Introduction
The association between anxiety and allergic disorders, including allergic rhinitis (AR), is well-documented1–3. Allergic individuals show higher levels of anxiety symptoms relative to non-allergic individuals3, 4, and clinical anxiety disorders are over-represented in AR populations1, 2. Anxiety symptoms include rumination and worry about stressful events, which exacerbate and prolong negative emotional arousal and physiological stress activation5. Biopsychosocial models of allergic disease underscore stress-related endocrine and immune alterations in the exacerbation of clinical symptoms6, 7. Indeed, psychological stress and negative emotions amplify immune-mediated clinical symptoms in individuals with allergic disorders (e.g.,8–10).
We, too, observed an important role for anxiety in allergy-related immune function. In a study of individuals with AR, anxiety enhanced the impact of stress on allergen-induced histamine release in response to skin prick tests11. Skin prick testing is a major diagnostic tool in the clinic and can serve to confirm whether patient symptomatology is due to allergy12, 13. The findings from our original study were based on an examination of wheal responses to antigens for which individuals met diagnostic criteria for allergy, that is, they reported a symptom history consistent with allergy and also showed clinically positive skin prick test (SPT) responses at baseline. In the current examination, we determined whether these findings translated to clinical implications. In our original study, a subset of individuals who had negative SPT responses to particular allergens at baseline, when retested after a laboratory stressor, showed a positive response to at least one of these allergens. For the current analysis, we examined in these individuals whether anxiety in combination with stress exposure increased the incidence of positive SPT responses to allergens previously testing negative. Subsequently, we used participants’ self-reported clinical history of allergies to determine how to interpret potential stress-related alterations in SPT testing; such findings may suggest two possibilities. First, anxiety and stress, in susceptible individuals could increase risk for acute allergic responses (that is, mast cell derived histamine release) after allergen exposure. Alternatively, in individuals without clinical symptoms in response to specific allergens, stress-related enhancement of positive SPT responses to these allergens may have implications for stress and anxiety impacting the validity and reliability of SPT testing. Finally, we examined, similar to our original analyses, whether anxiety modulated the impact of stress on magnitude of wheal responses to common allergens, but extended this analysis to allergens that previously tested negative in the sample.
Methods
Participants
The participants, 10 men and 18 women (mean age: 24.73, SD=4.35, range=18–33 years), were recruited using ads seeking healthy individuals ages 18–40 who had a history of seasonal nasal allergies (hay fever); for details, see11. The majority of participants were White, non-Hispanic (n = 22), 4 were African American, and 2 were Asian. Most (n = 26) had at least some college education. Exclusion criteria included active allergy immunotherapy, cardiovascular medications (statins, beta blockers, etc.), psychotropic medications, excessive alcohol use, smoking, asthma or other illnesses with immunological or endocrinological components other than allergy, or medications with obvious consequences for these systems or for allergies. We restricted recent use of a number of allergy medications in accord with recommendations designed to optimize skin testing results14, 15. Subjects were asked to refrain from use of vitamin C supplements for at least 24 hours prior to all study sessions.
A screening visit included allergen skin tests to 9 common allergens and questionnaires. Subjects were selected for participation in the study based on clinical history and SPTs. To be eligible, participants had to meet skin prick test (SPT) criteria for sensitivity to house dust mite (Der P 1) due to research objectives for the primary study11 (details of the SPT battery and criteria are detailed under Measures, Skin Prick Testing). This study was approved by the Ohio State Biomedical Research Review Committee and all participants provided written informed consent to participate.
Study Sessions: General Clinical Research Center (GCRC)
Two GCRC laboratory sessions were scheduled at least 2 weeks apart (mean=38.72 days, SD=45.74). Both visits followed the same timeline, differing only in the stressor/control condition randomization for that visit. On arrival a heparin well was placed in one arm for subsequent serial blood draws. The participant then ate a standardized breakfast (after fasting since midnight) and completed questionnaires. Next, he or she sat quietly (or listened to soothing classical music) for a 30-minute relaxation period, after which a nurse obtained blood and saliva samples from the participant. The nurse then applied the pre-task SPTs, which were followed by the Trier Social Stress Test (TSST) or a control task, a post-task SPTs performed on the opposite arm to the pre-task skin prick test and blood and saliva sampling. Participants returned for a 1.5-hour follow-up GCRC visit on the subsequent morning to undergo final skin prick testing.
