Abstract
Objectives
Primary carcinomas of the urethra (PCU) are rare and often advanced when diagnosed. Treatment standards are lacking. We studied treatment response and survival in a cohort of patients with PCU, with emphasis on modern platinum-containing chemotherapy regimens plus surgery for advanced disease.
Materials and methods
This was a retrospective chart review of consecutive patients with PCU seen by medical oncologists at our institution over a recent 5-year period. Outcome was measured as best response to chemotherapy. Kaplan-Meier estimates were generated for survival and Cox proportional hazard was used for prognostic factors for survival.
Results
The 44 patients (64% women) included had a median age at diagnosis of 66.5 years. The most prevalent histologic subtypes of PCU were squamous cell carcinoma and adenocarcinoma. At diagnosis, 43% already had lymph node-positive [lymph node (LN)+] disease, and 16% had distant metastases. The entire cohort's overall survival (OS) was 31.7 months. The response rate to platinum-containing neoadjuvant chemotherapy was 72%. Twenty-one patients with locally advanced or LN+ PCU underwent chemotherapy plus surgery. Their median OS from chemotherapy initiation was 25.6 months. Four of 9 patients (44%) with LN+ PCU at diagnosis were alive at our review, with a minimum follow-up of more than 3 years.
Conclusions
Modern platinum-containing regimens appear to be effective in advanced PCU. Preoperative chemotherapy is associated with prolonged disease-free survival in a subgroup of LN+ cases.
Keywords: Urethral carcinoma, Cisplatin chemotherapy, Survival outcomes
1. Introduction
Urothelial cancers are the fourth most common malignancy in men in the USA [1]. The subset of urothelial carcinomas that arise from the urethra accounts for only a small percentage of cases; far more common are those that arise in the bladder [2–4]. A recent Surveillance, Epidemiology, and End Results (SEER) database review of primary carcinomas of the urethra (PCU) in the USA established annual age-adjusted incidence rates in men and women of 4.3 and 1.5 cases of PCU per million population, respectively [5].
PCUs manifest a variety of histologic types, distinguishing them from bladder cancers, which are mainly urothelial carcinomas [5]. The most common histologic types of PCU in the SEER review of 1,615 patients were urothelial carcinoma (55%), squamous cell carcinoma (21.5%), and adenocarcinoma (16.4%) [5].
Clinical management and prognosis depends on the clinical stage and location of the lesion. Despite aggressive surgical treatment of PCU, recurrence is still a problem, especially in cases of locally advanced or posteriorly located tumors. A subgroup of patients (usually men) with tumors of the anterior urethra have better outcomes, with reported disease-free survival of 52% after a mean follow-up of 50 months [6]. Distal urethral tumors tend to be of low stage, and cure rates of 70% to 90% after local surgical excision and radiation therapy have been reported in some series [7]. Most cases (66%) of PCU, however, involve the proximal urethra, and they tend to be more advanced and are associated with a 5-year disease-free survival rate of only 20% to 30% with surgery alone [7].
Owing to the small number of patients who develop these urethral tumors, standardized treatment options are lacking, and no consensus has been reached about the best approach to take. There is especially a paucity of published data on outcomes when newer chemotherapy regimens are used as part of a multimodal treatment strategy for patients with potentially curable urethral carcinomas. At our institution, treatment of locally advanced PCU has evolved to include newer polychemotherapy regimens, including cisplatin, gemcitabine, and ifosfamide (CGI) [8] and gemcitabine, 5-fluorouracil, leukovorin, and cisplatin (Gem-FLP) [9], but the outcomes with these regimens have not been reported thus far. Therefore, the objective of this study was to analyze the data on treatment responses and survival outcomes for patients with advanced PCU who were seen over a recent 5-year period by medical oncologists at our institution.
2. Materials and methods
2.1. Patients
We performed a retrospective chart review of all patients with carcinomas of the urethra in our database and identified all patients with a diagnosis of PCU who had been seen from January 1, 2005, through December 31, 2009. Only patients who were eligible for chemotherapy and thus referred to the Department of Genitourinary Medical Oncology were included. Patients with small tumors who underwent surgery only and were never seen by a medical oncologist were not captured in the database and not considered in this study. Patients with a diagnosis of noncarcinoma (e.g., melanoma, lymphoma of the urethra), prostatic adenocarcinoma, and primary bladder cancer with extension into the urethra were excluded (Fig. 1). The charts of all patients with a diagnosis of “urethral” or “prostatic urethral” carcinomas were reviewed to identify all the patients who had had actual PCU.
