Abstract
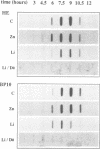
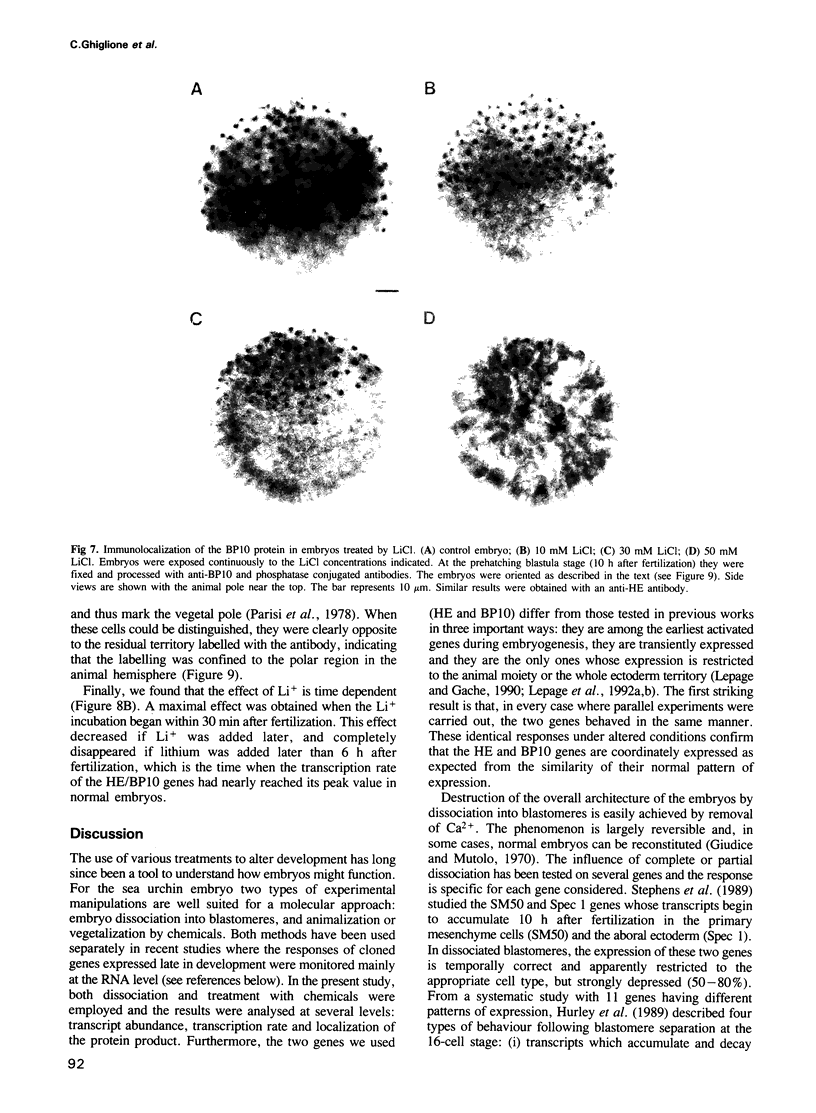
The expression of two zygotic genes (HE and BP10) during sea urchin embryogenesis was previously found to be early, transient, spatially restricted and controlled at the transcriptional level. Here we studied how the expression of these genes is affected when cell interactions are abolished by dissociating blastomeres and when development is perturbed by treatment with Li+. We found that in isolated blastomeres, transient transcriptional activity (HE) is unchanged and both genes apparently function in the appropriate cell type. Thus HE/BP10 expression is largely cell-autonomous and should rely on maternal factors unevenly distributed in the egg. Treatment with lithium does not affect the temporal control but decreases the transcriptional activity and the size of the domain of expression of the HE/BP10 genes. As the Li+ concentration increases, the border of the domain is progressively shifted towards the animal pole. This alteration of the spatial pattern is the earliest molecular evidence of a change in cell fate detectable only much later by morphological criteria, and reveals a gradient of sensitivity to Li+ along the animal--vegetal axis. These results suggest that the activity of the HE/BP10 genes is strongly dependent on spatially organized maternal information controlling early development.