Each of these two 4-hour laboratory appointments thus included two skin tests using alternate arms (pre-task and post-task), as well as a third skin test at a 1 ½-hour appointment the following morning.
Stressor Task
We used the TSST, a well-validated laboratory stressor16, to induce an acute stress condition. Saliva samples for cortisol (a primary stress hormone) were obtained throughout the procedure (see11 for relevant findings). In brief, participants were escorted to a room with a microphone stand and video camera, and a seated “audience” panel of 2–3 individuals, and informed that they would make a speech and perform mental arithmetic in front of the panel. For the speech, the participants were told to imagine that they had applied for a position and were about to be interviewed by the selection committee; they had 10 minutes to prepare a speech about why they would be best for the job, and 5 minutes to deliver the speech, followed by a 5 minute mental arithmetic task, subtracting serially the number 13 from 1,022 as fast and as accurately as possible They were told that at least one member of the panel was trained in behavioral observation, and would rate their speech’s content and style. Participants were also told that they would watch the videotape of their speech, without anyone else present, to review the performance upon which the committee would base their evaluation17.
The participant was then taken to another room for their speech preparation. They returned to the first room and performed the speech and serial subtraction task. After participants watched their speech and math videotape in private, a nurse performed the post-task SPT.
Control task
The control task served as the contrast condition for the TSST in this crossover design study. Participants silently read a magazine section for 10 minutes before reading the same material out loud while being audiotaped. Afterwards they listened to an audiotape, without anyone else present, of someone else reading the material. They were told that they were being asked to read aloud and listen to the audiotape to control for the effects of speaking and listening related to other experimental tasks; we emphasized that their performance was not being evaluated.
Measures
State anxiety
The 20-item state anxiety scale of the Spielberger State-Trait Anxiety Scale (STAI) is a widely used, reliable and valid measure of the current state of anxiety with excellent norms18. Participants report how they feel at the moment, with adjectives such as calm, tense, at ease, and upset rated on a scale from 1–4. The scale administered at the beginning of each 4-hour session, before the interaction tasks were introduced. The correlation between the two was r=.85, p<.0001, and thus we used the mean of the two administrations as the summary anxiety measure for each subject.
Skin Prick Allergy Testing
Skin prick testing12, 13, was used to assess allergic status to a battery of 9 allergens: house dust mite (Dermatophagoides pteronyssinus (Der P 1)), North American dust mite (Dermatophagoides farinae), ragweed, mold mix, weed mix, tree mix, grass mix, cat dander, and sagebrush. All participants met skin prick test (SPT) criteria for sensitivity to Der P 1 due to research objectives for the primary study11.
The battery was performed on the volar aspect of both forearms (alternating between tests) using the DermaPIK skin test system19. Histamine sulfate, 6mg/ml, served as the positive control14, and glycerinated saline was the negative control20. The prick tests were applied using a DermaPIK and read 20 minutes later by measuring the largest diameter of the wheal and flare (in mm). SPT data are expressed as the difference between wheal size produced by specific allergens and the concurrent saline control. The nurses who performed and read the tests were blind to the subject’s assigned condition for the day.
A wheal ≥ 3 mm larger than the concurrent saline control provided evidence of an allergen-specific IgE response14, and was considered a “positive” SPT. Participants were considered to be nonsensitized for a given antigen if a wheal < 3mm larger than the concurrent saline control (considered a “negative” SPT) was evident from SPTs performed at screening and the pre-task assessment at both visits (that is, at all 3 baseline testing timepoints). A “positive SPT” count variable was calculated as the total number of SPTs ≥ 3 mm larger than the concurrent saline control either immediately or the day after the stress or non-stress tasks for allergens that previously tested negative at the multiple baseline assessments. In addition, mean wheal diameters were calculated across all of the allergens for which participants tested negative (wheal < 3 mm larger than concurrent saline) at the 3 baseline assessments.
Self-reported allergies and comparison with SPTs
Participants self-reported whether they had an allergy to a series of 7 allergen categories (dust; weed, tree, or grass; cat; mold; ragweed; food) that represented 8 of the 9 allergens used in the SPT panel, in addition to 5 other allergens not used in the panel (dog; pollen; animal; feather; food). Specifically, participants who responded “yes” to having nasal symptoms (sneezing, itching, runny nose/postnasal drainage and/or nasal congestion) after exposure to environments rich with the specific allergen (such as outdoor exposure during a known pollen season in the Columbus, OH area) were considered allergic to that allergen.