Fig. 1.
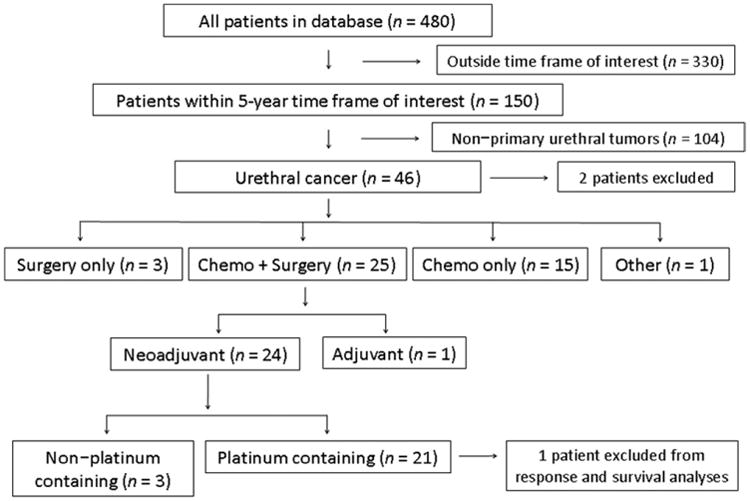
Flow diagram illustrates patient selection and disposition. Of the total of 480 patients in our database with carcinomas of the urethra and the prostatic urethra, 150 were treated at M. D. Anderson Cancer Center during the period from 2005 through 2009. Among those 150, 46 had a diagnosis of primary carcinoma of the urethra. Twenty-five patients received multimodality treatment, including chemotherapy and surgery. In total, 21 patients were treated with platinum-based preoperative chemotherapy followed by surgery.
2.2. Response to chemotherapy
Responses to platinum-containing chemotherapy regimens were defined as the best clinical response to first-line chemotherapy (either preoperatively or for metastatic disease) documented on either radiology or intraoperative reports of evaluation and examination. Responses were determined based on the official radiology reports and characterized as complete (CR), partial (PR), or minor (MR) responses, or as stable (SD) or progressive disease (PD). As this was a retrospective chart analysis, no centralized review of radiographic response was performed. The classification of response was taken verbatim from the official radiology reports, all of which were generated at our institution. Patients with missing records were excluded from analysis.
2.3. Survival outcome and statistical analyses
Survival status was determined on the basis of clinic notes, correspondence documented in charts, and/or the online Social Security Death Index (http://ssdi.rootsweb.ancestry.com/cgi-bin/ssdi.cgi). Data for surviving patients were censored at the time of their last documented visit.
Overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow-up. For patients who received preoperative (neoadjuvant) platinum-containing chemotherapy, a second OS measure was calculated from the first date of chemotherapy to the date of death or last follow-up. For that same subgroup of patients, progression-free survival (PFS; as detected on computed tomography or magnetic resonance imaging) was also calculated from the start of chemotherapy to the date of documented PD, the date of the last assessment for PD, or the date of death. For PFS, both PD and death were considered events.
The product-limit estimator of Kaplan and Meier [10] was used to calculate OS and PFS. Cox proportional hazards regression analysis [11] was used to model the effect of potential prognostic factors on OS from the start of chemotherapy.
All analyses were performed with S-PLUS 7.0 for Windows software (Insightful Corp., Seattle, WA).
3. Results
3.1. Patients
Forty-six patients were eligible for our analysis (Fig. 1). Two patients were excluded from our subsequent analyses: 1 who had had a concomitant diagnosis of acute myeloid leukemia and was not treated for his urethral carcinoma, and another who had only high-grade dysplasia.
