fat absorption or other outcomes based on structured diets and timed stool collections are difficult to perform in infants and toddlers. Development of a non-stool based measure of PERT efficacy is sorely needed.
■ Assessing growth (e.g., rate of weight gain) in a dose-comparison study might provide additional evidence for PERT efficacy in infants and young children. Longer study duration and close monitoring would be required.
Acknowledgments
This work was funded by the gracious support of the NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant P30 DK027651. The content is solely the responsibility of the authors and does not represent the views of the NIDDK or the NIH. M Konstan discloses a consulting relationship with Digestive Care, Inc., Abbott, Aptalis, and Eli Lilly, all of whom develop, manufacture and/or market pancreatic enzyme replacement therapy products. D Borowitz discloses a consulting relationship with Eli Lilly and CF Foundation Therapeutics, Inc., for a Phase IV study evaluating the incidence of fibrosing colonopathy, which is co-sponsored by Abbott, Aptalis and Johnson & Johnson. W Morgan is chair of the national Cystic Fibrosis Foundation Data Safety Monitoring Board, which has overseen pancreatic enzyme replacement therapy studies over the past 6 years. B Ramsey is director of the Cystic Fibrosis Foundation Therapeutics Development Network Coordinating Center, which provided support to Eurand (Aptalis) and Digestive Care, Inc., for the implementation of their studies.
Footnotes
Disclosure
These recommendations were developed with support from the Cystic Fibrosis Foundation.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
References/Websites
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1■.Borowitz D, Hendeles H. Pancreatic enzyme products. Pediatr. Allergy Immunol. Pulmonol. 2010;23:291–294. [Provides an overview of pancreatic enzyme replacement therapy (PERT) products and US FDA regulation.] [Google Scholar]
- 2■■.Guidance for Industry: Exocrine pancreatic insufficiency drug products – Submitting NDAs. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2006. [FDA guidance forming the basis for recommendations of study design and considerations made by the authors of this paper.] [Google Scholar]
- 3■■.Brady MS, Garson JL, Krug SK, et al. An enteric-coated high-buffered pancrelipase reduces steatorrhea in patients with cystic fibrosis: a prospective, randomized study. J. Am. Diet. Assoc. 2006;106:1181–1186. doi: 10.1016/j.jada.2006.05.011. [Enteric-coated bicarbonate-buffered PERT was significantly more effective than enteric-coated PERT without bicarbonate in reducing steatorrhea in cystic fibrosis (CF) patients when administered at equivalent doses.] [DOI] [PubMed] [Google Scholar]
- 4.Stern RC, Eisenberg JD, Wagener JS, et al. A comparison of the efficacy and tolerance of pancrelipase and placebo in the treatment of steatorrhea in cystic fibrosis patients with clinical pancreatic insufficiency. Am. J. Gastroenterol. 2000;95:1932 –1938. doi: 10.1111/j.1572-0241.2000.02244.x. [DOI] [PubMed] [Google Scholar]
- 5.Konstan MW, Stern RC, Trout JR, et al. Ultrase MT12 and Ultrase MT20 in the treatment of exocrine pancreatic insufficiency in cystic fibrosis: safety and efficacy. Aliment. Pharmacol. Ther. 2004;20:1365–1371. doi: 10.1111/j.1365-2036.2004.02261.x. [DOI] [PubMed] [Google Scholar]
- 6■■.Trapnell BC, Maguiness K, Graff GR, Boyd D, Beckmann K, Caras S. Efficacy and safety of Creon 24,000 in subjects with exocrine pancreatic insufficiency due to cystic fibrosis. J. Cyst. Fibros. 2009;8:370–377. doi: 10.1016/j.jcf.2009.08.008. [A crossover design with placebo that was included in the New Drug Application (NDA) submission for the approval of CREON® 24,000.] [DOI] [PubMed] [Google Scholar]
- 7■■.Wooldridge JL, Heubi JE, Amaro-Galvez R, et al. EUR-1008 pancreatic enzyme replacement is safe and effective in patients with cystic fibrosis and pancreatic insufficiency. J. Cyst. Fibros. 2009;8:405–417. doi: 10.1016/j.jcf.2009.07.006. [A crossover design with placebo that was included in the NDA submission for the approval of ZENPEP®.] [DOI] [PubMed] [Google Scholar]