Using these self-reports, we determined the extent to which the positive post-task SPTs were false positives or conversion of a previously false negative SPT. A positive post-task SPT was considered a false positive if a participant self-reported no clinical symptoms after exposure to the allergen that was negative at baseline but then positive at post-task SPT assessments. If the participant clinical history was consistent with specific allergen sensitivity, the positive SPT at post-task was considered to be a conversion of a previous false negative SPT at baseline.
Data Analysis
All data were analyzed using the Statistical Package for the Social Sciences (SPSS) software (version 19); tests were two-tailed with alpha set at .05. A generalized linear model was used to examine whether stress and anxiety influenced the total number (count) of post-task positive SPT to allergens for which individuals previously tested negative. The dependent variable was total number of positive SPT; the independent variables were visit (stress versus control) and baseline anxiety (noted as “anxiety”). This model, using poisson distributions, accounted for the non-normally distributed count data. The percentage of positive post-task SPTs that were false positives or corrections of false negatives was calculated to inform the interpretation of new positive SPTs. To examine whether stress and anxiety more generally affected wheal size to negative SPTs at baseline, a mixed model was fit that accounted for the correlation in measurements from the same subject across each time point. The dependent variable in the model was mean wheal across all allergens for which the individual tested negative at screening and pre-task assessments. Independent variables in the mixed model included visit (stress versus control), time, and baseline anxiety (noted as “anxiety”), as well as their interactions. Gender and the baseline wheal measure served as covariates (see (11)). Wheal data were log-transformed to achieve normality. Separate, post hoc models probing significant interactions from the full mixed and generalized linear models were Bonferroni adjusted to reduce familywise error. Effects of visit order (stress versus control) were not apparent for any outcomes (see11), and, thus, order was not included in final models.
Results
Descriptives
More than half of participants (n = 17; 60.7%) had at least one positive post-task SPT response (wheal ≥ 3 mm larger than saline control immediately for post-task or day 2 SPTs) at the stress or non-stress visit for an antigen that tested negative at baseline (i.e., at screening and both pre-task assessments). Across these 17 participants, there were a total of 40 SPT that tested positive post-task after previously testing negative at baseline (range = 1 – 6; M = 2.38, SD = 1.78). Eight of the 10 (80%) men and 9 of the 18 (50%) women had at least 1 positive SPT post-task response, proportions that were not significantly different (χ2 = 2.43, p = .23). Mean STAI18 anxiety scores did not statistically differ between participants who showed any positive post-task SPT responses to a previously identified negative SPT (M = 33.59; SD = 8.75) and those with no new post-task SPT conversions (M = 30.82; SD = 6.09; t(26) = −.91; p = .37). Further, individuals’ mean anxiety scores (M = 32.5, SD = 7.81) were not associated with the total number of previously negative SPT that tested positive following the stress or non-stress tasks (r = .09; p = .66).