Table 1 summarizes the characteristics of the 44 patients (64% female) in the study cohort. Their median age at diagnosis was 66.5 years. Squamous cell carcinoma and adenocarcinoma were most prevalent, followed by urothelial carcinomas (both pure and mixed). Most patients had either locally advanced or lymph node (LN)-only metastatic disease.
Table 1. Patients' characteristics.
| Characteristic | Number of patients | Percentage |
|---|---|---|
| Median age 66.5, years (36-81) | ||
| Race | ||
| White | 23 | 52 |
| Black | 14 | 32 |
| Other | 7 | 16 |
| Female gender | 28 | 64 |
| Histologic subtype | ||
| SCC | 17 | 39 |
| Adenocarcinoma | 13 | 30 |
| Total UC | 8 | 19 |
| UC | 4 | 9 |
| Papillary UC | 2 | 5 |
| Mixed morphology, SCC, and UC | 2 | 5 |
| Undifferentiated carcinoma | 5 | 11 |
| Other | 1 | 2 |
| N. stage | ||
| N0 | 18 | 41 |
| N1 | 19 | 43 |
| M1 | 7 | 16 |
| T. stage | ||
| T1–2 | 1 | 2 |
| T3–4 | 43 | 98 |
SCC = squamous cell carcinoma; UC = urothelial carcinoma.
3.2. Chemotherapy treatments and responses
Response rates (CR+PR+MR) to the most commonly administered chemotherapy regimens, CGI (n = 19) and Gem-FLP (n = 9), were 85% and 67%, respectively. In general, the choice of chemotherapy regimen was based on the histologic subtype. Accordingly, 85% of the patients with squamous cell carcinoma had received CGI, and 15% had received ifosfamide, paclitaxel, cisplatin (ITP). All the patients with adenocarcinoma had been treated with the Gem-FLP regimen. Patients with transitional cell carcinoma received methotrexate, vinblastine, doxorubicin, cisplatin (MVAC), CGI, or ITP.
At least an MR was achieved in 83% of the patients who received platinum-containing chemotherapy (n = 36), and 5 of 36 patients (14%) had a clinical CR (Table 2). Only 1 patient had a pathologic CR after preoperative chemotherapy. The overall response rate (CR + PR) for the various chemotherapy regimens together was 72%.
Table 2. Response rates to platinum-containing chemotherapy regimens*.
| Regimen | Complete response no. (%) | Partial response no. (%) | Minor response no. (%) | Stable disease no. (%) | Progressive disease no. (%) |
|---|---|---|---|---|---|
| CGI | 2 (11) | 12 (63) | 2 (11) | 3 (16) | 0 |
| Gem-FLP | 1 (11) | 5 (56) | 0 | 2 (22) | 1 (11) |
| ITP | 1 (25) | 2 (50) | 1 (25) | 0 | 0 |
| MVAC | 1 (50) | 1 (50) | 0 | 0 | 0 |
| Other | 0 | 1 (50) | 1 (50) | 0 | 0 |
| Total | 5 (14) | 21 (58) | 4 (11) | 5 (14) | 1 (3) |
CGI = cisplatin, gemcitabine, and ifosfamide; Gem-FLP = cisplatin, gemcitabine, 5-fluorouracil, and leucovorin; ITP = ifosfamide, paclitaxel, and cisplatin; MVAC = methotrexate, vinblastine, doxorubicin, and cisplatin.
Thirty-six patients received a platinum-containing regimen.
We also evaluated the pathologic responses in all patients who had undergone surgery (n = 27), which included the subgroup of patients (n = 21) who had received platinum-containing preoperative chemotherapy. As shown in Table 3, 67% of all the patients had undergone LN dissection, and 37% had undergone radical cystectomy as part of the procedure. The rates of positive margins and presence of LN metastasis at the time of surgery in all patients were 33% and 19%, respectively.