- 8.Graff GR, Maguiness K, McNamara J, et al. Efficacy and tolerability of a new formulation of pancrelipase delayed-release capsules in children ages 7 to 11 years with exocrine pancreatic insufficiency and cystic fibrosis: a multicenter, randomized, double-blind, placebo-controlled, two-period crossover, superiority study. Clin. Ther. 2010;32:89–103. doi: 10.1016/j.clinthera.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 9■■.Konstan MW, Liou TG, Strausbaugh SD, et al. Efficacy and safety of a new formulation of pancrelipase (Ultrase MT20) in the treatment of malabsorption in exocrine pancreatic insufficiency in cystic fibrosis. Gastroenterol. Res. Pract. 2010:898193. doi: 10.1155/2010/898193. [A crossover design with placebo that was included in the NDA submission for the approval of ULTRESA™.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10■■.Trapnell BC, Strausbaugh SD, Woo MS, et al. Efficacy and safety of PANCREAZE® for treatment of exocrine pancreatic insufficiency due to cystic fibrosis. J. Cyst. Fibros. 2011;10:350–356. doi: 10.1016/j.jcf.2011.04.005. [A parallel withdrawal design with placebo that was included in the NDA submission for the approval of PANCREAZE®.] [DOI] [PubMed] [Google Scholar]
- 11.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H, Clinical Practice Guidelines on Growth and Nutrition Subcommittee and the Ad Hoc Working Group Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J. Am. Diet. Assoc. 2008;108:832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 12■■.Borowitz D, Durie PR, Clarke LL, et al. Gastrointestinal Outcomes and Confounders in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2005;41:273–285. doi: 10.1097/01.mpg.0000178439.64675.8d. [Special report, from a Cystic Fibrosis Foundation Workshop that included experts from the USA and Europe from both academia and industry, provides an overview of CF gastrointestinal disease, PERT and methods to measure fat absorption.] [DOI] [PubMed] [Google Scholar]
- 13.van de Kamer JH. Total fatty acids in stool. In: Seligson D, editor. Standard Methods of Clinical Chemistry. Vol. 2. Academic Press; New York, NY: 1958. pp. 34–39. [Google Scholar]
- 14.Jeejeebhoy KN, Ahmad S, Kozak G. Determination of fecal fats containing both medium and long chain triglycerides and fatty acids. Clin. Biochem. 1970;3:157–163. [PubMed] [Google Scholar]
- 15.Kloke KM, Ward JN, Brown JE, Chezick PA, McConnell JP. Simple and Rapid Determination of Fecal Fat by Nuclear Magnetic Resonance Spectroscopy.. Presented at: American Association for Clinical Chemistry, 56th National Meeting; Los Angeles, CA, USA. July 2004.pp. 25–29. [Google Scholar]
- 16.Korpi-Steiner NL, Ward JN, Kumar V, McConnell JP. Comparative analysis of fecal fat quantitation via nuclear magnetic resonance spectroscopy (1H NMR) and gravimetry. Clin. Chim. Acta. 2009;400(1–2):33–36. doi: 10.1016/j.cca.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Fomon SJ, Ziegler EE, Thomas LN, Jensen RL, Filer LJ. Excretion of fat by normal full-term infants fed various milks and formulas. Am. J. Clin. Nutr. 1970;23:1299–1313. doi: 10.1093/ajcn/23.10.1299. [DOI] [PubMed] [Google Scholar]
- 18.van den Neuckere, Pestel N, Tran TM, et al. Clinical use of acid steatocrit. Acta Pediatr. 1997;86:466–469. doi: 10.1111/j.1651-2227.1997.tb08914.x. [DOI] [PubMed] [Google Scholar]
- 19.Wagner MH, Bowser EK, Sherman JM, Francisco MP, Theriaque D, Novak DA. Comparison of steatocrit and fat absorption in persons with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2002;35:202–205. doi: 10.1097/00005176-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Schuette SA, Janghorbani M, Cohen MB, et al. Dysprosium chloride as a nonabsorbable gastrointetstinal marker for studies of stable-isotope-labeled triglyceride excretion in man. J. Am. Coll. Nutr. 2003;22:379–387. doi: 10.1080/07315724.2003.10719321. [DOI] [PubMed] [Google Scholar]
- 21.Schuette SA, Janghorbani M, Cohen MB, et al. Effect of triglyceride structure on fecal excretion of 13C-labeled triglycerides. J. Am. Coll. Nutr. 2003;22:511–518. doi: 10.1080/07315724.2003.10719329. [DOI] [PubMed] [Google Scholar]
- 22.Ritz MA, Fraser RJ, Di Matteo AC, et al. Evaluation of the 13C-triolein breath test for fat malabsorption in adult patients with cystic fibrosis. J. Gastroenterol. Hepatol. 2004;19:448–453. doi: 10.1111/j.1440-1746.2003.03310.x. [DOI] [PubMed] [Google Scholar]