Using self-reported allergies along with positive SPT incident counts, it was determined that the majority of incidents were conversions of false negatives at baseline: across participants, 15 out of 20 allergens (75%) that were positive at post-task, but tested negative at baseline, were allergens to which participants reported being allergic.1
Anxiety and Stress Influences on Positive SPT Responses to Allergens Testing Negative at Baseline
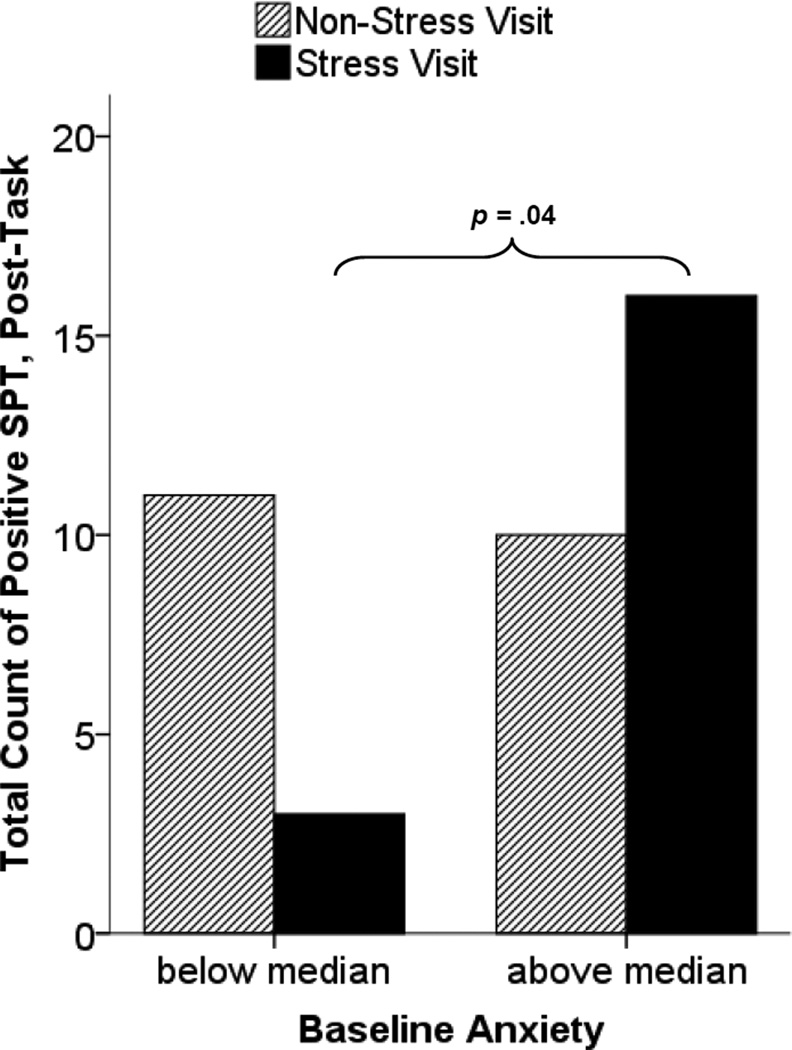
Results of the generalized linear model supported a combined role for anxiety and stress on the total count of positive, post-task SPTs (shown by a significant visit × anxiety interaction (χ2(1) = 4.10, p = .043). After exposure to the stressor, individuals with higher baseline anxiety had a higher incidence of positive SPTs for allergens testing negative at baseline relative to individuals with lower anxiety (padj = .032). Anxiety was not associated with number of positive, post-task SPT at any SPT assessment during the non-stress visit (padj = .512). Figure 1 depicts these findings, using anxiety categories of high (above the median anxiety score) and low (below the median anxiety score) for illustration. The significant difference noted in figure 1 between high and low anxious participants (p = .04) was derived from a Mann-Whitney U test comparing total new positive SPTs across post-task and day 2 between the stress and non-stress visit.
Figure 1.
Total number of SPT that tested negative at multiple baselines (screening and each CRC visit) but tested positive following the task by stress condition and anxiety category.
Interpretation of Post-Stressor Positive SPTs using Clinical History
We next explored how to interpret the stress-related enhancement of positive SPTs among the more anxious atopic individuals using their self-reported clinical history of allergies. Among these participants, 8 out of 10 SPTs (80%) that were positive at post-stress task but tested negative at baseline were allergens to which participants reported being allergic, suggesting that the stress task primarily converted false negative SPTs21 observed at screening and both pre-task assessments among the more anxious, atopic individuals.
