Abstract
The muscarinic cholinergic receptor system has been implicated in the pathophysiology of depression, with physiological evidence indicating this system is overactive or hyperresponsive in depression and with genetic evidence showing that variation in genes coding for receptors within this system are associated with higher risk for depression. In studies aimed at assessing whether a reduction in muscarinic cholinergic receptor function would improve depressive symptoms, the muscarinic receptor antagonist scopolamine manifested antidepressant effects that were robust and rapid relative to conventional pharmacotherapies. Here, we review the data from a series of randomized, double-blind, placebo-controlled studies involving subjects with unipolar or bipolar depression treated with parenteral doses of scopolamine. The onset and duration of the antidepressant response are considered in light of scopolamine's pharmacokinetic properties and an emerging literature that characterizes scopolamine's effects on neurobiological systems beyond the cholinergic system that appear relevant to the neurobiology of mood disorders. Scopolamine infused at 4.0 μg/kg intravenously produced robust antidepressant effects versus placebo, which were evident within 3 days after the initial infusion. Placebo-adjusted remission rates were 56% and 45% for the initial and subsequent replication studies, respectively. While effective in male and female subjects, the change in depression ratings was greater in female subjects. Clinical improvement persisted more than 2 weeks following the final infusion. The timing and persistence of the antidepressant response to scopolamine suggest a mechanism beyond that of direct muscarinic cholinergic antagonism. These temporal relationships suggest that scopolamine-induced changes in gene expression and synaptic plasticity may confer the therapeutic mechanism.
Keywords: Antidepressant, bipolar, cholinergic, muscarinic, scopolamine, unipolar
In the management of patients suffering from mood disorders, a great need persists for the availability of rapid antidepressant therapies. A series of studies has demonstrated the ability of novel approaches—including the N-methyl-D-aspartate antagonist, ketamine (1,2), and sleep deprivation therapy (3–5)—to provide significant symptom improvement within hours, with symptoms typically returning within a period of days after discontinuation of the acute intervention. Here, we review a series of randomized, controlled, clinical trials conducted in the National Institute of Mental Health Intramural Research Program, indicating that intravenous administration of the muscarinic cholinergic receptor antagonist, scopolamine (4.0 μg/kg), exerts relatively rapid antidepressant effects. We review these data within the context of an earlier literature that implicated the muscarinic system in the pathophysiology of depression.
Muscarinic Cholinergic Antagonists in the Treatment of Mood Disorders
Interest in the muscarinic cholinergic system in mood disorders stemmed initially from evidence suggesting that hyper-sensitivity of the cholinergic system plays a role in the pathophysiology of depression (6). Researchers showed that increasing cholinergic activity using the anticholinesterase inhibitor, physostigmine, provided a challenge uniquely capable both of exacerbating depressive symptoms in currently depressed subjects with major depressive disorder (MDD) and inducing depressive symptoms and reversing manic symptoms in manic subjects with bipolar disorder (BD) (6–10). The neuroendocrine and pupillary responses to physostigmine (11–13) also were abnormally increased in depressed individuals. The muscarinic cholinergic receptor system specifically was implicated by evidence showing that polysomnographic responses to selective muscarinic agonists (14–16) were exaggerated in depressed versus control samples, suggesting that muscarinic receptor supersensitivity exists in depressed individuals. Furthermore, within the muscarinic receptor system, variation in the type 2 muscarinic (M2) cholinergic receptor gene (CHRM2) was associated with an elevated incidence or severity of unipolar depression and with abnormal reductions in M2 receptor binding in bipolar depression (17–19).
Some abnormalities in cholinergic receptor function in mood disorders showed sex effects. For example, sex differences manifested in the baseline and cholinergically stimulated plasma hormone measures that differed between depressed and control samples, suggesting that heightened cholinergic sensitivity exists preferentially in premenopausal females with MDD (13,20,21). Comings et al. (18) found that genetic variation in CHRM2 gene (A/T 1890) was associated with MDD specifically in female subjects. In rodents, estrogen enhanced choline acetyltransferase activity and acetylcholine release (22,23), and M2 receptor stimulation mediated the estrogen-induced enhancement of N-methyl-D-aspartate receptor (NMDAR) function (24). These observations complement evidence reviewed below that women are more likely than men to show an antidepressant response to scopolamine.
Putative animal models of depression also supported a role for elevated muscarinic cholinergic function. Flinders Sensitive Line rats, bred selectively for increased sensitivity of muscarinic receptors, showed putative behavioral analogs of depression such as lethargy, reductions in self-stimulation, and increased behavioral despair in the forced swim test in response to agents that increase central cholinergic function (25). Moreover, antimuscarinic agents (including scopolamine) produced antidepressant-like effects by reducing the behavioral despair induced via this test (26,27).
