Abstract
Several phlebotomine sand fly species have been regarded as putative or proven vectors of parasites of the genus Leishmania in Brazil, but data for the northeastern region remains incipient. In this study, a total of 600 phlebotomine sand flies were grouped in pools of 10 specimens each and tested by a Leishmania genus-specific PCR and by a PCR targeting Leishmania (Leishmania) infantum. Fourteen out of 60 pools were positive by the genus-specific PCR, being five pools of L. migonei, seven of L. complexa, one of L. sordellii and one of L. naftalekatzi, which correspond to a minimal infection rate of 2.3% (14/600). Our results, associated with their known anthropophily and their abundance, suggest the participation of L. migonei and L. complexa as vectors of Leishmania in northeastern Brazil. Remarkably, this is the first time in this country that the detection of Leishmania DNA in L. sordellii and L. naftalekatzi has been reported, but future studies are necessary to better understand the significance of these findings.
Keywords: Phlebotomine sand flies, Leishmania, Transmission
Abstract
Vários flebotomíneos têm sido considerados como possíveis vetores suspeitos ou comprovados de parasitas do gênero Leishmania no Brasil, mas os dados para região nordeste continuam incipientes. Neste estudo, 600 flebotomíneos foram agrupados em grupos de 10 espécimes e testados por uma PCR específica para o gênero Leishmania e por outra PCR para Leishmania (Leishmania) infantum. Quatorze dos 60 grupos foram positivos por PCR gênero-específica, sendo cinco de L. migonei, sete L. complexa, um de L. sordellii e um de L. naftalekatzi, o que corresponde a uma taxa mínima de infecção de 2,3% (14/600). Nossos resultados, associados à antropofilia e abundância dessas espécies, sugerem a participação de L. migonei e L. complexa como vetores de Leishmania no nordeste do Brasil. Notavelmente, a detecção de DNA de Leishmania em L. sordellii e L. naftalekatzi é relatada pela primeira vez no Brasil, mas futuros estudos são necessários para compreender melhor o significado desses achados.
Leishmania parasites are the causative agents of leishmaniasis, a group of diseases with a broad clinical spectrum, which may be present in cutaneous and visceral forms, according to the parasite species involved and the host response to infection. Brazil represents one of the largest foci of leishmaniasis from a worldwide perspective2, with several thousand cases notified every year and sporadic outbreaks reported in both rural and urban centers.
The most important causative agents of American cutaneous leishmaniasis (ACL) and zoonotic visceral leishmaniasis (VL) are Leishmania (Viannia) braziliensis and Leishmania (Leishmania) infantum (syn. L. chagasi), respectively. Their transmission occurs as the result of the bite of phlebotomine sand fly females, such as Lutzomyia whitmani, L. intermedia, L. migonei, L. wellcomei and L. complexa for L. (V.) braziliensis and L. longipalpis for L. (L.) infantum 13. Indeed, several phlebotomine sand flies have been regarded as putative or proven vectors of L. (V.) braziliensis in Brazil11,13, but studies have been limited to particular regions of the country and data from northeastern Brazil remains incipient.
ACL is the most prevalent form of leishmaniasis in Pernambuco State, northeastern Brazil9. Since the 1980s, the number of VL cases has been on the rise and a 5-fold increase in the number of municipalities that report one or more VL cases was recorded between 1990 and 20016. It means that both forms of leishmaniasis are widespread in Pernambuco, with overlapping distribution in some areas, which provides us with the opportunity to study the diversity of potential vectors for both L. (V.) braziliensis and L. (L.) infantum in sympatric areas. In this perspective, we have recently conducted an entomological survey to study the fauna of phlebotomine sand flies in an area where ACL and VL occur in sympatry in northeastern Brazil9. Herein, we assessed the infection by Leishmania spp. in phlebotomine sand flies collected in the framework of our previous study.
