Abstract
Purpose of review
While allogeneic hematopoietic stem cell transplantation (allo-SCT) is potentially curative for a number of hematologic malignancies, its use is limited by the development of acute and chronic graft-versus-host disease (GVHD). This potentially fatal complication occurs in approximately 50% of allo-SCT recipients. Its pathogenesis is poorly understood, methods to prevent it are largely unchanged over the past two decades, and response to front-line treatment with corticosteroids is suboptimal.
Recent findings
The pathogenesis of acute and chronic GVHD remains poorly understood, methods to prevent it are largely unchanged over the past two decades, and response to front-line treatment with corticosteroids is suboptimal. For patients with steroid-refractory disease, response to second-line treatment is dismal. The prospective clinical studies evaluating new agents for GVHD have been hampered by inconsistencies in design, making generalization difficult, and few multicenter studies have been conducted.
Summary
Advances have been made over the past decade in grading both acute and chronic GVHD, with the development of biomarkers that provide improved prognostic information in acute GVHD and NIH Consensus Criteria for improved grading of chronic GVHD. This, along with the broad understanding of the need to conduct prospective studies with uniform inclusion criteria and endpoints leading to multicenter studies will hopefully lead to advancements in the prevention of GVHD in the near future.
Keywords: allogeneic stem cell transplantation, acute graft-versus-host disease, chronic graft-versus-host disease
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (allo-SCT) is a potentially curative immunotherapy for many hematologic malignancies.(1) A recent Center for International Blood and Marrow Transplant Research (CIBMTR) analysis showed that despite increasing use of unrelated donors, peripheral blood and cord blood grafts, and older recipient age, survival has significantly increased over time, likely due to better donor selection with improved HLA typing and improved supportive care.(2) Acute graft-versus-host disease (aGVHD) and chronic GVHD (cGVHD) remain major causes of non-relapse mortality (NRM); approximately 50% of SCT recipients develop GVHD.(3) The graft-versus-tumor (GVT) effect is critical for the success of allo-SCT,(4) but separating this from GVH reactions may result in an increased risk of relapse or infection. This article will examine advances made in understanding, preventing, and treating GVHD, as well as why more progress has not been made in combating this significant barrier to the success of transplantation.
UNDERSTANDING THE PATHOGENESIS OF GVHD
T cell alloreactivity has been established as the primary cause of GVHD.(5) Conditioning causes tissue damage and release of pro-inflammatory cytokines, leading to activation of antigen-presenting cells (APC). APCs present processed antigen to T cells, leading to donor T cell activation.(6) Then, cytokines and cellular effectors mediate target cell injury and apoptosis (Figure 1).(7) The immunopathology underlying development of cGVHD is poorly understood but involves three main mechanisms: autoantibody production, pro-fibrotic pathways, and defective thymic function.(8) A recent excellent review provides further detail, which is outside the scope of this article.(9)
Figure 1.
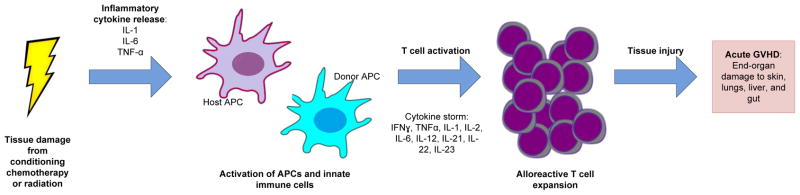
Pathogenesis if acute GVHD. Tissue damage from conditioning chemotherapy or raditation leads to immune priming and activation of antigen presenting cells (APC), follwed by activation and expansion of alloreactive T cells. These T Cells traffic to target organs and medicate tissue injury resulting in acute GVHD.