Table 3. Procedure performed and status of surgical margins and lymph nodes at surgery after neoadjuvant chemotherapy.
| All patients who had undergone surgery (n = 27) | Subgroup given neoadjuvant platinum-containing chemotherapy (n = 21) | |||
|---|---|---|---|---|
| Variable | n | % | n | % |
| Surgical procedure (some patients underwent >1) | ||||
| Urethrectomy | 12 | 44 | 9 | 43 |
| Radical cystectomy or cystoprostatectomy | 10 | 37 | 6 | 29 |
| Lymph node dissection | 18 | 67 | 14 | 67 |
| Anterior pelvic exenteration | 6 | 22 | 6 | 29 |
| Penectomy | 5 | 19 | 5 | 24 |
| Prostatectomy | 3 | 11 | 2 | 10 |
| Other* | 2 | 7 | 1 | 5 |
| Unknown† | 3 | 11 | 3 | 14 |
| Margin status | ||||
| Positive | 9 | 33 | 7 | 33 |
| Negative | 14 | 53 | 11 | 53 |
| Unknown† | 4 | 14 | 3 | 14 |
| Lymph node status | ||||
| Positive | 5 | 19 | 5 | 24 |
| Negative | 16 | 59 | 12 | 57 |
| Unknown† | 3 | 11 | 3 | 14 |
| Not applicable‡ | 3 | 11 | 1 | 5 |
Scrotectomy, bilateral orchiectomy (n = 1); diverticulectomy (n = 1).
Patient underwent surgery at a different institution and surgical reports were unavailable, or reports in the medical chart did not comment on margin or lymph node status.
Lymph node dissection was not performed.
3.3. Survival
The median OS for all 44 eligible patients was 31.7 months (surgery, 46.9 months vs. no surgery, 21.7 months). Median follow-up time from diagnosis for all 44 patients was 27.3 months (range, 2.8–70.9 months), and for 17 patients who had still been alive at last follow-up 38.4 months (range, 2.8–60.7 months) (Fig. 2).
Fig. 2.
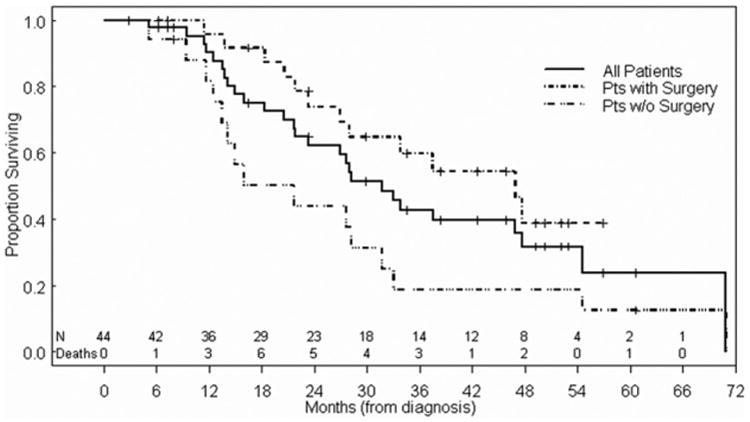
Kaplan-Meier plots of overall survival from time of diagnosis in the entire patient cohort. Patients who underwent surgery had a significantly better overall survival than did patients who did not (P = 0.02).
We then focused our attention on the subgroup of patients who had received platinum-containing neoadjuvant chemotherapy followed by salvage surgery because this group includes those patients who might have had a curative outcome. The median survival time from the start of chemotherapy for 20 patients with available data were 25.6 months (range, 2.3–55.7 months), and that for those patients alive at the last follow-up was 42.0 months (range, 2.3–55.7 months). The effects of several potential prognostic factors on OS and PFS times from the start of chemotherapy are summarized in Tables 4 and 5 (no significant associations with either OS or PFS). Kaplan-Meier survival curves for the OS and PFS of these patients are shown in Fig. 3.
Table 4. Effects of several potential prognostic factors on overall survival, from start of chemotherapy.