The study was carried out in a rural community located in the municipality of São Vicente Férrer (07°35′27″S, 35°29′27′″W) in the northern rainforest area of Pernambuco, where both visceral and cutaneous leishmaniasis are endemic. The municipality has an area of 110,489 sq km and an estimated population of 17,000 inhabitants. The climate is tropical with a mean average annual temperature of 23 °C. Throughout the years, this area has been affected by an intense process of deforestation and the primary vegetation was largely substituted by banana tree plantations and rural properties, in which the presence of animal shelters (e.g., chicken coops and stables) near remnants of Atlantic rainforest is commonly observed.
Insects were captured monthly from September 2009 to September 2010 using CDC light traps (33 traps per month on average), from 18:00 to 6:00, during four consecutive nights. Traps were placed either in the peridomicile or forest remnants, and the specimens captured were identified morphologically16. In total, 600 unfed female phlebotomine sand flies belonging to four species (L. migonei, L. complexa, L. sordellii and L. naftalekatzi) were grouped in pools of 10 specimens each and stored at -70 °C until DNA extraction.
DNA extraction was effected as described elsewhere1 with some modifications. Pools of insects were macerated in 1.5 mL tubes containing one mL of PBS, which were then centrifuged at 12000 g for two min. After removing the supernatant, 100 µL of lysis solution type 1 (GenomicPrep Cells and Tissue DNA isolation kits, GE Healthcare, Piscataway, NJ, USA) were added and the tubes shaken vigorously for 15 s. After adding 10 µL of proteinase K (30 mg/mL) the tubes were shaken again for 15 s and incubated at 56 °C for one hour. Then, the suspension was once again incubated at 70 °C for 10 min and then centrifuged at 6000 g for 10 min. The supernatants were transferred to 1.5 mL tubes and frozen in absolute ethanol (in a volume twice as much the supernatant recovered). The material was stored at -20 °C for 18 h and, after that, the tubes were centrifuged at 6500 g for five min and after discharging the supernatants, the sediments were dried at room temperature. The extracted DNA was re-suspended in 50 µL 0.1 × TE buffer (pH 8.0) at 70 °C and stored at -20 °C. The concentration and purity of DNA samples were assessed using a spectrophotometer (Ultrospec 3000, Pharmacia Biotech).
The quality of DNA samples was assessed by PCR using the primers 5Llcac (5′-GTGGCCGAACATAATGTTAG-3′) and 3Llcac (5′-CCACGAACAAGTTCAACATC-3′)10, which amplify a 220 bp fragment of the cacophony gene of Lutzomyia spp3. PCR products were resolved on 1.5% agarose gel and visualized by ethidium bromide staining.
For Leishmania spp. detection, we used the primers LITSR (5′-CTGGATCATTTTCCGATG-3′) and L5.8S (5′-TGATACCACTTATCGCACTT-3′)7, which amplifies the internal transcription spacer 1 (ITS-1), a noncoding region placed at SSUrRNA, bounded by the genes 18S and 5.8S, which produce a 300-350 bp fragment of Leishmania spp. Amplification reactions were performed in a 50 µL volume containing 100 mM Tris-HCl, 250 mM KCl, 2.5 mM MgCl2, 250 µM dNTPs, 50 pmol of each primer, 2.5 U of Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA) and 2 µL of the template DNA. The amplification conditions consisted of 35 cycles of 95 °C for 40 s, 53 °C for 45 s and 72 °C for one min, which were preceded of initial denaturation of 95 °C for three min DNA extracted from L. (V.) braziliensis promastigotes (MHOM/BR/75/M2903) was used as positive control. PCR products were resolved on 2% agarose gels and visualized by ethidium bromide staining, using 100 bp ladder DNA (GibcoBRL-Life Technologies) as a molecular marker.