DEFINING GVHD AND IDENTIFYING THOSE AT HIGHEST RISK
aGVHD is diagnosed clinically but should be confirmed by biopsy whenever possible.(4) Historically defined as occurring within the first 100 days post-transplant, now termed “classic aGVHD,” it is recognized to occur later, termed resistent, recurrent, or late aGVHD. The Glucksberg criteria, initially published in 1974 and revised in 1995 (the Consensus criteria), remain the most commonly used grading criteria.(10)(11) Each of 3 organs are scored and combined to give an overall grade. There is significant intraobserver variability, especially for grade II. The CIBMTR criteria retain the objective organ staging of the Consensus criteria, but simplify grading.(11) Among patients receiving myeloablative SCT, the overall κ coefficient between the systems is 0.78 (95% CI 0.77–0.87), indicating strong agreement. Maximum grade is significantly associated with mortality, with grade D in the CIBMTR system and IV in the Consensus system conferring higher odds of 100-day mortality (OR 3.60, p<0.001, and OR 4.30, p<0.001 respectively).(12) The Ann Arbor grading system uses biomarkers (ST2, TNFR1, REG3α) in conjunction with Consensus grading to better define risk groups. The addition of biomarkers improved stratification, with a subset of patients with grade I/II aGVHD recategorized as Ann Arbor grade 3 with a 59% 6 month NRM, and another subset with grade III/IV aGVHD recategorized as Ann Arbor grade 1, with a 0% 6 month NRM {citation needed when abstracts published}. The role of microRNAs in the development of aGVHD is actively being investigated and may provide further prognostic or predictive information.(13, 14)
cGVHD is also primarily a clinical diagnosis. It is divided into “classic cGVHD” and “overlap syndrome,” which includes features of both acute and chronic GVHD. Previously, based on a study of 20 patients published in 1980, it was staged as “limited,” with localized involvement, or “extensive,” with generalized skin involvement, aggressive hepatitis, or any other target organ involvement.(15) In 2005, new NIH Consensus Criteria were published, designed to provide more detailed information about individual organ involvement. A global score of mild, moderate, or severe is generated.(16) The development of the NIH Consensus Criteria has been one of the most important advancements in the study and treatment of cGVHD, providing a platform to monitor response to treatment.
GVHD PROPHYLAXIS
Efforts to prevent aGVHD without compromising GVT are a major research objective.(17) Methotrexate (MTX) was the original GVHD prophylaxis evaluated in the 1970s.(18) Studies comparing cyclosporine (CSA) and MTX demonstrated equivalency,(19) but a combination of MTX/CSA compared with MTX alone resulted in a significant reduction in the cumulative incidence of grades II-IV aGVHD; no difference in the incidence of cGVHD was noted.(20, 21) Two large phase III studies comparing tacrolimus with MTX to CSA/MTX demonstrated reduced incidence of aGVHD with tacrolimus without difference in the incidence of cGVHD.(22, 23) Mycophenolate mofetil (MMF) in combination with calcineurin inhibitors has evidence of efficacy, but has no advantage over a calcineurin inhibitor with MTX.(24, 25)
In vivo T cell depletion with polyclonal or monoclonal anti-T-cell antibodies effectively reduce aGVHD, but increase risks of relapse, infection and graft failure. In a randomized study of patients who received unrelated donor stem cells after myeloablative conditioning, patients who received rabbit ATG with CSA/MTX had significant reductions of grade III-IV aGVHD compared with those who received CSA/MTX alone.(26) ATG was associated with a decrease in the 3-year incidence of extensive cGVHD (45% vs 12.2%).(27) CIBMTR data appears to confirm concerns about the increased risk of relapse among RIC recipients.(28) Ex vivo T cell depletion has been evaluated in a phase II study of AML patients receiving myeloablative conditioning without post-transplant immune suppression, and has been shown to be feasible, with low incidences of aGVHD and cGVHD.(29)