| Prognostic factor | Number of patients (number of deaths) | Median survival (months) | P | Hazard ratio | 95% CI |
|---|---|---|---|---|---|
| Surgery (all patients) | |||||
| Yes | 27 (12) | 46.9 | 0.02 | 0.4 | 0.18–0.87 |
| No | 17 (15) | 21.7 | — | 1 | — |
| Pathologic type | |||||
| Nonsquamous cell carcinoma | 8 (6) | 23.4 | — | 1 | — |
| Squamous cell carcinoma | 12 (4) | NR | 0.13 | 0.38 | 0.10–1.35 |
| Lymph node status at initial visit | |||||
| Negative | 11 (5) | 44.2 | — | 1 | — |
| Positive | 9 (5) | 35.6 | 0.55 | 1.47 | 0.42–5.23 |
| Response to chemotherapy | |||||
| No response | 7 (4) | 44.2 | — | 1 | — |
| Complete or partial response | 13 (6) | 25.4 | 0.48 | 1.6 | 0.44–5.79 |
| Margin status at surgery | |||||
| Negative | 11 (5) | 35.6 | — | 1 | — |
| Positive | 7 (4) | 44.2 | 0.69 | 1.31 | 0.35–4.91 |
| Unknown* | 2 (1) | — | — | — | — |
| Lymph node status at surgery | |||||
| Negative | 12 (4) | NR | — | 1 | — |
| Positive | 5 (4) | 17.7 | 0.09 | 3.36 | 0.83–13.7 |
| Unknown* | 3 (2) | — | — | — | — |
Prognostic factor (subset of patients treated with platinum-containing neoadjuvant chemotherapy).
CI = confidence interval; NR = not reached.
Patient underwent surgery at a different institution and surgical reports were unavailable, or reports in the medical chart did not comment on margin or lymph node status.
Table 5. Effects of several potential prognostic factors on progression-free survival of patients given platinum-containing neoadjuvant chemotherapy, from start of chemotherapy.
| Prognostic factor | Number of patients (number of deaths) | Median survival (months) | P | Hazard ratio | 95% CI |
|---|---|---|---|---|---|
| Pathologic type | |||||
| Nonsquamous cell carcinoma | 8 (7) | 12 | — | 1 | — |
| Squamous cell carcinoma | 12 (5) | 35.2 | 0.13 | 0.4 | 0.13–1.29 |
| Lymph node status at initial visit | |||||
| Negative | 11 (6) | 14.9 | — | 1 | — |
| Positive | 9 (6) | 20.6 | 0.82 | 1.14 | 0.37–3.56 |
| Response to chemotherapy | |||||
| No response | 7 (4) | 35.2 | — | 1 | — |
| Complete or partial response | 13 (8) | 14.9 | 0.44 | 1.61 | 0.48–5.41 |
| Margin status at surgery | |||||
| Negative | 11 (6) | 27.3 | — | 1 | — |
| Positive | 7 (5) | 12.4 | 0.33 | 1.89 | 0.53–6.79 |
| Unknown* | 2 (1) | — | — | — | — |
| Lymph node status at surgery | |||||
| Negative | 12 (6) | 19.4 | — | 1 | — |
| Positive | 5 (4) | 15.3 | 0.72 | 1.26 | 0.35–4.47 |
| Unknown* | 3 (2) | — | — | — | — |
CI = confidence interval.
Patient underwent surgery at a different institution and surgical reports were unavailable, or reports in the medical chart did not comment on margin or lymph node status.
Fig. 3.
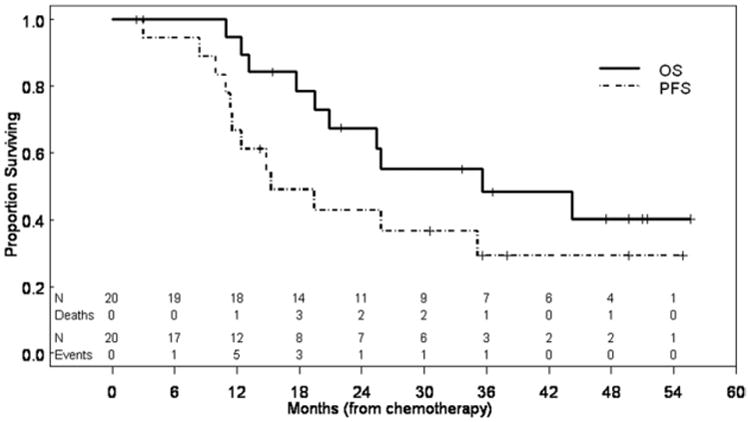
Kaplan-Meier plots for the subgroup of patients who received platinum-based neoadjuvant chemotherapy (n = 21) illustrate PFS and OS calculated from the date of initiation of chemotherapy.