All samples were also tested by a PCR targeting the kDNA of L. (L.) infantum using the primers Linf 1B 23F (5′-TCCCAAACTTTTCTGGTCCT-3′) and Linf 1B 154R (5′-TTACACCAACCCCCAGTTTC-3′)12. The reaction was carried out in a 25 µL final volume containing 10 mM Tris-HCl, 50 nM KCl, 1.5 MgCl2, 0.2 mM dNTPs, 5 pmol of each primer, 2.5 U of Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA) and 2 µL of the template DNA. Amplification conditions consisted of 35 cycles of 94 °C for 30 s, 67 °C for one min and 72 °C for 30 s, which were preceded by initial denaturation of 94 °C for one min. DNA extracted from L. (L.) infantum promastigotes (MHOM/BR/1974/PP75) was used as positive control. PCR products were resolved on 2% agarose gels and visualized by ethidium bromide staining, using 100 bp ladder DNA (GibcoBRL-Life Technologies) as a molecular marker.
All 60 pools of phlebotomine sand flies belonging to four species (Lutzomyia migonei, Lutzomyia complexa, Lutzomyia sordellii, and Lutzomyia naftalekatzi) were analyzed and all samples were positive for the fragment of 220 bp corresponding to cacophony gene, confirming the quality of the DNA obtained (Fig. 1). A total of 14 pools were positive by Leishmania genus-specific PCR, being five pools of L. migonei, seven of L. complexa, one of L. sordellii and one of L. naftalekatzi (Fig. 2), which corresponds to a minimal infection rate of 2.3% (14/600) (Table 1).
Fig. 1 -. Agarose gel electrophoresis showing PCR amplification of cacophony gene IVS6 region of phlebotomine sand flies. MW: molecular weight marker (100 bp DNA Ladder); C1: no DNA; C2: pools of phlebotomine sand flies (positive control); lanes 1-17: positive samples of phlebotomine sand flies.

Fig. 2 -. Agarose gel electrophoresis stained showing PCR amplification of Leishmania spp. DNA from phlebotomine sand flies. MW: molecular weight marker (100 bp DNA Ladder); C1: no DNA; C2: L. (V.) braziliensis DNA (positive control); lanes 1-5: L. migonei samples; lane 6-12: L. complexa; lane 13: L. sordellii; lane 14: L. naftalekatzi.
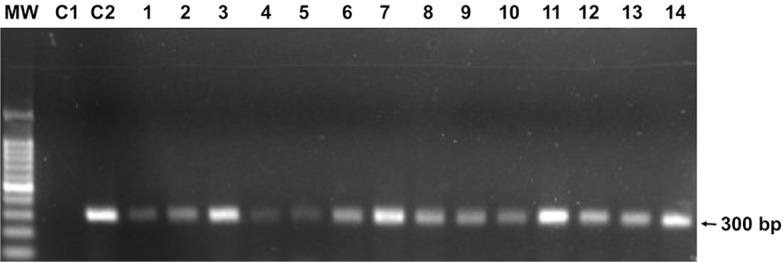
Table 1. Positivity of Leishmania DNA in phlebotomine sand flies species tested by the Leishmania genus-specific polymerase chain reaction (PCR), Northeastern Brazil.
| Species | Infected specimens/total specimens tested | Minimum infection rate (%)a | Collection site |
|---|---|---|---|
| Lutzomyia migonei | 5/190 | 2.63 | Animal shelters |
| Lutzomyia complexa | 7/370 | 1.89 | Forest remnant |
| Lutzomyia sordellii | 1/20 | 5.0 | Forest remnant |
| Lutzomyia naftalekatzi | 1/20 | 5.0 | Forest remnant |
| Total | 14/600 | 2.33 |
Minimum infection rate (MIR) = [(minimum number of infected specimens/total specimens tested) x 100].