Anxiety, Stress Condition, and Wheal Responses to Allergens Testing Negative at Baseline
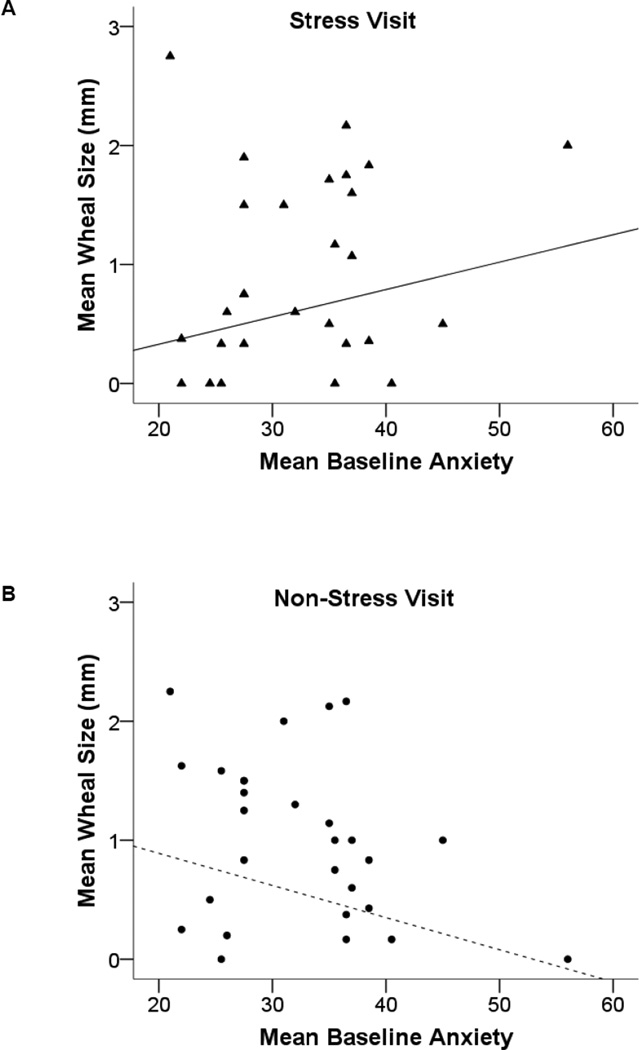
We next examined whether stress and anxiety influenced average wheal size across all allergens to which participants tested negative at baseline. From the mixed model, there was again support for the combined effect of anxiety and stress exposure on SPTs (indicated by a significant anxiety by visit interaction (F(1, 59) = 6.93, p = .011)). Specifically, following stressor exposure, higher anxiety was associated with larger mean wheals for the allergens testing negative at baseline, although this association was non-significant (padj = .08; figure 2A). At the non-stress visit, higher anxiety was associated with smaller post-task mean wheals at the non-stress visit, although this association again was non-significant (padj = .10; figure 2B). The participant with the highest mean anxiety score had one outlying wheal response to an allergen for which he tested negative at baseline (sagebrush); when we excluded that response from the calculation of his average wheal response, this participant had no apparent influence on model fit and so was included in final models.
Figure 2.
(A and B). Mean SPT wheal response (allergen minus saline) for all allergens that tested negative at baseline assessments as a function of anxiety (lines represent associations between anxiety and wheal response derived from regression models).
To determine whether effects of stress and anxiety were specific to allergen SPTs, separate mixed effect model tested stress and anxiety effects on histamine (the positive control) and saline (negative control) SPT responses. There were no significant effects of stressor exposure, anxiety or their interactions for either SPT control (saline: p = .26; histamine: p = .60).
Discussion
The role of stress in the disease activity of patients with various allergic illnesses has long been recognized. Too, stress appears to have immediate influence on allergic symptoms, possibly by impacting mast cell activity. As such, patients’ stress levels at the time of SPT could have important clinical implications in interpreting these mast cell-mediated SPT results. The current study addresses this issue by examining whether sensitivity and/or specificity of SPT is affected by stress exposure and anxiety levels. Across the stress visit, higher anxiety was associated with higher total counts of positive SPT following the stress task. Anxiety had no apparent influence on SPT thresholds at the non-stress visit, suggesting that anxiety and stress in combination can lead to SPTs that are interpreted as positive test results in individuals with atopy. The fundamental question raised by these findings is whether the newly positive SPT results represent increased sensitivity to stressful situations or represent a false positive that would be important for clinicians to anticipate when interpreting these results for clinical action (ie. avoidance/environmental control measures, allergen specific immunotherapy prescriptions). Our preliminary data in this pilot trial suggest that among more anxious individuals, SPT tests are more likely to correspond with their reported clinical allergy history when these individuals have recently experienced significant psychological stress. These findings underscore implications for accuracy in the clinical identification of specific allergic sensitivity. Future studies are needed that more directly evaluate associations among stress and anxiety, SPT responses, and individuals’ day-to-day clinical symptom levels.