Fifty percent (n = 10) of the patients in this subgroup were still alive at the time of our analysis in February 2011, and 8 of those 10 patients had been followed for more than 3 years (range, 37.2–72.4 months). Four of those 10 patients had LN-metastatic disease when they were first seen; 3 of them had been followed for more than 5 years without recurrence. Eight patients had experienced PD since their surgery, as documented by imaging. Three of those 8 (37.5%) had local recurrence only (n = 1 each: dome of the bladder, vagina, and penile stump). Four others experienced distant recurrence (n = 1 each: lung, femur, retroperitoneal LN, and abdominal soft tissue). The eighth patient, who had previously undergone superficial and deep inguinal LN dissection, experienced recurrence in external iliac, inguinal, and retroperitoneal LN.
Focusing on those patients who underwent chemotherapy followed by surgery, 4 of the 9 patients (44%) with LN-metastatic disease at diagnosis experienced disease-free survival of more than 3 years with the combined treatment of platinum-based chemotherapy followed by salvage surgery.
4. Discussion
In this study, we characterized our single-institution experience with a cohort of contemporary patients with PCU for whom details of the treatment and outcomes are known. Our results can potentially serve as a guiding framework for selecting a treatment approach for patients with locally advanced urethral carcinoma: we emphasize a multidisciplinary approach that includes both combination chemotherapy and surgery.
In our cohort, squamous cell carcinoma, adenocarcinoma, and urothelial carcinoma were the majority of histologic types. The authors of the SEER database review on the incidence of PCU found that urothelial carcinoma was the most prevalent histologic type [5], although they pointed out that because their study was based on data reporting, some misclassification might have occurred. Only 2% of the cases were T1 or T2 lesions, and they were treated with surgery only. Another 16% of the patients had metastatic disease beyond the LN at diagnosis, which means that slightly more than 80% had had locally advanced or LN+ metastatic disease.
Our cohort is enriched with patients with advanced disease referred to the medical oncology department for possible systemic therapy. These are the patients who probably need the greatest effort in terms of a multidisciplinary approach to ensure the best clinical outcomes. Earlier reports of comparable cohorts emphasized a radiation-based approach (with or without surgery and/or chemotherapy) [3,12–14]. However, those patients experienced significant radiation-related toxicity and, in addition, the studies contained relatively small numbers of patients and covered time spans as long as several decades, rendering decision-making regarding these patients difficult.
Within our institution, a preoperative approach to chemotherapy is preferred to adjuvant treatment for several reasons: preoperative systemic therapy downstages the tumor, thus likely improving the results of the surgery; the response of the primary tumor to chemotherapy can be monitored, thus providing an “in vivo sensitivity test” that might guide the prognosis; and finally, possible complications from surgery and associated recovery might prevent a proportion of the patients eligible for systemic therapy from ever receiving appropriate adjuvant therapy. Given these reasons, at our institution all patients with pT3b and T4 and selected high-risk pT2 or pT3a tumors are considered for neoadjuvant chemotherapy.
Comparison of the outcomes between radiotherapy and the neoadjuvant approach described in our series is difficult for several reasons. The historical series of radiotherapy for PCU are more than 10 years old, thus not accounting for modern radiation techniques, which might result in improved outcomes. There are also likely to be differences in patient selection. Randomized prospective comparison is not feasible, however, because PCU is an uncommon malignancy. With those limitations in mind, we note that the historical median survival time for female patients treated with radiotherapy was 24 months [13], whereas in our cohort of 44 consecutive patients, the median OS was more than 31 months, and that for patients selected to undergo surgery (n = 27) approached 4 years.
This study has some limitations, mainly a result of the fact that it is a retrospective review. Unfortunately, the rare nature of this disease makes it very difficult to perform large, randomized prospective studies. Also, the small numbers of each histologic subtype of the disease make it difficult to make valid statements about the possible predictive relationship between distinct histologic type and survival, although our univariate analysis revealed a trend toward better survival of patients with squamous cell carcinoma treated with CGI. Histologic analysis was not centralized for this study, but all cases were originally reviewed at M. D. Anderson by experienced pathologists with a focus on genitourinary cancers. Since most of the patients were not enrolled in a clinical trial, there was no centralized assessment of response by a single radiologist. However, our response criteria were based on the readings of the radiologists and were sufficient to classify the qualitative nature of the response to treatment. Finally, it is important to note that all but 1 patient with locally advanced (T3 or T4) disease had received multimodal treatment. This indicates that similar stages of disease were treated similarly, thus reducing selection bias.