Full text
PDF




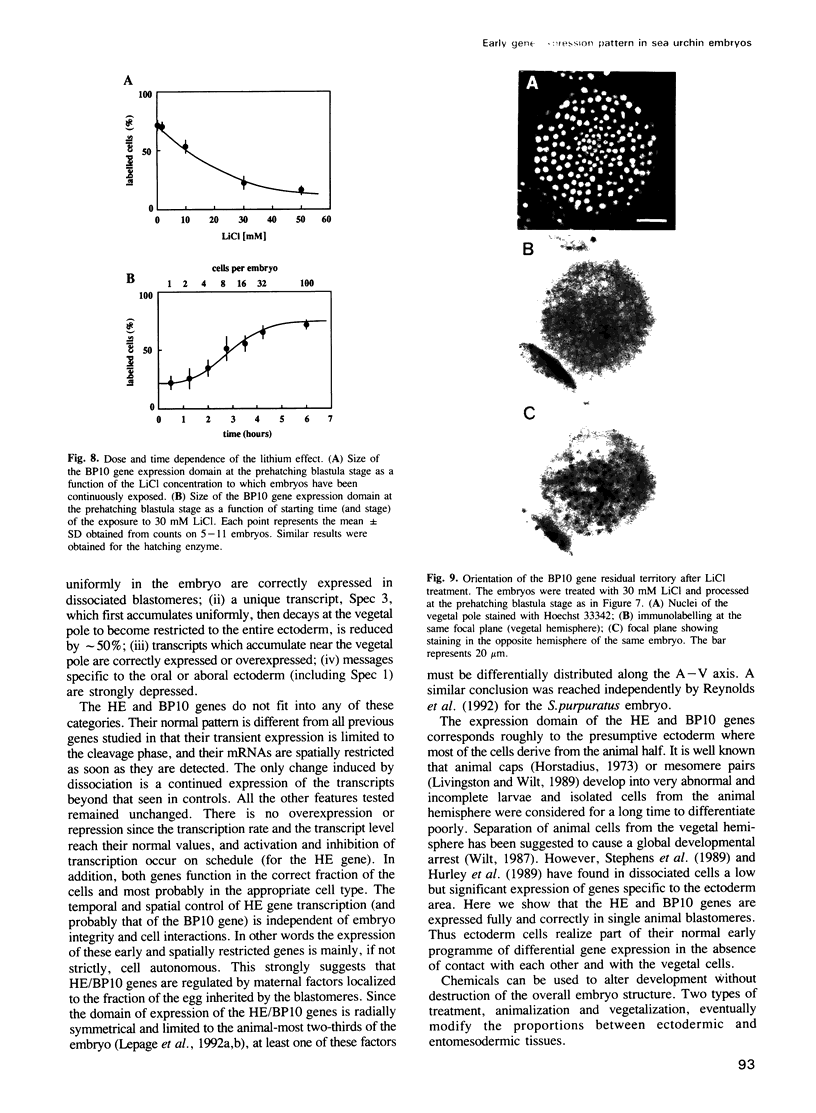



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander C. M., Werb Z. Proteinases and extracellular matrix remodeling. Curr Opin Cell Biol. 1989 Oct;1(5):974–982. doi: 10.1016/0955-0674(89)90068-9. [DOI] [PubMed] [Google Scholar]
- Angerer R. C., Davidson E. H. Molecular indices of cell lineage specification in sea urchin embryos. Science. 1984 Dec 7;226(4679):1153–1160. doi: 10.1126/science.6594757. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989 Nov 3;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Cetta A., Davidson E. H. The single-copy DNA sequence polymorphism of the sea urchin Strongylocentrotus purpuratus. Cell. 1978 Dec;15(4):1175–1186. doi: 10.1016/0092-8674(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Gimlich R. L. Lithium-induced teratogenesis in frog embryos prevented by a polyphosphoinositide cycle intermediate or a diacylglycerol analog. Dev Biol. 1989 Apr;132(2):315–324. doi: 10.1016/0012-1606(89)90228-5. [DOI] [PubMed] [Google Scholar]
- Cameron R. A., Fraser S. E., Britten R. J., Davidson E. H. Segregation of oral from aboral ectoderm precursors is completed at fifth cleavage in the embryogenesis of Strongylocentrotus purpuratus. Dev Biol. 1990 Jan;137(1):77–85. doi: 10.1016/0012-1606(90)90009-8. [DOI] [PubMed] [Google Scholar]
- Cameron R. A., Fraser S. E., Britten R. J., Davidson E. H. The oral-aboral axis of a sea urchin embryo is specified by first cleavage. Development. 1989 Aug;106(4):641–647. doi: 10.1242/dev.106.4.641. [DOI] [PubMed] [Google Scholar]
- Cameron R. A., Hough-Evans B. R., Britten R. J., Davidson E. H. Lineage and fate of each blastomere of the eight-cell sea urchin embryo. Genes Dev. 1987 Mar;1(1):75–85. doi: 10.1101/gad.1.1.75. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. Lineage-specific gene expression and the regulative capacities of the sea urchin embryo: a proposed mechanism. Development. 1989 Mar;105(3):421–445. doi: 10.1242/dev.105.3.421. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. Spatial mechanisms of gene regulation in metazoan embryos. Development. 1991 Sep;113(1):1–26. doi: 10.1242/dev.113.1.1. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gagnon M. L., Angerer L. M., Angerer R. C. Posttranscriptional regulation of ectoderm-specific gene expression in early sea urchin embryos. Development. 1992 Feb;114(2):457–467. doi: 10.1242/dev.114.2.457. [DOI] [PubMed] [Google Scholar]
- Gee N. S., Ragan C. I., Watling K. J., Aspley S., Jackson R. G., Reid G. G., Gani D., Shute J. K. The purification and properties of myo-inositol monophosphatase from bovine brain. Biochem J. 1988 Feb 1;249(3):883–889. doi: 10.1042/bj2490883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice G., Mutolo V. Reaggregation of dissociated cells of sea urchin embryos. Adv Morphog. 1970;8:115–158. doi: 10.1016/b978-0-12-028608-9.50007-7. [DOI] [PubMed] [Google Scholar]