Other strategies to prevent aGVHD are under investigation. Sirolimus, an mTOR inhibitor that preserves regulatory T cell function, has been evaluated in combination with tacrolimus with or without MTX after both RIC and myeloablative conditioning with reductions in the rates of aGVHD.(30–33) BMT CTN 0402 is a recently conducted phase III study of sirolimus/MTX aGVHD prophylaxis compared with tacrolimus/MTX, and no difference between the treatment arms was noted.(34) Post-transplant cyclophosphamide deletes rapidly dividing alloreactive T cells and has been evaluated as a sole prophylaxis after myeloablative conditioning with busulfan and cyclophosphamide. While the incidence of grades II-IV aGVHD with this approach was just over 40%, the cumulative incidence of cGVHD was strikingly low at 10%.(35) Recently published studies of bortezomib (36), atorvastatin (37), vorinostat (38), and the CCR5 antagonist maraviroc (39) also appear promising. With infliximab, the incidence of aGVHD was not lowered, but the incidence of bacterial and invasive fungal infections increased.(40)
The development of cGVHD is associated with lower risk of relapse and lower OS.(41) Only two randomized studies designed to evaluate the prevention of cGVHD have been conducted. In the first, hydroxychloroquine was administered up to a year after transplant, with no differences in the incidences of aGVHD or cGVHD compared with placebo.(42) In the second, patients treated with thalidomide had an increased rate of cGVHD and poor survival compared with a placebo control.(43) A phase III study of Fresenius ATG (NCT01295710) is ongoing and may provide more information. Better understanding of the pathophysiology of cGVHD will lead to better prevention studies, though, as demonstrated by a recent phase II study evaluating post-transplant rituximab. The cumulative incidences of cGVHD and steroid-requiring cGVHD were significantly lower than in a control cohort, and 4 year OS was superior (71% vs 56%, p=0.05).(44)
GVHD TREATMENT
There is no FDA-approved treatment for acute or chronic GVHD. The frontline treatment of choice for both for patients needing systemic therapy is steroids. For patients with aGVHD, the recommended starting dose is 2 mg/kg/day methylprednisolone or equivalent along with continued calcineurin-based prophylaxis. Lower doses (1 mg/kg) have been evaluated in patients with grade II aGVHD, with dose escalation to 2 mg/kg/day with progression. A large retrospective study of the use of 1 mg/kg/day demonstrated no adverse effect on outcome, but patients with more severe initial presentations were likely started on 2 mg/kg, making direct comparisons of efficacy difficult.(45) Prospective randomized data shows no advantage to using higher doses of steroids than 2 mg/kg/day initially.(46) There is no benefit with a prolonged steroid taper after an initial response is achieved compared with a more rapid taper.(47) Typically, tapering begins once manifestations of aGVHD start showing a significant improvement, then continues at 10% of the starting dose every 3–5 days,(48) but the ideal schedule is not well-defined.
Attempts to improve on initial responses have proven frustrating. Multiple studies have compared additional agents to steroids alone, including studies of IL-2R antibodies, horse ATG, etanercept, infliximab, MMF, pentostatin, and sirolimus, and in most cases, no additional benefit was seen.(48) In the case of daclizumab, 100-day and one year survivals were significantly worse, owing both to relapse-related and GVHD-related mortality.(49) BMT CTN 0302 was a 4-arm randomized phase II study of MMF, etanercept, denileukin, or pentostatin with corticosteroids for the initial therapy of aGVHD. MMF was most promising, and was taken to a phase III study, BMT CTN 0802. This study closed after a planned futility analysis determined that it would not meet its primary endpoint of improving GVHD-free survival. The inclusion of patients with early acute GVHD and timing of initiation of MMF were factors that may have contributed to its failure.
aGVHD becomes steroid-refractory when it progresses within 3 days of initial treatment with corticosteroids or is not improved after 5–7 days. There is no standard second-line therapy, and a clinical trial should be offered wherever possible. Steroid-refractory aGVHD has a dismal prognosis, and second-line treatments have high failure rates. One-year survival rates are approximately 20–30%.(48) There have been few prospective studies of second-line agents and due to lack of standardization, their results are difficult to generalize. Studies of MTX, MMF, extracorporeal photopheresis (ECP), IL-2R antibodies, alemtuzumab, horse ATG, etanercept, infliximab, and sirolimus have been conducted, and while the average response rate was approximately 50%, the median survival was only about 6 months, and no agent was clearly superior.(48) Further immune suppression with second line therapy is associated with increased infectious risk, particularly viral reactivation, contributing to the risk of mortality.