- 23.Amarri S, Harding M, Coward WA, Evans TJ, Weaver LT. 13Carbon mixed triglyceride breath test and pancreatic enzyme supplementation in cystic fibrosis. Arch. Dis. Child. 1977;76:349–351. doi: 10.1136/adc.76.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Boeck K, Delbeke I, Eggermont E, Veereman-Wauters G, Ghoos Y. Lipid digestion in cystic fibrosis: comparison of conventional and high-lipase enzyme therapy using the mixed-triglyceride breath test. J. Pediatr. Gastroenterol. Nutr. 1998;26:408–411. doi: 10.1097/00005176-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Lepage G, Yesair DW, Ronco N, et al. Effect of an organized lipid matrix on lipid absorption and clinical outcomes in patients with cystic fibrosis. J. Pediatr. 2002;141(2):178–185. doi: 10.1067/mpd.2002.124305. [DOI] [PubMed] [Google Scholar]
- 26.Stallings VA, Mondick JT, Schall JI, Barrett JS, Wilson M, Mascarenhas MR. Diagnosing malabsorption with systemic lipid profiling: pharmacokinetics of pentadecanoic acid and triheptadecanoic acid following oral administration in healthy subjects and subjects with cystic fibrosis. Int. J. Clin. Pharmacol. Ther. 2013;51(4):263–273. doi: 10.5414/CP201793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munck A, Duhamel J-F, Lamireau T, et al. Pancreatic enzyme replacement therapy for young cystic fibrosis patients. J. Cyst. Fibr. 2009;8:14–18. doi: 10.1016/j.jcf.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Kalnins D, Ellis L, Corey M, et al. Enteric-coated pancreatic enzyme with bicarbonate is equal to standard enteric-coated enzyme in treating malabsorption in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2006;42:256–261. doi: 10.1097/01.mpg.0000189356.93784.01. [DOI] [PubMed] [Google Scholar]
- 29■■.Baker SS, Borowitz D, Duffy L, Fitzpatrick L, Gyamfi J, Baker RD. Pancreatic enzyme therapy and clinical outcomes in patients with cystic fibrosis. J. Pediatr. 2005;146(2):189–193. doi: 10.1016/j.jpeds.2004.09.003. [Assessed the relationship between PERT and growth, abdominal pain, constipation, gassiness and number of stools in 1110 patients with CF.] [DOI] [PubMed] [Google Scholar]
- 30.Borowitz D, Konstan MW, Goss C, Limauro SE, Murray FT, the ALTU-135 Study Group Treatment with ALTU-135 results in a positive inverse relationship between coefficient of fat absorption with stool weight in subjects with cystic fibrosis-related pancreatic insufficiency. J. Cyst. Fibr. 2006;5:S56. [Google Scholar]
- 31.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and Validation of the Cystic Fibrosis Questionnaire (CFQ) in the United States: a health-related quality of life measure for cystic fibrosis. Chest. 2005;128:2347–2354. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 32.Borowitz D, Goss CH, Stevens C, et al. Safety and preliminary clinical activity of a novel pancreatic enzyme preparation in pancreatic insufficient patients with cystic fibrosis. Pancreas. 2006;32:258–263. doi: 10.1097/01.mpa.0000202952.10612.21. [DOI] [PubMed] [Google Scholar]
- 33.Durie P, Kalnins D, Ellis L. Uses and abuses of enzyme therapy in cystic fibrosis. J. Royal Soc. Med. 1997;91(Suppl. 34):2–13. doi: 10.1177/014107689809134s02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34■■.Konstan MW, Accurso FJ, Nasr SZ, Ahrens RC, Graff GR. Efficacy and safety of a unique enteric-coated bicarbonate-buffered pancreatic enzyme replacement therapy in children and adults with cystic fibrosis. Clin. Investig. 2013;3(8):723–729. doi: 10.4155/cli.13.62. [A cross-over design with placebo that was included in the NDA submission for PERTZYE®.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francisco MP, Wagner MH, Sherman JM, et al. Ranitidine and omeprazole as adjuvant therapy to pancrelipase to improve fat absorption in patients with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2002;35:79–83. doi: 10.1097/00005176-200207000-00017. [DOI] [PubMed] [Google Scholar]