- Goldstein B. Induction of gut in Caenorhabditis elegans embryos. Nature. 1992 May 21;357(6375):255–257. doi: 10.1038/357255a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Embryonic induction--molecular prospects. Development. 1987 Mar;99(3):285–306. doi: 10.1242/dev.99.3.285. [DOI] [PubMed] [Google Scholar]
- Hall H. G. Hardening of the sea urchin fertilization envelope by peroxidase-catalyzed phenolic coupling of tyrosines. Cell. 1978 Oct;15(2):343–355. doi: 10.1016/0092-8674(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Hurley D. L., Angerer L. M., Angerer R. C. Altered expression of spatially regulated embryonic genes in the progeny of separated sea urchin blastomeres. Development. 1989 Jul;106(3):567–579. doi: 10.1242/dev.106.3.567. [DOI] [PubMed] [Google Scholar]
- Johnson M. H., Ziomek C. A. Cell interactions influence the fate of mouse blastomeres undergoing the transition from the 16- to the 32-cell stage. Dev Biol. 1983 Jan;95(1):211–218. doi: 10.1016/0012-1606(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Klein S. L., Moody S. A. Lithium changes the ectodermal fate of individual frog blastomeres because it causes ectopic neural plate formation. Development. 1989 Jul;106(3):599–610. doi: 10.1242/dev.106.3.599. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lepage T., Gache C. Early expression of a collagenase-like hatching enzyme gene in the sea urchin embryo. EMBO J. 1990 Sep;9(9):3003–3012. doi: 10.1002/j.1460-2075.1990.tb07493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage T., Gache C. Purification and characterization of the sea urchin embryo hatching enzyme. J Biol Chem. 1989 Mar 25;264(9):4787–4793. [PubMed] [Google Scholar]
- Lepage T., Ghiglione C., Gache C. Spatial and temporal expression pattern during sea urchin embryogenesis of a gene coding for a protease homologous to the human protein BMP-1 and to the product of the Drosophila dorsal-ventral patterning gene tolloid. Development. 1992 Jan;114(1):147–163. doi: 10.1242/dev.114.1.147. [DOI] [PubMed] [Google Scholar]
- Lepage T., Sardet C., Gache C. Spatial expression of the hatching enzyme gene in the sea urchin embryo. Dev Biol. 1992 Mar;150(1):23–32. doi: 10.1016/0012-1606(92)90004-z. [DOI] [PubMed] [Google Scholar]
- Livingston B. T., Wilt F. H. Lithium evokes expression of vegetal-specific molecules in the animal blastomeres of sea urchin embryos. Proc Natl Acad Sci U S A. 1989 May;86(10):3669–3673. doi: 10.1073/pnas.86.10.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston B. T., Wilt F. H. Range and stability of cell fate determination in isolated sea urchin blastomeres. Development. 1990 Mar;108(3):403–410. doi: 10.1242/dev.108.3.403. [DOI] [PubMed] [Google Scholar]