For patients with cGVHD, the NIH consensus conference recommends systemic treatment with corticosteroids for those with moderate or severe disease.(16) One mg/kg prednisone or equivalent is standard, but no randomized studies comparing this with an alternate dose exist. There are no consistent guidelines for tapering steroids for patients who have achieved a response. The Seattle group has reported on an alternate day dosing regimen.(50, 51) The median duration of therapy is 2–3 years.(52) A randomized study of CSA/prednisone versus prednisone alone allowed for sparing of steroids in the combination arm, but no survival benefit was noted.(53) As in aGVHD, attempts to improve on initial response rates have proven frustrating. Two randomized studies of the addition of thalidomide to CSA and prednisone demonstrated no additional benefit,(54, 55) and a large randomized multicenter study evaluating the addition of MMF to initial systemic therapy was closed early because it was unlikely to meet its primary end-point of 2-year survival off of systemic immunosuppression.(56)
Half of patients require second-line treatment. NRM increases from 12% to 27% for patients requiring a therapy change within 4 months of the start of treatment.(57) There is no uniformly accepted definition of steroid-refractory cGVHD, resulting in different inclusion criteria across studies.(58) Evidence-based guidelines determined that the strength of recommendation for the majority of treatment options is category C, with only ECP, pentostatin, rituximab, and thalidomide having level II recommendations.(58) Responses on the mostly smaller phase II studies of these agents have ranged from 25–80%, but are often incomplete and transient, and results need to be further evaluated in multicenter studies, as in the recent CALGB study of pentostatin.(58) Mesenchymal stromal cells have been evaluated in small studies for the prevention and treatment of GVHD, but proof of efficacy is lacking.(59) More recent research has focused on targeted interventions based on the pathophysiology of cGVHD, and studies of low-dose IL-2,(60) imatinib,(61) and rituximab (62) have been promising.
Conclusions
Our ability to prevent or treat GVHD has not clearly improved over the past two decades. In part, this has been due to poor understanding of the pathogenesis of GVHD, vague grading criteria, and a failure to standardize clinical studies, follow single-institution studies up with prospective multicenter studies, and report negative studies.
We rely predominantly on the Consensus criteria for aGVHD grading, which provides limited prognostic information. The addition of biomarkers promises to further define which patients are at highest risk. These patients, defined as high-risk by Ann Arbor grading, represent an ideal group for clinical studies of novel therapeutic agents. Likewise, clinical trials evaluating minimization of treatment can target low-risk patients. MicroRNAs may be able identify patients prior to clinically evident disease, allowing for pre-emptive therapies. The development of the NIH Consensus Criteria for cGVHD represents a significant advance, providing a clinically useful severity measure for use in clinical trials.(16) Prospective data is emerging from the Chronic GVHD Consortium studies confirming risk factors identified in the CIBMTR study (3) and may define patients less likely to respond to initial steroid therapy.
Responses to treatment are suboptimal, and patients should be offered a clinical trial whenever possible. Variations in donor type and matching, stem cell source, and conditioning, as well as inconsistencies in study design, have hampered interpretation of prospective aGVHD studies, and the best endpoint for an aGVHD prophylaxis study has not been defined. Day 28 has emerged as a standard response evaluation point in aGVHD studies because it also correlates with NRM.(63) Likewise, clinical studies in cGVHD have been hampered by a failure to specify a time for response assessment, define objective criteria for response, account for concomitant treatments, or identify a historical benchmark to identify a null hypothesis.(64) Standardization of methods, response criteria, and inclusion and exclusion criteria would allow for better comparisons across studies. Because of the limited number of patients and the expense associated with conducting phase III studies, phase II studies in GVHD should be performed with the highest rigor to identify the best agents to take forward. Institutional practice variations impact outcomes in single-institution studies, and multicenter studies are vital to the advancement of GVHD research. The BMT CTN and the Chronic GVHD Consortium provide avenues for the conduct of multicenter phase II studies which can be confidently taken forward to phase III studies. Two such examples, BMT CTN 1203, evaluating maraviroc, bortezomib, or post-transplant cyclophosphamide as aGVHD prophylaxis following RIC, and BMT CTN 1301, evaluating post-transplant cyclophosphamide, ex vivo T cell depletion, and a control arm following myeloablative conditioning in patients with acute leukemia and myelodysplastic syndrome are currently in development.
Moving forward, the identification of novel hypotheses regarding the prevention and treatment of GVHD as well as development of agents to test these hypotheses will be critical.(65) In addition to the trials mentioned, aGVHD prophylaxis studies with RGI-2001, a liposomal formulation of alpha-GalCer, a CD1d ligand, which induces regulatory T cells, and milatuzumab, an anti-CD74 monoclonal antibody that targets dendritic cells, are ongoing. Both trials are being conducted with the support of pharmaceutical companies, which will be crucial owing to the expense of many novel therapeutics. The atorvastatin data is particularly intriguing in part because of its availability and ease of use, and further studies are ongoing.(66)
GVHD remains a major barrier to the success of allo-SCT. While progress in its prevention and treatment has been limited, advancements have been made in understanding its pathogenesis, as well as in grading and risk prediction, particularly in aGVHD. These advances and an understanding of the need to conduct well-defined multicenter studies will hopefully translate to improvements in patient outcomes in the near future.