- 36■■.Borowitz D, Konstan M, O'Rourke A, Cohen M, Hendeles L, Murray F. Coefficient of fat and nitrogen absorption in healthy subjects and individuals with cystic fibrosis. J. Pediatr. Pharamcol. Ther. 2007;12:47–52. doi: 10.5863/1551-6776-12.1.47. [Provides guidance regarding timed stool collections and the use of coefficient of fat absorption, including its limitations, as an outcome measure for PERT trials in CF.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller FG, Wendler D, Wilfond B. When do federal regulations allow placebo-controlled trials in children? J. Pediatr. 2003;142:102–107. doi: 10.1067/mpd.2003.43. [DOI] [PubMed] [Google Scholar]
- 38.WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards: Growth velocity based on weight, length and head circumference: methods and development. World Health Organization; Geneva, Switzerland: 2009. [Google Scholar]
- 39.Borowitz D, Robinson KA, Rosenfeld M, et al. Cystic Fibrosis Foundation Evidence-Based Guidelines for Management of Infants with Cystic Fibrosis. J. Pediatr. 2009;155(6 Suppl.):S73–S93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stapleton FB, Kennedy J, Nousia-Arvanitakis S, Linshaw MA. Hyperuricosuria due to high-dose pancreatic extract therapy in cystic fibrosis. N. Engl. J. Med. 1976;295:246–248. doi: 10.1056/NEJM197607292950503. [DOI] [PubMed] [Google Scholar]
- 41.Baldree LA, Stapleton FB. Uric acid metabolism in children. Pediatr. Clin. North Am. 1990;37:391–418. doi: 10.1016/s0031-3955(16)36876-6. [DOI] [PubMed] [Google Scholar]
- 42.Goss CH, Mayer-Hamblett N, Kronmal RA, Williams J, Ramsey BW. Laboratory parameter profiles among patients with cystic fibrosis. J. Cyst. Fibr. 2007;6:117–123. doi: 10.1016/j.jcf.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Smyth RL, van Velzen D, Smyth AR, Lloyd DA, Heaf DP. Strictures of ascending colon in cystic fibrosis and high-strength pancreatic enzymes. Lancet. 1994;343(8889):85–86. doi: 10.1016/s0140-6736(94)90817-6. [DOI] [PubMed] [Google Scholar]
- 44.Schwarzenberg SJ, Wielinski CL, Shamieh I. Cystic fibrosis-associated colitis and fibrosing colonopathy. J. Pediatr. 1995;127(4):565–570. doi: 10.1016/s0022-3476(95)70113-3. [DOI] [PubMed] [Google Scholar]
- 45■■.FitzSimmons SC, Burkhart GA, Borowitz D. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N. Engl. J. Med. 1997;336(18):1283–1289. doi: 10.1056/NEJM199705013361803. [Supports previous reports that high doses of PERT products were associated with fibrosing colonopathy in patients with CF. This finding ultimately led to the FDA determination that PERT products must be subjected to the NDA process.] [DOI] [PubMed] [Google Scholar]
- 46.Papas AM. Vitamin E: Tocopherols and Tocotrienols. In: Papas AM, editor. Antioxidant Status, Diet, Nutrition and Health. CRC Press; Boca Raton, FL, USA: 1998. [Google Scholar]
- 47.Cheeseman KH, Holley AE, Kelly FJ, Wasil M, Hughes L, Burton G. Biokinetics in humans of RRR-alpha-tocopherol: the free phenol, acetate ester, and succinate ester forms of vitamin E. Free Radic. Biol. Med. 1995;19(5):591–598. doi: 10.1016/0891-5849(95)00083-a. [DOI] [PubMed] [Google Scholar]
- 48.Borowitz D, Goss CH, Limauro S, et al. A Phase 2, randomized, double-blind, parallel dose-ranging study of a novel pancreatic enzyme replacement therapy (ALTU-135) in pancreatic insufficient subjects with cystic fibrosis. J. Pediatr. 2006;149:658–662. doi: 10.1016/j.jpeds.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 49.Houwen RHJ, van der Doef HP, Sermet I, The ESPGHAN Cystic Fibrosis Working Group Defining DIOS and constipation in cystic fibrosis with a multicenter study on the incidence, characteristics and treatment of DIOS. J. Pediatr. Gastroenterol. Nutr. 2010;50(1):38–42. doi: 10.1097/MPG.0b013e3181a6e01d. [DOI] [PubMed] [Google Scholar]
- 101.Prescribing information for CREON®. www.rxabbott.com/pdf/creon_PI.pdf.
- 102.Prescribing information for PANCREAZE®. www.pancreaze.net/pdf/PANCREAZE.pdf.
- 103.Prescribing information for PERTZYE®. www.digestivecare.com/PDFs/NDA%20022175%20APPROVAL%2017MAY2012_PI&MG.pdf.
- 104.Prescribing information for ULTRESA™. www.aptalispharma.com/pdf/ultresa_info.pdf.
- 105.Prescribing information for ZENPEP®. www.zenpep.com/pdf/prescribing-info.pdf.
- 106.FDA guidance for industry for submitting NDAs for exocrine pancreatic insufficiency drug products. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071651.pdf.