Collectively, these data suggested the hypothesis that antimuscarinic drugs produce antidepressant effects and, based upon the rapidity of the depressogenic effects of physostigmine, raised expectations that mood improvements may manifest rapidly. Nevertheless, early studies exploring effects of antimuscarinic agents reported modest and inconsistent antidepressant responses (reviewed in [28]), although these were primarily uncontrolled studies. In one open-label study, significant antidepressant effects were observed the day following the administration of scopolamine .4 mg intramuscular (29), but the magnitude of this effect was small. Based upon the lower bioavailability during intramuscular administration, this dose approximates 2 μg/kg intravenous (IV) in a 100 kg individual (30). The results of the studies presented below suggest that a higher scopolamine dose may be required to obtain an antidepressant effect size of sufficient magnitude to encourage further development. In another study, scopolamine was administered in depressed subjects at doses up to .5 mg IV, and no change in mood ratings (assessed using Profile of Mood States [POMS] depression factor scores) was observed by 120 minutes postinfusion (28). The highest dose used in that study was comparable with the dose used in our studies (4.0 μg/kg), and the clinical result also was similar, insofar as we observed no significant change in POMS depression factor ratings up to 150 minutes after scopolamine administration. As the clinical effects were assessed only acutely (120 minutes postinfusion) (28), the robust antidepressant effects described below were established 3 to 5 days following drug administration.
Pilot Dose-Finding Study Suggests Rapid Antidepressant Response to Scopolamine
In a pilot study designed to evaluate the role of the muscarinic system in the cognitive symptoms associated with depression, Furey and Drevets (31) observed a rapid and robust antidepressant response to scopolamine. We specifically aimed to investigate whether the impairments of selective attention manifest in depressed subjects would improve under antimuscarinic challenge. Muscarinic cholinergic mechanisms facilitate the processing of sensory information and play a major role in selective attention processing, such that deviations from an optimal range of cholinergic function in either direction can impair attention (32– 36). Based upon the evidence that muscarinic receptors are hypersensitive in depressed patients, we hypothesized that selective attention would improve under antimuscarinic challenge.
To test this hypothesis, we selected scopolamine because of its selectivity for muscarinic receptors, high potency for all five muscarinic receptor subtypes (KD ranges from .4 to 2.1 nmol/L for M1 through M5), slow dissociation rate from central muscarinic cholinergic receptors, and advantageous pharmacokinetic profile for rapidly entering brain (37–39). We designed a dose-finding study using a scopolamine dose range that previously was associated with cognitive effects but without toxic effects such as delirium (e.g., [40,41]). Eight MDD patients participated in four testing sessions performed in random order under double-blind conditions, in which participants received a 15-minute IV infusion of saline placebo and each of three doses of scopolamine: 2.0, 3.0, and 4.0 μg/kg.
The mean Montgomery-Åsberg Depression Rating Scale (MADRS) scores differed across assessments (p = .005), and the MADRS scores obtained following 4.0 μg/kg of scopolamine were lower than both the baseline (p = .0015) and the pre-4.0 μg/kg measures (p = .018). Moreover, there was a larger reduction in MADRS scores pre-4.0 μg/kg versus post-4.0 μg/kg of scopolamine than preplacebo versus postplacebo (p = .01), where postassessments were obtained at the subsequent session, 3 to 5 days later. No other difference was significant. The mean change in MADRS score between the pretreatment baseline and the evaluation following session 4 was −13.8 ± 7.7 (p < .002). Five subjects showed a >50% reduction in the MADRS score, and three remitted (MADRS < 10).
The improvement observed, particularly following the 4.0 μg/kg dose, suggested robust antidepressant responses to scopolamine. The effects occurred rapidly, as depressive symptoms were improved during the 3 to 5 days between infusions. Nonetheless, these promising results were unexpected, and the study was not designed to evaluate an antidepressant response. A second study was designed to test the hypothesis that the antidepressant response to scopolamine would exceed that to placebo.
Randomized Controlled Trial Confirms Rapid Antidepressant Response to Scopolamine
In a double-blind, placebo-controlled, crossover clinical trial (n = 18), depressed subjects with MDD (n = 9) or BD (n = 9) underwent multiple sessions in which they received 15-minute IV infusions of placebo (P) or scopolamine (S) (4.0 μg/kg) (31). Following a single-blind placebo lead-in, participants entered either a P/S sequence or a S/P sequence, where P was a series of three placebo sessions and S was a series of three scopolamine sessions. The sessions were separated by 3 to 5 days. Clinical ratings were acquired before each infusion.
Volunteers 18 to 45 years of age were assessed for eligibility if they met DSM-IV criteria for recurrent MDD or BD. Exclusion criteria included exposure to psychotropic drugs or other medications likely to affect cholinergic function within 3 weeks, current smoking, serious risk of suicide, current psychosis, lifetime history of substance dependence or substance abuse within 1 year, major medical or neurological disorders, narrow angle glaucoma, hypersensitivity to anticholinergic agents, hepatic dysfunction, or weight >125 kg. Pregnant or nursing female subjects were excluded. Subjects provided written informed consent as approved by the National Institute of Mental Health Institutional Review Board.