KEY POINTS.
The success of allogeneic stem cell transplantation has been limited by the development of acute and chronic graft-versus-host disease
Little progress has been made over the past 2 decades with respect to preventing and treating this potentially fatal complication
Better understanding of the pathogenesis of GVHD and development of biomarkers has led to better risk-stratification in patients with acute GVHD, and development of NIH Consensus Criteria for grading chronic GVHD has provided a platform for uniform response evaluation.
There is an urgent need for rationally designed, uniformly conducted prospective studies of novel therapeutic agents which can be brought forward to multicenter studies within the BMT CTN or the Chronic GVHD Consortium.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES/RECOMMENDED READING
- 1.Appelbaum FR. Hematopoietic-cell transplantation at 50. The New England journal of medicine. 2007;357(15):1472–5. doi: 10.1056/NEJMp078166. Epub 2007/10/12. [DOI] [PubMed] [Google Scholar]
- **2.Hahn T, McCarthy PL, Jr, Hassebroek A, Bredeson C, Gajewski JL, Hale GA, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(19):2437–49. doi: 10.1200/JCO.2012.46.6193. Epub 2013/05/30. Analysis demonstrating improved survival over a period of increasing use of PBSCT and cord blood transplants in increasingly older recipients, likely due to better donor selection, improved HLA typing, and better supportive care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *3.Pavletic SZ, Fowler DH. Are we making progress in GVHD prophylaxis and treatment? Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2012;2012:251–64. doi: 10.1182/asheducation-2012.1.251. Epub 2012/12/13. This is a good review of the current state of the science in the prevention and treatment of GVHD. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61. doi: 10.1016/S0140-6736(09)60237-3. Epub 2009/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korngold R, Sprent J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. The Journal of experimental medicine. 1978;148(6):1687–98. doi: 10.1084/jem.148.6.1687. Epub 1978/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. Epub 1998/04/01. [DOI] [PubMed] [Google Scholar]
- 7.Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119(22):5088–103. doi: 10.1182/blood-2011-11-364091. Epub 2012/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroeder MA, DiPersio JF. Mouse models of graft-versus-host disease: advances and limitations. Disease models & mechanisms. 2011;4(3):318–33. doi: 10.1242/dmm.006668. Epub 2011/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **9.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nature reviews Immunology. 2012;12(6):443–58. doi: 10.1038/nri3212. Epub 2012/05/12. This is a good review of the biology of GVHD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. Epub 1974/10/01. [DOI] [PubMed] [Google Scholar]
- 11.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. British journal of haematology. 1997;97(4):855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. Epub 1997/06/01. [DOI] [PubMed] [Google Scholar]
- 12.Cahn JY, Klein JP, Lee SJ, Milpied N, Blaise D, Antin JH, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106(4):1495–500. doi: 10.1182/blood-2004-11-4557. Epub 2005/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranganathan P, Heaphy CE, Costinean S, Stauffer N, Na C, Hamadani M, et al. Regulation of acute graft-versus-host disease by microRNA-155. Blood. 2012;119(20):4786–97. doi: 10.1182/blood-2011-10-387522. Epub 2012/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao B, Wang Y, Li W, Baker M, Guo J, Corbet K, et al. Plasma microRNA signature as a non-invasive biomarker for acute graft-versus-host disease. Blood. doi: 10.1182/blood-2013-06-510586. Epub 2013/09/18. blood-2013-06-510586 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. The American journal of medicine. 1980;69(2):204–17. doi: 10.1016/0002-9343(80)90380-0. Epub 1980/08/01. [DOI] [PubMed] [Google Scholar]
- 16.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. Epub 2005/12/13. [DOI] [PubMed] [Google Scholar]