5. Conclusions
We obtained reasonable response rates to front-line cisplatin-based chemotherapy among patients with PCU. When treated with the combination of chemotherapy and surgery, a considerable proportion of patients with LN-metastatic disease remained disease-free after such treatment, which may serve as a starting point for a treatment guideline for this disease.
Acknowledgments
The authors thank Karen Phillips, ELS, for editorial assistance.
Footnotes
This work was supported in part by the National Institutes of Health through M. D. Anderson's Cancer Center Support Grant, P30 CA016672.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA: J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Cheon J, Kim CS, Lee ES, et al. Survey of incidence of urological cancer in South Korea: a 15-year summary. Int J Urol. 2002;9:445–54. doi: 10.1046/j.1442-2042.2002.00500.x. [DOI] [PubMed] [Google Scholar]
- 3.Forman JD, Lichter AS. The role of radiation therapy in the management of carcinoma of the male and female urethra. Urol Clin North Am. 1992;19:383–9. [PubMed] [Google Scholar]
- 4.Mostofi FK, Davis CJ, Jr, Sesterhenn IA. Carcinoma of the male and female urethra. Urol Clin North Am. 1992;19:347–358. [PubMed] [Google Scholar]
- 5.Swartz MA, Porter MP, Lin DW, et al. Incidence of primary urethral carcinoma in the United States. Urology. 2006;68:1164–8. doi: 10.1016/j.urology.2006.08.1057. [DOI] [PubMed] [Google Scholar]
- 6.Dinney CP, Johnson DE, Swanson DA, et al. Therapy and prognosis for male anterior urethral carcinoma: an update. Urology. 1994;43:506–14. doi: 10.1016/0090-4295(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 7.Thyavihally YB, Tongaonkar HB, Srivastava SK, et al. Clinical outcome of 36 male patients with primary urethral carcinoma: a single center experience. Int J Urol. 2006;13:716–20. doi: 10.1111/j.1442-2042.2006.01392.x. [DOI] [PubMed] [Google Scholar]
- 8.Pagliaro LC, Millikan RE, Tu SM, et al. Cisplatin, gemcitabine, and ifosfamide as weekly therapy: a feasibility and phase II study of salvage treatment for advanced transitional-cell carcinoma. J Clin Oncol. 2002;20:2965–70. doi: 10.1200/JCO.2002.11.114. [DOI] [PubMed] [Google Scholar]
- 9.Siefker-Radtke A. Systemic chemotherapy options for metastatic bladder cancer. Expert Rev Anticancer Ther. 2006;6:877–85. doi: 10.1586/14737140.6.6.877. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat, American Statistical Assoc. 1958;53:457–81. [Google Scholar]
- 11.Cox D. Regression models and life tables (with discussion) J Royal Stat Soc: B (Statistical Methodology) 1972;34:187–220. [Google Scholar]
- 12.Cohen MS, Triaca V, Billmeyer B, et al. Coordinated chemoradiation therapy with genital preservation for the treatment of primary invasive carcinoma of the male urethra. J Urol. 2008;179:536–41. doi: 10.1016/j.juro.2007.09.068. Discussion: 541. [DOI] [PubMed] [Google Scholar]
- 13.Dalbagni G, Donat SM, Eschwège P, et al. Results of high dose rate brachytherapy, anterior pelvic exenteration, and external beam radiotherapy for carcinoma of the female urethra. J Urol. 2001;166:1759–61. [PubMed] [Google Scholar]
- 14.Gheiler EL, Tefilli MV, Tiguert R, et al. Management of primary urethral cancer. Urology. 1998;52:487–93. doi: 10.1016/s0090-4295(98)00199-x. [DOI] [PubMed] [Google Scholar]