The primary outcome measure used to assess the antidepressant response was the change in MADRS scores. Using conventional criteria (42), patients were characterized as achieving full response (>50% reduction in MADRS score from baseline) and/or remission (posttreatment MADRS score <10). Secondary outcome measures included the Hamilton Anxiety Rating Scale, Clinical Global Impressions, and POMS. The mean area under the curve concentrations of scopolamine did not differ significantly across the three 4.0 μg/kg scopolamine infusions.
Following completion of the initial study block, the group receiving scopolamine first (S/P) showed a greater reduction in MADRS scores than the group who received placebo first (P/S) (the placebo-adjusted reduction in MADRS scores under scopolamine was 52%; p < .0001; Cohen's d = 2.7). Similarly, within-group analyses in the P/S group showed lower MADRS scores in block 2 as compared with both the baseline block (p < .0001; Cohen's d = 3.2) and block 1 (the placebo-adjusted reduction in MADRS scores under scopolamine was 66%; p < .0001, Cohen's d = 3.4). In both the P/S and S/P subgroups, improvement was significant at the first evaluation that followed scopolamine administration (i.e., 3 to 4 days following the initial administration) (Figure 1). Anxiety ratings also decreased under scopolamine versus placebo (p < .001). Notably, the reductions in depression and anxiety ratings that the S/P group experienced following scopolamine persisted throughout the subsequent placebo series, well beyond the expected duration of scopolamine's direct action at muscarinic receptors (Figure 1).
Figure 1.
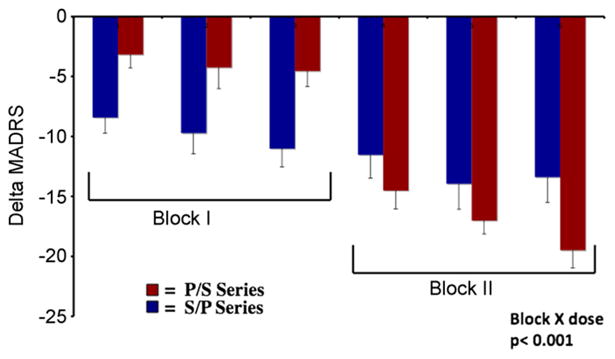
Mean change in Montgomery-Åsberg Depression Rating Scale (MADRS) over sessions relative to the first baseline session is shown for the group who received placebo (P) in block 1 and scopolamine (S) in block 2 (P/S; red bars) and the group receiving scopolamine in block 1 and placebo in block 2 (S/P; blue bars). The 52 participants whose data are represented in this graph were pooled from the randomized controlled trials described in the text (31,43,44). Errors bars reflect standard error.
Replication in Independent Sample
These findings were replicated in an independent sample limited to depressed subjects with recurrent MDD (43). Twenty-three depressed subjects were randomized into the study, of whom 22 were included in the analysis (one dropped out before receiving scopolamine). Using the double-blind, placebo-controlled, crossover design described above, subjects were randomized into either a P/S (n = 11) or S/P (n = 11) sequence. Upon completion of the first block, the group receiving scopolamine first (S/P) showed a 32% reduction in MADRS scores (p < .001) that exceeded the corresponding change of 6.5% under placebo (P/S) (p = .009; Cohen's d = 1.38). Improvement was significant at the first evaluation that followed scopolamine administration (p = .011). In the second block, the P/S group showed a 53% reduction in MADRS scores (p = .001) following scopolamine versus baseline, while the reduction seen in S/P subjects who received scopolamine first persisted, as they received placebo in block 2 (i.e., the MADRS scores in the S/P group decreased nonsignificantly [7.0%] by the end of block 2 versus the end of block 1 [t = .65; p = .53], such that at the end of the placebo phase, their mean reduction in MADRS scores relative to baseline was 38%).
Sex Effects in the Response to Scopolamine
In a third study, we assessed sex differences in antidepressant response to scopolamine (44). A total of 52 (male [n = 21] and female [n = 31]) outpatients meeting criteria for recurrent MDD or BD participated using the same experimental design. The sample included the 18 subjects from our initial study, the 22 subjects from the replication study, and 12 newly studied subjects. The treatment group × block interaction was significant in male subjects (p = .043) and female subjects (p < .001) separately, although a block × gender interaction (p = .009) indicated that the response magnitude was larger in women. The Hamilton Anxiety Rating Scale scores similarly showed a block × gender interaction indicating a greater antianxiety response to scopolamine in women (p = .001). Neither the area under the curve plasma concentration for scopolamine nor the drug-induced reductions in heart rate and blood pressure (BP) (which putatively are centrally mediated) differed significantly between male and female subjects (44).
These data add to other literature reporting sex differences in the response to some antidepressant classes. Although disagreement remains (45), when such differences are reported, women show an enhanced response to selective serotonin reuptake inhibitors (SSRIs) versus either the selective norepinephrine reuptake inhibitor, reboxetine, or the tricyclic antidepressants (TCA) imipramine and maprotiline (which predominantly inhibit norepinephrine transporters), while men show a better response to imipramine versus SSRI and no difference in their response to reboxetine or maprotiline versus SSRI (46–48).