Skin prick testing is characterized by two stages of skin responses to antigen – an immediate reaction occurring within the first 20 minutes after allergen exposure and a so-called late phase reaction that manifests 3–24 hrs later with no additional allergen exposure21. The immediate reaction to allergen is due to IgE-mediated activation of mast cells that release histamine, resulting in the characteristic wheal and flair skin reaction. In our initial examination22, we found that anxiety increased immediate wheal responses to SPT positive allergens after stressor exposure11. In the current analysis, among more anxious atopic individuals, SPT wheal responses to previously identified “nonallergens” were enhanced following a laboratory stressor. Together, these findings suggest that more anxious atopic individuals in stressful environments have alterations in immune function that enhance histamine release to allergens, including allergens that show low immunogenicity when tested under non-stressful conditions. Translated to clinical practice, information about anxiety and stress exposure may allow the clinician to help patients identify symptom “triggers,” as well as consider stress management approaches in the therapeutic plans for these patients.
The cellular and molecular mechanisms linking anxiety and stress to enhanced allergen-induced histamine release remain to be identified. Increased sensitivity of mast cells to antigen-induced activation remains an intriguing possibility. For instance, mast cells can be activated in response to stress23, which may help explain, at least in part, observed relationships between anxiety and psychological stress and enhanced atopic symptoms24. Our observations do not support either increased nonspecific mast cell degranulation or increased skin responsiveness to histamine as primary mechanisms for these results. There were no significant differences caused by either instrinsic histamine release by glycerinated saline (negative control) or skin responsiveness to a standard dose of histamine (positive control). Further, the number of new positive post-task SPT results did not correlate with anxiety scores, and none of the individuals in our study had all previously negative allergens turn positive after the stress task, regardless of anxiety level. It is more likely that individuals had allergen-specific IgE production, but not in sufficient quantity to cause a positive baseline SPT. With higher anxiety and the stress associated with the laboratory task, it is plausible that mast cell sensitivity increased enough to result in a positive SPT. Indeed, this could be a contributing mechanism to the well-known clinical phenomenon of priming whereby lower allergen concentrations trigger mast cell activity after an initial response25.
The combination of environmental stressors and anxiety may be particularly important to understanding these mechanisms. We found that among less anxious individuals, there was a trend toward decreased post task SPT positives at the stress compared to the non-stress visit (Figure 1), and under less stressful conditions, higher anxiety was associated with lower post-task mean wheals from SPTs (Figure 2). We suggest that these findings may point to conditions under which physiological arousal, especially catecholamine release, may be optimal for regulating allergic responses. For example, β2 adrenergic receptor-mediated suppression of mast cell activity has been reported26 and allergic rhinitis symptoms can be improved after moderate intensity exercise, which increases catecholamine levels27. Under the most stressful conditions, however, exacerbated or dysregulated physiological responses may inadequately regulate IgE production, upregulate, mast cell sensitivity, and increase vulnerability among the most anxious atopic individuals experiencing stressful events.
The current findings require replication with a larger sample. Further, our sample was predominately White, non-Hispanic, young, and college educated. Additional studies are needed to determine whether the current findings generalize to other demographic groups. Finally, our analysis of self-reported allergy should be considered preliminary as all of the allergens used in the SPT battery were not assessed in the self-reported clinical history. Future studies should undergo more extensive clinical history assessment that parallels SPT in order to determine the extent to which stress and anxiety influence sensitivity of SPT. In addition, although SPT is a widely-used standard, studies evaluating the influence of stress and anxiety on other allergy testing approaches, such as intradermal testing or nasal challenge, would help strengthen the clinical relevance of our findings.
Allergy skin testing is a major tool used by allergists to confirm the clinical diagnosis of atopy. The test has high sensitivity and specificity and results from SPTs are used to devise specific treatment strategies12. Our results suggest that, in anxious atopic patients, whether specific allergens test positively or negatively in the clinic may be influenced by the patient’s recent stress levels. Further, it does not appear that the change from negative to positive status for a given allergen was a function of repeated testing. Under non-stressful conditions, anxiety was unrelated to whether a negative allergen later tested positive. This implies that repeated skin prick testing within a short period can be performed without concerns about reliability and validity. More generally, these data suggest possible mechanisms linking high stress levels, in individuals who are more anxious, to increased symptoms in allergic conditions such as rhinitis, asthma, atopic dermatitis and urticaria/angioedema28, 29. In contrast, these data may also suggest that inquiry into acute stress (i.e. exercise or experiencing an acutely stressful event) exposure immediately before SPT may be important for assessing sensitivity of SPT, particularly when these tests fail to confirm strong clinical suspicions of allergen sensitivity in a particular patient. Assessment of current anxiety and stress at the time of skin prick testing, along with clinical history, may provide valuable information about the allergic status of the patient and aid in clinical decision-making.