- 17.Ram R, Storb R. Pharmacologic prophylaxis regimens for acute graft-versus-host disease: past, present and future. Leukemia & lymphoma. 2013;54(8):1591–601. doi: 10.3109/10428194.2012.762978. Epub 2013/01/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Flournoy N, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49(4):511–33. Epub 1977/04/01. [PubMed] [Google Scholar]
- 19.Storb R, Deeg HJ, Fisher L, Appelbaum F, Buckner CD, Bensinger W, et al. Cyclosporine v methotrexate for graft-v-host disease prevention in patients given marrow grafts for leukemia: long-term follow-up of three controlled trials. Blood. 1988;71(2):293–8. Epub 1988/02/01. [PubMed] [Google Scholar]
- 20.Storb R, Deeg HJ, Farewell V, Doney K, Appelbaum F, Beatty P, et al. Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986;68(1):119–25. Epub 1986/07/01. [PubMed] [Google Scholar]
- 21.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. The New England journal of medicine. 1986;314(12):729–35. doi: 10.1056/NEJM198603203141201. Epub 1986/03/20. [DOI] [PubMed] [Google Scholar]
- 22.Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–14. Epub 1998/09/25. [PubMed] [Google Scholar]
- 23.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–8. Epub 2000/09/09. [PubMed] [Google Scholar]
- 24.Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone marrow transplantation. 2004;34(7):621–5. doi: 10.1038/sj.bmt.1704647. Epub 2004/08/10. [DOI] [PubMed] [Google Scholar]
- 25.Perkins J, Field T, Kim J, Kharfan-Dabaja MA, Fernandez H, Ayala E, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2010;16(7):937–47. doi: 10.1016/j.bbmt.2010.01.010. Epub 2010/01/28. [DOI] [PubMed] [Google Scholar]
- 26.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. The lancet oncology. 2009;10(9):855–64. doi: 10.1016/S1470-2045(09)70225-6. Epub 2009/08/22. [DOI] [PubMed] [Google Scholar]
- 27.Socie G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–82. doi: 10.1182/blood-2011-01-329821. Epub 2011/04/07. [DOI] [PubMed] [Google Scholar]
- 28.Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–70. doi: 10.1182/blood-2011-01-332007. Epub 2011/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devine SM, Carter S, Soiffer RJ, Pasquini MC, Hari PN, Stein A, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2011;17(9):1343–51. doi: 10.1016/j.bbmt.2011.02.002. Epub 2011/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antin JH, Kim HT, Cutler C, Ho VT, Lee SJ, Miklos DB, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102(5):1601–5. doi: 10.1182/blood-2003-02-0489. Epub 2003/05/06. [DOI] [PubMed] [Google Scholar]
- 31.Cutler C, Li S, Ho VT, Koreth J, Alyea E, Soiffer RJ, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–14. doi: 10.1182/blood-2006-09-046219. Epub 2006/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alyea EP, Li S, Kim HT, Cutler C, Ho V, Soiffer RJ, et al. Sirolimus, tacrolimus, and low-dose methotrexate as graft-versus-host disease prophylaxis in related and unrelated donor reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14(8):920–6. doi: 10.1016/j.bbmt.2008.05.024. Epub 2008/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho VT, Aldridge J, Kim HT, Cutler C, Koreth J, Armand P, et al. Comparison of Tacrolimus and Sirolimus (Tac/Sir) versus Tacrolimus, Sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem. doi: 10.1016/j.bbmt.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Cutler C, Logan BR, Nakamura R, Johnston L, Choi SW, Porter DL, et al. Tacrolimus/Sirolimus Vs. Tacrolimus/Methotrexate for Graft-Vs.-Host Disease Prophylaxis After HLA-Matched, Related Donor Hematopoietic Stem Cell Transplantation: Results of Blood and Marrow Transplant Clinical Trials Network Trial 0402. ASH Annual Meeting Abstracts. 2012;120(21):739. Recent results of a CTN study comparing a sirolimus-containing prophylaxis regimen versus a standard regimen. Presented as an abstract at the ASH 2012 Annual Meeting, no differences in the two arms were observed. [Google Scholar]