Adverse and Side Effects
Compared with placebo, scopolamine administration resulted in higher rates of drowsiness, blurred vision, dry mouth, and light-headedness, but these effects were sufficiently transient (resolving within 2 to 4 hours) and well tolerated that no subject dropped out due to a side effect. No medically serious adverse event was encountered; in particular, no subject developed delirium, psychosis, overt confusion, clinically significant cardiovascular effects, or treatment-emergent suicidal ideation. The heart rate, systolic BP, and diastolic BP decreased following scopolamine relative to placebo, consistent with scopolamine's central effects on parasympathetic autonomic function, although no subject developed symptoms of hypotension or evidence of cardiovascular insufficiency (Figure 2).
Figure 2.
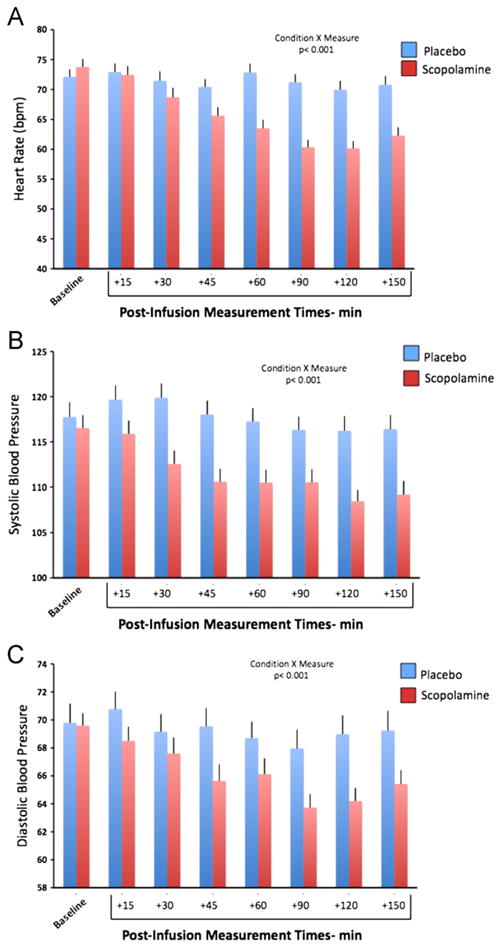
In the dose range studied, scopolamine administration reduced heart rate and blood pressure, putatively reflecting enhancement of the central parasympathetic autonomic outflow (50–52). (A) Mean heart rate (± SE) is shown for the placebo (blue) and the scopolamine (red) sessions averaged over all subjects. Bars reflect baseline, as well as heart rate, at each time point indicated, relative to infusion start time. (B) Mean systolic blood pressure (± SE) is shown for the placebo (blue) and the scopolamine (red) sessions averaged over all subjects. Bars reflect baseline, as well as systolic pressure, at each time point indicated, relative to infusion start time. (C) Diastolic blood pressure (± SE) is shown for the placebo (blue) and the scopolamine (red) sessions averaged over all subjects. Bars reflect baseline, as well as diastolic pressure, at each time point indicated, relative to infusion start time.
No subject developed hypomania during the study. The mean Young Mania Rating Scale score decreased (p < .006) between baseline and study end. If confirmed in a larger sample of BD subjects, the lack of switching in the bipolar group as they received a putatively effective antidepressant would be of particular interest to the clinical psychopharmacology field.
With respect to neuropsychological testing, scopolamine's effects on performance on selective attention tasks were neither generalized nor unidirectional (34).
Timing of Onset of Scopolamine's Antidepressant Effects
Although we established that scopolamine's antidepressant action was evident by 3 days after administration, no ratings were obtained between the initial infusion and the first follow-up evaluation (days 3–5). Nevertheless, those participants who observed an improvement in their depression severity generally reported relief from their depressive symptoms on the first morning after scopolamine infusion (i.e., within 24 hours of drug exposure). In contrast, no improvement in mood was evident within 150 minutes of scopolamine infusion based upon the POMS (43). Further studies are needed to characterize more precisely the timing of the onset of scopolamine's antidepressant effect (Supplement 1).
Effect Size of the Antidepressant Response
The Cohen's d values for the within-group comparisons of scopolamine versus placebo for blocks 1 and 2 were 2.2 and 3.4 in our first study and 1.2 and 1.7 in our replication study, respectively. These effect sizes compared favorably with those typically observed in antidepressant treatment studies, which ranged from .5 to 1.1 in moderately and severely depressed cases, respectively (49) (the participants in our studies manifested depression severity in the moderate-to-severe range).
Most of the subjects who responded to scopolamine also achieved remission (MADRS score <10), irrespective of whether they had MDD or BD (Table 1). The placebo-adjusted remission rates (rate under scopolamine minus rate under placebo) were 56% and 45% in our first and second studies, respectively. These results compare favorably with the 10% to 20% placebo-adjusted remission rates reported for SSRIs (50). Nevertheless, no study has compared directly the efficacy of scopolamine with that for SSRIs.