Lutzomyia migonei has been implicated as a vector of L. (V.) braziliensis in different Brazilian regions13. This phlebotomine sand fly is widespread in Brazil, including in northeastern Brazil. In our study, L. migonei was the most abundant species (71.8%), being found both indoors and in the peridomicile, especially in animal shelters (chicken pen and stables)9. In southeastern Brazil, it has also been found naturally infected by L. (V.) braziliensis and regarded as a possible secondary vector13. Lutzomyia migonei has also been considered to be a putative vector of L. (L.) infantum 11, including in the area where the present study was carried out, where the primary vector (L. longipalpis) is absent5. In a study conducted in Rio de Janeiro near 10 years ago, the absence of L. longipalpis in six areas where VL is endemic provided circumstantial evidence for the participation of L. migonei as a vector of L. (L.) infantum 14. In the present study, all specimens of L. migonei were negative for L. (L.) infantum, which strongly indicates that they were infected by L. (V.) braziliensis.
The positivity of L. complexa to Leishmania sp. and negativity to L. (L.) infantum is in line with previous studies and indicates the participation of this species as a vector of L. (V.) braziliensis in forested environments in different Brazilian regions13. In fact, this species predominates in areas of the Atlantic rainforest and it displays strong anthropophilic behavior. On the other hand, the detection of Leishmania DNA in pools of L. sordellii and L. naftalekatzi has been reported, for the first time, in Brazil. Recently, females of L. sordellii were dissected and flagellates were seen in two of them, but an attempt to isolate the parasite failed4. L. sordellii is widespread in the country being found in many habitats, such as tree trunks, rock crevices, caves, animal shelters (e.g. chicken coops, pigsties, corrals) and households of different areas of Brazil9. However, L. sordellii is known to feed on cold-blooded rather than warm-blooded vertebrates15, which probably indicates that it plays no role in the transmission of Leishmania parasites. Conversely, L. naftalekatzi apparently has a more restricted distribution8 and there is limited information on its biology, including feeding habits, but so far there is no evidence suggesting its participation in the transmission of Leishmania parasites. In this regard, further studies with a larger number of specimens would be interesting to assess the actual prevalence of Leishmania infection in these phlebotomine sand flies, but also to identify the parasite species they carry.
The high degree of anthropophily of L. migonei and its overlapping distribution with ACL cases may suggest its participation in the zoonotic transmission cycle of ACL in the study area. In the same way, our data suggests the involvement of L. complexa in the enzootic cycle of L. (V.) braziliensis, mainly considering its close association with forested environments. For future studies, it will be important to focus on the isolation and characterization of Leishmania parasites in the aforementioned phlebotomine species, as well as to assess their feeding source, as this information might be of great epidemiological relevance.
ACKNOWLEDGEMENTS
Thanks to Kyldman Thais da Silva, Kamila Gaudêncio da Silva and Rafael Acioli Medeiros for their support with some laboratory work.
Funding Statement
This research was supported by Fundação de Amparo à Pesquisa de Pernambuco (FACEPE), APQ-0630-2.13/08.
Footnotes
FINANCIAL SUPPORT This research was supported by Fundação de Amparo à Pesquisa de Pernambuco (FACEPE), APQ-0630-2.13/08.