- 35.Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–30. doi: 10.1182/blood-2009-11-251595. Epub 2010/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koreth J, Stevenson KE, Kim HT, McDonough SM, Bindra B, Armand P, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(26):3202–8. doi: 10.1200/JCO.2012.42.0984. Epub 2012/08/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Hamadani M, Gibson LF, Remick SC, Wen S, Petros W, Tse W, et al. Sibling Donor and Recipient Immune Modulation With Atorvastatin for the Prophylaxis of Acute Graft-Versus-Host Disease. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013 doi: 10.1200/JCO.2013.50.8747. Epub 2013/10/30. This study demonstrates that atorvastatin has efficacy in preventing GVHD, which, given the ease of availability of atorvastatin, could be practice changing if confirmed in a larger, multicenter study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi SW, DiPersio JF, Braun TM, Hou G, Stockerl-Goldstein K, Tawara I, et al. Targeting Histone Deacetylases As a New Strategy for Graft Versus Host Disease Prevention. ASH Annual Meeting Abstracts. 2012;120(21):740. [Google Scholar]
- *39.Reshef R, Luger SM, Hexner EO, Loren AW, Frey NV, Nasta SD, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. The New England journal of medicine. 2012;367(2):135–45. doi: 10.1056/NEJMoa1201248. Epub 2012/07/13. This early study of maraviroc is mechanistically very interesting and is leading to an upcoming aGVHD prophylaxis study being conducted through the CTN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamadani M, Hofmeister CC, Jansak B, Phillips G, Elder P, Blum W, et al. Addition of infliximab to standard acute graft-versus-host disease prophylaxis following allogeneic peripheral blood cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14(7):783–9. doi: 10.1016/j.bbmt.2008.04.006. Epub 2008/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **41.Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18(11):1727–33. doi: 10.1016/j.bbmt.2012.06.014. Epub 2012/07/07. This analysis demonstrates the effect of acute and chronic GVHD on relapse-free survival in allogeneic transplant recipients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fong T, Trinkaus K, Adkins D, Vij R, Devine SM, Tomasson M, et al. A randomized double-blind trial of hydroxychloroquine for the prevention of chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2007;13(10):1201–6. doi: 10.1016/j.bbmt.2007.06.012. Epub 2007/09/25. [DOI] [PubMed] [Google Scholar]
- 43.Chao NJ, Parker PM, Niland JC, Wong RM, Dagis A, Long GD, et al. Paradoxical effect of thalidomide prophylaxis on chronic graft-vs.-host disease. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 1996;2(2):86–92. Epub 1996/05/01. [PubMed] [Google Scholar]
- 44.Cutler C, Kim HT, Bindra B, Sarantopoulos S, Ho VT, Chen YB, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122(8):1510–7. doi: 10.1182/blood-2013-04-495895. Epub 2013/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mielcarek M, Storer BE, Boeckh M, Carpenter PA, McDonald GB, Deeg HJ, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113(13):2888–94. doi: 10.1182/blood-2008-07-168401. Epub 2008/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Lint MT, Uderzo C, Locasciulli A, Majolino I, Scime R, Locatelli F, et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood. 1998;92(7):2288–93. Epub 1998/09/25. [PubMed] [Google Scholar]
- 47.Hings IM, Filipovich AH, Miller WJ, Blazar BL, McGlave PB, Ramsay NK, et al. Prednisone therapy for acute graft-versus-host disease: short- versus long-term treatment. A prospective randomized trial. Transplantation. 1993;56(3):577–80. doi: 10.1097/00007890-199309000-00016. Epub 1993/09/01. [DOI] [PubMed] [Google Scholar]
- **48.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18(8):1150–63. doi: 10.1016/j.bbmt.2012.04.005. Epub 2012/04/19. This is a concise summary of recommendations for first- and subsequent-line treatment of acute GVHD from the ASBMT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SJ, Zahrieh D, Agura E, MacMillan ML, Maziarz RT, McCarthy PL, Jr, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104(5):1559–64. doi: 10.1182/blood-2004-03-0854. Epub 2004/05/13. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan KM, Witherspoon RP, Storb R, Deeg HJ, Dahlberg S, Sanders JE, et al. Alternating-day cyclosporine and prednisone for treatment of high-risk chronic graft-v-host disease. Blood. 1988;72(2):555–61. Epub 1988/08/01. [PubMed] [Google Scholar]