Table 1. Scopolamine Response vs. Remission (Rates Based on MADRS)a.
| Diagnosis | Response (≥50% Improvement) | Remission (Mean of Final Two Sessions ≤10) |
|---|---|---|
| MDD | ||
| n/ntotal (%) | 26/38 (68) | 18/38 (47) |
| BD | ||
| n/ntotal (%) | 10/15 (67) | 9/15 (60) |
BD, bipolar disorder; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder.
One BD and no MDD subjects met response criteria to placebo.
Duration of Antidepressant Effect: Potential Benefits of Repeated Administration
The antidepressant response persisted beyond the final scopolamine administration by at least several weeks. In individuals who received scopolamine during block 1, the improvement seen during drug administration persisted, as they received placebo during block 2, indicating the antidepressant effects persisted at least 10 to 14 days after the final scopolamine administration (31,43). This carryover effect was confirmed by demonstrating that depression ratings did not differ between the S/P and P/S groups in the final study block, when both groups showed improvement relative to the pretreatment baseline. Subjects showed further improvement across the scopolamine block (which consisted of three scopolamine infusions each separated by 3 to 5 days), suggesting that repeated administrations provided additional benefit. Nevertheless, whether the persistence in the antidepressant response depends on repeated administrations of scopolamine remains unclear.
Preliminary Observations Using Scopolamine in Treatment-Resistant Depression
Within the study samples described above, 11 participants met criteria for treatment-resistant depression (Supplement 1). Preliminary data suggest that previous nonresponse to conventional antidepressants may not predict nonresponse to scopolamine. While confirmation in a larger sample of treatment-resistant subjects is critical, these observations are compatible with pharmacological and gene expression data indicating that the therapeutic mechanism of scopolamine differs from that of SSRIs.
Previous Literature Using Other Antimuscarinics in Depression
Of treatments reported to produce antidepressant responses within 1 week, namely electroconvulsive therapy (ECT), high-dose tricyclic antidepressant administration, total sleep deprivation, and ketamine, both ECT and high-dose tricyclic antidepressants are associated with potent antimuscarinic effects. Electroconvulsive therapy is routinely preceded by administration of the antimuscarinic agent, atropine, to reduce salivation and stabilize autonomic responses. Whether atropine contributes to the antidepressant efficacy of ECT is unclear, particularly since atropine has poor penetration across the blood brain barrier. In contrast, scopolamine rapidly enters the brain (37).
In the case of the TCAs, the IV administration of amitriptyline (AMT) and clomipramine resulted in antidepressant effects within 1 week (52,53), although the extent to which antimuscarinic effects contribute to the efficacy of TCAs has not been established. While several TCAs have sufficient muscarinic receptor affinity to produce peripheral anticholinergic side effects, putatively these effects are mediated at peripheral, parasympathetic neuroeffector junctions in various organs, which have much higher sensitivity to antimuscarinic drugs than central muscarinic receptors (54). Among the TCAs, AMT has the highest potency for muscarinic receptors and is one of the only TCAs with an affinity for muscarinic receptors that is similar in magnitude to its affinity for monoamine transporters (55,56). Thus, at therapeutic doses of AMT, where most of the serotonin transporter sites should be occupied, a large proportion of muscarinic sites also would be occupied (57). Because the scopolamine-induced antidepressant effect appeared dose-dependent in our pilot study, this difference in muscarinic receptor affinity across TCAs may hold relevance for the finding that AMT was the only nonselective antidepressant drug that proved more effective than more selective agents (e.g., SSRI) (58). In clinical practice, however, the AMT dose is gradually titrated upward because of side effects, so potentially rapid responses to full therapeutic AMT doses may not have been detected using oral dosing. In contrast, the more rapid antidepressant effects observed during IV administration of AMT may have depended, at least partly, on the rapid antimuscarinic action achieved (52).
Whether the mechanism of action underlying scopolamine's antidepressant effect depends upon a specific muscarinic receptor subtype or some combination of subtypes remains unclear. The only controlled study of a selective antimuscarinic agent other than scopolamine found no significant difference in the antidepressant response to biperiden relative to glycopyrrolate (an antimuscarinic agent that only weakly crosses the blood brain barrier) (59). Biperiden is relatively selective for M1 muscarinic receptors, whereas scopolamine has high potency at all five muscarinic subtypes (60), suggesting the hypothesis that the antidepressant response at least partly involves muscarinic receptors other than M1.
Possible Mechanisms of Action Underlying Scopolamine's Antidepressant Effect
Although scopolamine's antidepressant efficacy appears consistent with the hypothesis that hypersensitivity of the cholinergic system plays a central role in the pathophysiology of mood disorders (6), the latency of the antidepressant response and its persistence well after scopolamine's clearance from plasma (elimination half-life = 2–4 hours) suggests a mechanism beyond the direct pharmacological actions on muscarinic receptors. The delayed onset and persistence of response until well beyond the resolution of anticholinergic side effects appear compatible with an effect on gene transcription or synaptic plasticity.