REFERENCES
- 1.Adamson RE, Ward RD, Feliciangeli MD, Maingon R. The application of random amplified polymorphic DNA for sandfly species identification. Med Vet Entomol. 1993;7:203–7. doi: 10.1111/j.1365-2915.1993.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLos One. 2012;7:1–12. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottecchia M, Oliveira SG, Bauzer LG, Souza NA, Ward RD, Garner KJ, et al. Genetic divergence in the cacophony IVS6 intron among five Brazilian populations of Lutzomyia longipalpis . J Mol Evol. 2004;58:754–61. doi: 10.1007/s00239-004-2586-y. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho GML, Brazil RP, Saraiva L, Quaresma PF, Botelho HA, Ramos MCNF, et al. Hourly activity and natural infection of sandflies (Diptera: Psychodidae) captured from the aphotic zone of a cave, Minas Gerais State, Brazil. Plos One. 2012;7:1–6. doi: 10.1371/journal.pone.0052254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho MR, Valença HF, Silva FJ, Pita-Pereira D, Pereira TA, Britto C, et al. Natural Leishmania infantum infection in Migonemyia migonei (Franca, 1920) (Diptera: Psychodidae: Phlebotominae) the putative vector of visceral leishmaniasis in Pernambuco State, Brazil. Acta Trop. 2010;116:108–10. doi: 10.1016/j.actatropica.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Dantas-Torres F, Brandão SP., Filho Expansão geográfica da leishmaniose visceral no estado de Pernambuco. Rev Soc Bras Med Trop. 2006;39:352–6. doi: 10.1590/s0037-86822006000400007. [DOI] [PubMed] [Google Scholar]
- 7.El Tai NO, El Fari M, Mauricio I, Miles MA, Oskam L, El Safi S, et al. Leishmania donovani: intraspecific polymorphisms of Sudanese isolates revealed by PCR based analyses and DNA sequencing. Exp Parasitol. 2001;97:35–44. doi: 10.1006/expr.2001.4592. [DOI] [PubMed] [Google Scholar]
- 8.Falcão AL, Andrade JD, Filho, Almeida FA, Brandão SP., Filho Lutzomyia naftalekatzi, a new species of phlebotomine sand fly (Diptera: Psychodidae) from Zona da Mata region, Pernambuco, Brazil. Mem Inst Oswaldo Cruz. 2000;95:843–8. doi: 10.1590/s0074-02762000000600016. [DOI] [PubMed] [Google Scholar]
- 9.Guimarães VCFV, Costa PL, Silva FJ, Silva KT, Silva KG, Araújo AIF, et al. Phlebotomine sand flies (Diptera: Psychodidae) in São Vicente Férrer, a sympatric area to cutaneous and visceral leishmaniasis in Pernambuco, Brazil. Rev Soc Bras Med Trop. 2012;45:66–70. doi: 10.1590/s0037-86822012000100013. [DOI] [PubMed] [Google Scholar]
- 10.Lins RM, Oliveira SG, Souza NA, de Queiroz RG, Justiniano SC, Ward RD, et al. Molecular evolution of the cacophony IVS6 region in sandflies. Insect Mol Biol. 2002;11:117–22. doi: 10.1046/j.1365-2583.2002.00315.x. [DOI] [PubMed] [Google Scholar]
- 11.Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013;27:123–47. doi: 10.1111/j.1365-2915.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 12.Paiva-Cavalcanti M, Felinto de Brito ME, de Souza WV, de Miranda Gomes Y, Abath FG. The development of a real-time PCR assay for the quantification of Leishmania infantum DNA in canine blood. Vet J. 2009;189:356–8. doi: 10.1016/j.tvjl.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Rangel EF, Lainson R. Proven and putative vectors of American cutaneous leishmaniasis in Brazil: aspects of their biology and vectorial competence. Mem Inst Oswaldo Cruz. 2009;104:937–54. doi: 10.1590/s0074-02762009000700001. [DOI] [PubMed] [Google Scholar]
- 14.Souza MB, Marzochi MCA, Carvalho RW, Ribeiro PC, Pontes CS, Caetano JM, et al. Ausência da Lutzomyia longipalpis em algumas áreas de ocorrência de leishmaniose visceral no município do Rio de Janeiro. Cad Saúde Pública. 2003;19:1881–5. doi: 10.1590/s0102-311x2003000600033. [DOI] [PubMed] [Google Scholar]
- 15.Tesh RB, Chaniotis BN, Aronson MD, Johnson KM. Natural host preferences of Panamanian phlebotomine sandflies as determined by precipitin test. Am J Trop Med Hyg. 1971;20:150–6. doi: 10.4269/ajtmh.1971.20.150. [DOI] [PubMed] [Google Scholar]
- 16.Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae) Mem Am Entomol Inst. 1994;54:1–881. [Google Scholar]