- 51.Lee SJ, Flowers MED. Recognizing and Managing Chronic Graft-Versus-Host Disease. ASH Education Program Book. 2008;2008(1):134–41. doi: 10.1182/asheducation-2008.1.134. [DOI] [PubMed] [Google Scholar]
- 52.Stewart BL, Storer B, Storek J, Deeg HJ, Storb R, Hansen JA, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104(12):3501–6. doi: 10.1182/blood-2004-01-02002004-01-0200. Epub 2004/08/05. [pii] [DOI] [PubMed] [Google Scholar]
- 53.Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100(1):48–51. doi: 10.1182/blood.v100.1.48. Epub 2002/06/19. [DOI] [PubMed] [Google Scholar]
- 54.Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000;96(12):3995–6. Epub 2000/11/23. [PubMed] [Google Scholar]
- 55.Arora M, Wagner JE, Davies SM, Blazar BR, Defor T, Enright H, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001;7(5):265–73. doi: 10.1053/bbmt.2001.v7.pm11400948. Epub 2001/06/13. S1083879101500439 [pii] [DOI] [PubMed] [Google Scholar]
- 56.Martin PJ, Storer BE, Rowley SD, Flowers ME, Lee SJ, Carpenter PA, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113(21):5074–82. doi: 10.1182/blood-2009-02-202937. Epub 2009/03/10. blood-2009-02-202937 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flowers ME, Storer B, Carpenter P, Rezvani AR, Vigorito AC, Campregher PV, et al. Treatment change as a predictor of outcome among patients with classic chronic graft-versus-host disease. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14(12):1380–4. doi: 10.1016/j.bbmt.2008.09.017. Epub 2008/12/02. S1083-8791(08)00416-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolff D, Schleuning M, von Harsdorf S, Bacher U, Gerbitz A, Stadler M, et al. Consensus Conference on Clinical Practice in Chronic GVHD: Second-Line Treatment of Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 17(1):1–17. doi: 10.1016/j.bbmt.2010.05.011. Epub 2010/08/06. S1083-8791(10)00223-5 [pii] [DOI] [PubMed] [Google Scholar]
- 59.Baron F, Storb R. Mesenchymal stromal cells: a new tool against graft-versus-host disease? Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18(6):822–40. doi: 10.1016/j.bbmt.2011.09.003. Epub 2011/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *60.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, 3rd, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 365(22):2055–66. doi: 10.1056/NEJMoa1108188. Epub 2011/12/02. This study evaluating the use of low-dose IL-2 is a good, rationally designed study of a potentially very interesting agent for chronic GVHD that will likely be taken forward to multicenter studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olivieri A, Locatelli F, Zecca M, Sanna A, Cimminiello M, Raimondi R, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009;114(3):709–18. doi: 10.1182/blood-2009-02-204156. Epub 2009/05/01. blood-2009-02-204156 [pii] [DOI] [PubMed] [Google Scholar]
- 62.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–62. doi: 10.1182/blood-2006-01-0233. Epub 2006/03/23. 2006-01-0233 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pavletic SZ. Response as an end point in treatment trials for acute GVHD. Bone Marrow Transplant. 47(2):161–3. doi: 10.1038/bmt.2011.59. Epub 2012/02/09. bmt201159 [pii] [DOI] [PubMed] [Google Scholar]
- 64.Martin PJ, Inamoto Y, Carpenter PA, Lee SJ, Flowers ME. Treatment of chronic graft-versus-host disease: Past, present and future. Korean J Hematol. 46(3):153–63. doi: 10.5045/kjh.2011.46.3.153. Epub 2011/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolf D, von Lilienfeld-Toal M, Wolf AM, Schleuning M, von Bergwelt-Baildon M, Held SA, et al. Novel treatment concepts for graft-versus-host disease. Blood. 2012;119(1):16–25. doi: 10.1182/blood-2011-08-339465. Epub 2011/09/29. [DOI] [PubMed] [Google Scholar]
- 66.Hamadani M, Gibson LF, Remick SC, Wen S, Petros W, Tse W, et al. Sibling Donor and Recipient Immune Modulation With Atorvastatin for the Prophylaxis of Acute Graft-Versus-Host Disease. J Clin Oncol. doi: 10.1200/JCO.2013.50.8747. Epub 2013/10/30. JCO.2013.50.8747 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]