Scopolamine conceivably may alter synaptic plasticity or gene expression through a variety of direct or indirect mechanisms. In addition to producing antagonist effects at muscarinic receptors, scopolamine acutely increases acetylcholine release (via inhibi tion of release-controlling muscarinic autoreceptors) and thereby increases cholinergic effects on nicotinic receptor systems to an extent that conceivably may contribute to antidepressant or anti-inflammatory effects (61,62). In addition, changes in muscarinic tone specifically have been shown to affect other depression relevant systems, including the central dopamine, serotonin, and neuropeptide Y transmitter systems and the innate immune system (63). Thus, the antidepressant mechanism(s) of scopolamine potentially may involve a variety of systems.
One effect of scopolamine that is shared by some other somatic antidepressant treatments involves modulation of NMDAR function. The NMDAR gene expression is enhanced by muscarinic receptor stimulation in at least some brain structures (64), and thus, the elevated muscarinic receptor sensitivity identified in mood disorders (65) may contribute to an elevation in NMDAR transmission. Blocking muscarinic receptors via scopolamine administration reduces messenger RNA concentrations for NMDAR types 1A and 2A in the rat brain in vivo and protects hippocampal neurons from glutamate-mediated neurotoxicity in vitro (64,66). Chronic administration of TCAs and repeated electroconvulsive shock reduce cortical NMDAR function, and treatments associated with a rapid onset of antidepressant effects exert direct NMDAR antagonist effects (ketamine) or induce NMDAR internalization (sleep deprivation) (67). Given evidence that abnormal glutamatergic transmission is involved in the pathophysiology of depression, these data suggest that scopolamine's effect on reducing NMDAR gene expression may play a role in its antidepressant action.
Notably, an effect of ketamine on synaptic plasticity that is hypothesized to underlie its rapid antidepressant effects is at least partly shared by scopolamine. Li et al. (68) reported that ketamine administration rapidly activates the mammalian target of rapamycin (mTOR) pathway, leading to increased synaptic signaling protein expression and increased number and function of new spine synapses in the rodent prefrontal cortex. The blockade of mTOR signaling interrupted ketamine's induction of synaptogenesis and the associated antidepressant-like, behavioral responses in rodent models (68). The same group demonstrated that scopolamine also induces the mTOR pathway at a timing and magnitude similar to ketamine (69). Ketamine's enhancement of mTOR signaling appeared to depend upon the ketamine-induced elevation of extracellular glutamate concentrations and the associated increase in glutamate-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor activation (70,71). Similarly, scopolamine administration acutely increases extracellular glutamate concentrations in the rodent striatum (72). These data suggest the hypothesis that the rapid antidepressant effects of ketamine and scopolamine involve effects on synaptic plasticity (69).
Limitations of the Extant Data
The antidepressant efficacy of scopolamine (4.0 μg/kg IV) awaits replication by an independent laboratory. The generalizability of our findings is limited by the relatively small sample size studied to date (n = 52), the inclusion of both unipolar and bipolar depressives, and the exclusion of cigarette smokers and individuals under age 18 or over age 55. Our studies also were limited to outpatients and included a disproportionately high number of subjects who were naïve to antidepressant pharmacotherapy. Scopolamine's clinical utility in emergency room or inpatient settings has not been assessed.
The side effects experienced during scopolamine infusion conceivably may compromise the blind to drug versus placebo assignment. Nevertheless, the transient nature of the side effects aided the preservation of the double-blind, since the primary outcome measure was obtained at the beginning of each session when subjects were side-effect free (i.e., before they received the infusion for that day) and thus reflected the treatment received during the session that occurred 3 to 5 days previously. Another design feature that mitigated the likelihood of unblinding by side effects was that the placebo challenge, which involved IV infusion while sitting in a reclining hospital chair or bed, was associated with sedative side effects in 59% of subjects.
Whether scopolamine's utility as an antidepressant treatment can be achieved using other routes of administration remains unclear. Scopolamine is poorly and inconsistently absorbed via the oral route (73). While scopolamine is well absorbed transdermally, the gene expression changes observed in the mTOR pathway are concentration dependent (74), and it is unclear whether the maximum concentrations achieved under the IV route can be achieved under routes characterized by slower absorption, such as the transdermal route (75). Finally, scopolamine is well absorbed during intranasal administration (73), but it remains unclear whether the greater variability in absorption under the intranasal route will unacceptably increase the rate of adverse events (40). Nevertheless, parenteral routes other than the IV route ultimately may prove effective for obtaining antidepressant efficacy using scopolamine (e.g., [30]).
Conclusions
The studies reviewed here provide evidence that scopolamine (4.0 μg/kg IV) exerts rapid and robust antidepressant effects. Scopolamine was well tolerated at this dose, particularly since it was pulsed at semiweekly intervals so that the problematical antimuscarinic effects associated with daily use (e.g., constipation, urinary retention) were rare. Future studies are needed to independently replicate these findings and to examine whether a comparable antidepressant response can be achieved when scopolamine is delivered via other routes.