Acknowledgments
Source of Funding. This research was supported by a supplement to NIH grant P50 DE13749, NIH Training Grant T32 MH18831, by General Clinical Research Center Grant MO1-RR-0034, by Ohio State Comprehensive Cancer Center Core Grant CA16058, and by the Rochester Center for Mind- Body Research grant R24 AG031089-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest The authors have no conflicts of interest to declare.
Sagebrush was not included as participants were not asked to self-report an allergy to sagebrush.
Contributor Information
Kathi L. Heffner, University of Rochester School of Medicine and Dentistry, Department of Psychiatry, Rochester Center for Mind-Body Research
Janice K. Kiecolt-Glaser, The Ohio State University College of Medicine, Department of Psychiatry, The Institute for Behavioral Medicine Research, The Ohio State University Comprehensive Cancer Center
Ronald Glaser, The Ohio State University College of Medicine, Department of Molecular Virology, Immunology and Medical Genetics, The Institute for Behavioral Medicine Research, Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center
William B. Malarkey, The Ohio State University, The Institute for Behavioral Medicine Research, Department of Internal Medicine, The Ohio State University Comprehensive Cancer
Gailen D. Marshall, The University of Mississippi Medical Center, Division of Clinical Immunology and Allergy, Laboratory of Behavioral Immunology Research
References
- 1.Cuffel B, Wamboldt M, Borish L, Kennedy S, Crystal-Peters J. Economic consequences of comorbid depression, anxiety, and allergic rhinitis. Psychosomatics. 1999;40(6):491–496. doi: 10.1016/S0033-3182(99)71187-4. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin RD, Galea S, Perzanowski M, Jacobi F. Impact of allergy treatment on the association between allergies and mood and anxiety in a population sample. Clin Exp Allergy. 2012;42(12):1765–1771. doi: 10.1111/j.1365-2222.2012.04042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katon WJ, Richardson L, Lozano P, McCauley E. The relationship of asthma and anxiety disorders. Psychosom Med. 2004;66(3):349–355. doi: 10.1097/01.psy.0000126202.89941.ea. [DOI] [PubMed] [Google Scholar]
- 4.Stauder A, Kovacs M. Anxiety symptoms in allergic patients: identification and risk factors. Psychosom Med. 2003;65(5):816–823. doi: 10.1097/01.psy.0000088620.66211.b1. [DOI] [PubMed] [Google Scholar]
- 5.Brosschot JF. Markers of chronic stress: prolonged physiological activation and (un)conscious perseverative cognition. Neurosci Biobehav Rev. 2010;35(1):46–50. doi: 10.1016/j.neubiorev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Buske-Kirschbaum A, Ebrecht M, Hellhammer DH. Blunted HPA axis responsiveness to stress in atopic patients is associated with the acuity and severeness of allergic inflammation. Brain Behav Immun. 2010;24(8):1347–1353. doi: 10.1016/j.bbi.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007;21(8):993–999. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kodama A, Horikawa T, Suzuki T, et al. Effect of stress on atopic dermatitis: investigation in patients after the great hanshin earthquake. J Allergy Clin Immunol. 1999;104(1):173–176. doi: 10.1016/s0091-6749(99)70130-2. [DOI] [PubMed] [Google Scholar]
- 9.Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. Am J Respir Crit Care Med. 2002;165(8):1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- 10.Sandberg S, Paton JY, Ahola S, et al. The role of acute and chronic stress in asthma attacks in children. Lancet. 2000;356(9234):982–987. doi: 10.1016/S0140-6736(00)02715-X. [DOI] [PubMed] [Google Scholar]
- 11.Kiecolt-Glaser JK, Heffner KL, Glaser R, et al. How stress and anxiety can alter immediate and late phase skin test responses in allergic rhinitis. Psychoneuroendocrinology. 2009;34(5):670–680. doi: 10.1016/j.psyneuen.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bousquet PJ, Chatzi L, Jarvis D, Burney P. Assessing skin prick tests reliability in ECRHS-I. Allergy. 2008;63(3):341–346. doi: 10.1111/j.1398-9995.2007.01581.x. [DOI] [PubMed] [Google Scholar]