- Maslanski J. A., Leshko L., Busa W. B. Lithium-sensitive production of inositol phosphates during amphibian embryonic mesoderm induction. Science. 1992 Apr 10;256(5054):243–245. doi: 10.1126/science.1314424. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemer M. An altered series of ectodermal gene expressions accompanying the reversible suspension of differentiation in the zinc-animalized sea urchin embryo. Dev Biol. 1986 Mar;114(1):214–224. doi: 10.1016/0012-1606(86)90397-0. [DOI] [PubMed] [Google Scholar]
- Nemer M., Wilkinson D. G., Travaglini E. C. Primary differentiation and ectoderm-specific gene expression in the animalized sea urchin embryo. Dev Biol. 1985 Jun;109(2):418–427. doi: 10.1016/0012-1606(85)90468-3. [DOI] [PubMed] [Google Scholar]
- Nocente-McGrath C., McIsaac R., Ernst S. G. Altered cell fate in LiCl-treated sea urchin embryos. Dev Biol. 1991 Oct;147(2):445–450. doi: 10.1016/0012-1606(91)90302-j. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C. Determination of the embryonic axes of Drosophila. Dev Suppl. 1991;1:1–10. [PubMed] [Google Scholar]
- Parisi E., Filosa S., De Petrocellis B., Monroy A. The pattern of cell division in the early development of the sea urchin. Paracentrotus lividus. Dev Biol. 1978 Jul;65(1):38–49. doi: 10.1016/0012-1606(78)90177-x. [DOI] [PubMed] [Google Scholar]
- Peters D. J., Van Lookeren Campagne M. M., Van Haastert P. J., Spek W., Schaap P. Lithium ions induce prestalk-associated gene expression and inhibit prespore gene expression in Dictyostelium discoideum. J Cell Sci. 1989 May;93(Pt 1):205–210. doi: 10.1242/jcs.93.1.205. [DOI] [PubMed] [Google Scholar]
- Reynolds S. D., Angerer L. M., Palis J., Nasir A., Angerer R. C. Early mRNAs, spatially restricted along the animal-vegetal axis of sea urchin embryos, include one encoding a protein related to tolloid and BMP-1. Development. 1992 Mar;114(3):769–786. doi: 10.1242/dev.114.3.769. [DOI] [PubMed] [Google Scholar]
- Shimell M. J., Ferguson E. L., Childs S. R., O'Connor M. B. The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell. 1991 Nov 1;67(3):469–481. doi: 10.1016/0092-8674(91)90522-z. [DOI] [PubMed] [Google Scholar]
- Showman R. M., Foerder C. A. Removal of the fertilization membrane of sea urchin embryos employing aminotriazole. Exp Cell Res. 1979 May;120(2):253–255. doi: 10.1016/0014-4827(79)90385-9. [DOI] [PubMed] [Google Scholar]
- Stephens L., Kitajima T., Wilt F. Autonomous expression of tissue-specific genes in dissociated sea urchin embryos. Development. 1989 Oct;107(2):299–307. doi: 10.1242/dev.107.2.299. [DOI] [PubMed] [Google Scholar]
- Wilt F. H. Determination and morphogenesis in the sea urchin embryo. Development. 1987 Aug;100(4):559–576. doi: 10.1242/dev.100.4.559. [DOI] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Yang Q., Angerer L. M., Angerer R. C. Structure and tissue-specific developmental expression of a sea urchin arylsulfatase gene. Dev Biol. 1989 Sep;135(1):53–65. doi: 10.1016/0012-1606(89)90157-7. [DOI] [PubMed] [Google Scholar]