Supplementary Material
Footnotes
Drs. Furey and Drevets are named as co-inventors on the use patent application “Scopolamine in the Treatment of Depression” that is pending. Drs. Furey and Drevets have assigned their rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. While neither investigator has received a royalty or payment related to scopolamine, they have the possibility of doing so if the use patent ultimately is licensed to a commercial entity. Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government.
Supplementary material cited in this article is available online.
References
- 1.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 2.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 3.Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6:361–377. [PubMed] [Google Scholar]
- 4.Shelton RC, Loosen PT. Sleep deprivation accelerates the response to nortriptyline. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:113–123. doi: 10.1016/0278-5846(93)90036-r. [DOI] [PubMed] [Google Scholar]
- 5.Wu JC, Kelsoe JR, Schachat C, Bunney BG, DeModena A, Golshan S, et al. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66:298–301. doi: 10.1016/j.biopsych.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 7.Davis JL, Gerbrandt LK, Cherkin A. Retroactive amnesia induced in chicks by the proline analog L-baikiain, without EEG seizures or depression. Physiol Behav. 1978;21:653–658. doi: 10.1016/0031-9384(78)90144-0. [DOI] [PubMed] [Google Scholar]
- 8.Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Nurnberger JI, Jr, Jimerson DC, Simmons-Alling S, Tamminga C, Nadi NS, Lawrence D, et al. Behavioral, physiological, and neuroendocrine responses to arecoline in normal twins and “well state” bipolar patients. Psychiatry Res. 1983;9:191–200. doi: 10.1016/0165-1781(83)90043-4. [DOI] [PubMed] [Google Scholar]
- 10.Risch SC, Kalin NH, Janowsky DS. Cholinergic challenges in affective illness: Behavioral and neuroendocrine correlates. J Clin Psychopharmacol. 1981;1:186–192. doi: 10.1097/00004714-198107000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Dilsaver SC. Pathophysiology of “cholinoceptor supersensitivity” in affective disorders. Biol Psychiatry. 1986;21:813–829. doi: 10.1016/0006-3223(86)90246-5. [DOI] [PubMed] [Google Scholar]
- 12.Janowsky DS, Risch SC, Huey LY, Kennedy B, Ziegler M. Effects of physostigmine on pulse, blood pressure, and serum epinephrine levels. Am J Psychiatry. 1985;142:738–740. doi: 10.1176/ajp.142.6.738. [DOI] [PubMed] [Google Scholar]
- 13.Rubin RT, O'Toole SM, Rhodes ME, Sekula LK, Czambel RK. Hypothalamo-pituitary-adrenal cortical responses to low-dose physostigmine and arginine vasopressin administration: Sex differences between major depressives and matched control subjects. Psychiatry Res. 1999;89:1–20. doi: 10.1016/s0165-1781(99)00085-2. [DOI] [PubMed] [Google Scholar]
- 14.Berger M, Riemann D, Hochli D, Spiegel R. The cholinergic rapid eye movement sleep induction test with RS-86. State or trait marker of depression? Arch Gen Psychiatry. 1989;46:421–428. doi: 10.1001/archpsyc.1989.01810050035006. [DOI] [PubMed] [Google Scholar]
- 15.Gillin JC, Sitaram N, Duncan WC. Muscarinic supersensitivity: A possible model for the sleep disturbance of primary depression? Psychiatry Res. 1979;1:17–22. doi: 10.1016/0165-1781(79)90023-4. [DOI] [PubMed] [Google Scholar]
- 16.Riemann D, Hohagen F, Krieger S, Gann H, Muller WE, Olbrich R, et al. Cholinergic REM induction test: Muscarinic supersensitivity underlies polysomnographic findings in both depression and schizophrenia. J Psychiatr Res. 1994;28:195–210. doi: 10.1016/0022-3956(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 17.Cannon DM, Klaver JK, Gandhi SK, Solorio G, Peck SA, Erickson K, et al. Genetic variation in cholinergic muscarinic-2 receptor gene modulates M2 receptor binding in vivo and accounts for reduced binding in bipolar disorder. Mol Psychiatry. 2011;16:407–418. doi: 10.1038/mp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comings DE, Wu S, Rostamkhani M, McGue M, Iacono WG, MacMurray JP. Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. Am J Med Genet. 2002;114:527–529. doi: 10.1002/ajmg.10406. [DOI] [PubMed] [Google Scholar]
- 19.Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, et al. Evidence of common and specific genetic effects: Association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13:1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- 20.Riemann D, Hohagen F, Bahro M, Berger M. Sleep in depression: The influence of age, gender and diagnostic subtype on baseline sleep and the cholinergic REM induction test with RS 86. Eur Arch Psychiatry Clin Neurosci. 1994;243:279–290. doi: 10.1007/BF02191586. [DOI] [PubMed] [Google Scholar]