- 13.Tatar EC, Surenoglu UA, Saylam G, Isik E, Ozdek A, Korkmaz H. Is there any correlation between the results of skin-prick test and the severity of symptoms in allergic rhinitis? Am J Rhinol Allergy. 2012;26(1):e37–e39. doi: 10.2500/ajra.2012.26.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein IL, Storms WW. Practice parameters for allergy diagnostic testing. Joint Task Force on Practice Parameters for the Diagnosis and Treatment of Asthma. The American Academy of Allergy, Asthma and Immunology and the American College of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1995;75(6 Pt 2):543–625. [PubMed] [Google Scholar]
- 15.Niemeijer NR, Fluks AF, de Monchy JG. Optimization of skin testing. II. Evaluation of concentration and cutoff values, as compared with RAST and clinical history, in a multicenter study. Allergy. 1993;48(7):498–503. doi: 10.1111/j.1398-9995.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 16.Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 17.Heffner KL, Ginsburg GP, Hartley TR. Appraisals and impression management opportunities: Person and situation influences on cardiovascular reactivity. Int J Psychophysiol. 2002;44(2):165–175. doi: 10.1016/s0167-8760(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 18.Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc.; 1983. [Google Scholar]
- 19.Corder WT, Wilson NW. Comparison of three methods of using the DermaPIK with the standard prick method for epicutaneous skin testing. Ann Allergy Asthma Immunol. 1995;75(5):434–438. [PubMed] [Google Scholar]
- 20.Turkeltaub PC. Percutaneous and intracutaneous diagnostic tests of IgE-mediated diseases (immediate hypersensitivity) Clin Allergy Immunol. 2000;15:53–87. [PubMed] [Google Scholar]
- 21.Andersson M, Pipkorn U. Immediate and late phase allergic cutaneous reactions are not inducers of unspecific or specific local hyperreactivity. Allergy. 1988;43(8):597–602. doi: 10.1111/j.1398-9995.1988.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 22.Minai-Fleminger Y, Levi-Schaffer F. Mast cells and eosinophils: the two key effector cells in allergic inflammation. Inflamm Res. 2009;58(10):631–638. doi: 10.1007/s00011-009-0042-6. [DOI] [PubMed] [Google Scholar]
- 23.Harvima IT, Nilsson G. Stress, the neuroendocrine system and mast cells: current understanding of their role in psoriasis. Expert Rev Clin Immunol. 2012;8(3):235–241. doi: 10.1586/eci.12.1. [DOI] [PubMed] [Google Scholar]
- 24.Theoharides TC, Enakuaa S, Sismanopoulos N, et al. Contribution of stress to asthma worsening through mast cell activation. Ann Allergy Asthma Immunol. 2012;109(1):14–19. doi: 10.1016/j.anai.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Rosenwasser L. New insights into the pathophysiology of allergic rhinitis. Allergy Asthma Proc. 2007;28(1):10–15. doi: 10.2500/aap.2007.28.2977. [DOI] [PubMed] [Google Scholar]
- 26.Wang XS, Lau HY. Beta-adrenoceptor-mediated inhibition of mediator release from human peripheral blood-derived mast cells. Clin Exp Pharmacol Physiol. 2006;33(8):746–750. doi: 10.1111/j.1440-1681.2006.04435.x. [DOI] [PubMed] [Google Scholar]
- 27.Tongtako W, Klaewsongkram J, Jaronsukwimal N, Buranapraditkun S, Mickleborough TD, Suksom D. The effect of acute exhaustive and moderate intensity exercises on nasal cytokine secretion and clinical symptoms in allergic rhinitis patients. Asian Pac J Allergy Immunol. 2012;30(3):185–192. [PubMed] [Google Scholar]
- 28.Hashimoto M, Sato EF, Hiramoto K, Kasahara E, Inoue M. Role of the hypothalamo-pituitary-adrenal axis in the modulation of pollinosis induced by pollen antigens. Allergol Int. 2010;59(2):201–206. doi: 10.2332/allergolint.09-OA-0133. [DOI] [PubMed] [Google Scholar]
- 29.Rod NH, Kristensen TS, Lange P, Prescott E, Diderichsen F. Perceived stress and risk of adult-onset asthma and other atopic disorders: a longitudinal cohort study. Allergy. 2012;67(11):1408–1414. doi: 10.1111/j.1398-9995.2012.02882.x.. [DOI] [PubMed] [Google Scholar]