- 21.Rubin RT, Abbasi SA, Rhodes ME, Czambel RK. Growth hormone responses to low-dose physostigmine administration: Functional sex differences (sexual diergism) between major depressives and matched controls. Psychol Med. 2003;33:655–665. doi: 10.1017/s0033291703007426. [DOI] [PubMed] [Google Scholar]
- 22.Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124:809–816. doi: 10.1016/j.neuroscience.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 24.Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overstreet DH, Russell RW, Hay DA, Crocker AD. Selective breeding for increased cholinergic function: Biometrical genetic analysis of muscarinic responses. Neuropsychopharmacology. 1992;7:197–204. [PubMed] [Google Scholar]
- 26.Betin C, DeFeudis FV, Blavet N, Clostre F. Further characterization of the behavioral despair test in mice: Positive effects of convulsants. Physiol Behav. 1982;28:307–311. doi: 10.1016/0031-9384(82)90080-4. [DOI] [PubMed] [Google Scholar]
- 27.Browne RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58:331–334. doi: 10.1016/0014-2999(79)90483-7. [DOI] [PubMed] [Google Scholar]
- 28.Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thompson K, Mellow AM, et al. The effects of acute scopolamine in geriatric depression. Arch Gen Psychiatry. 1988;45:906–912. doi: 10.1001/archpsyc.1988.01800340028004. [DOI] [PubMed] [Google Scholar]
- 29.Gillin JC, Sutton L, Ruiz C, Darko D, Golshan S, Risch SC, Janowsky D. The effects of scopolamine on sleep and mood in depressed patients with a history of alcoholism and a normal comparison group. Biol Psychiatry. 1991;30:157–169. doi: 10.1016/0006-3223(91)90170-q. [DOI] [PubMed] [Google Scholar]
- 30.Ebert U, Grossmann M, Oertel R, Gramatte T, Kirch W. Pharmacokinetic-pharmacodynamic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. J Clin Pharmacol. 2001;41:51–60. doi: 10.1177/00912700122009836. [DOI] [PubMed] [Google Scholar]
- 31.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: A randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furey ML. The prominent role of stimulus processing: Cholinergic function and dysfunction in cognition. Curr Opin Neurol. 2011;24:364–370. doi: 10.1097/WCO.0b013e328348bda5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- 34.Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology. 2008;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez V, Parikh V, Sarter M. Sensitized attentional performance and Fos-immunoreactive cholinergic neurons in the basal forebrain of amphetamine-pretreated rats. Biol Psychiatry. 2005;57:1138–1146. doi: 10.1016/j.biopsych.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing in schizophrenia. Schizophr Bull. 2005;31:117–138. doi: 10.1093/schbul/sbi006. [DOI] [PubMed] [Google Scholar]
- 37.Frey KA, Koeppe RA, Mulholland GK, Jewett D, Hichwa R, Ehrenkaufer RL, et al. In vivo muscarinic cholinergic receptor imaging in human brain with [11C]scopolamine and positron emission tomography. J Cereb Blood Flow Metab. 1992;12:147–154. doi: 10.1038/jcbfm.1992.18. [DOI] [PubMed] [Google Scholar]
- 38.Hyttel J, Larsen JJ, Christensen AV, Arnt J. Receptor-binding profiles of neuroleptics. Psychopharmacology Suppl. 1985;2:9–18. doi: 10.1007/978-3-642-70140-5_2. [DOI] [PubMed] [Google Scholar]
- 39.Richelson E. Antimuscarinic and other receptor-blocking properties of antidepressants. Mayo Clin Proc. 1983;58:40–46. [PubMed] [Google Scholar]
- 40.Safer DJ. The concomitant effects of mild sleep loss and an anticholinergic drug. Psychopharmacologia. 1970;17:425–433. doi: 10.1007/BF00403813. [DOI] [PubMed] [Google Scholar]
- 41.Sunderland T, Tariot PN, Cohen RM, Weingartner H, Mueller EA, 3rd, Murphy DL. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose-response study. Arch Gen Psychiatry. 1987;44:418–426. doi: 10.1001/archpsyc.1987.01800170032006. [DOI] [PubMed] [Google Scholar]
- 42.Nierenberg AA, DeCecco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: A focus on treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):5–9. [PubMed] [Google Scholar]
- 43.Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: A randomized, placebo-controlled clinical trial. Biol Psychiatry. 2009;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35:2479–2488. doi: 10.1038/npp.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quitkin FM, Stewart JW, McGrath PJ. Gender differences in treatment response. Am J Psychiatry. 2001;158:1531–1533. doi: 10.1176/appi.ajp.158.9.1531-a. [DOI] [PubMed] [Google Scholar]
- 46.Berlanga C, Flores-Ramos M. Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J Affect Disord. 2006;95:119–123. doi: 10.1016/j.jad.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 47.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 48.Martenyi F, Dossenbach M, Mraz K, Metcalfe S. Gender differences in the efficacy of fluoxetine and maprotiline in depressed patients: A double-blind trial of antidepressants with serotonergic or norepinephrinergic reuptake inhibition profile. Eur Neuropsychopharmacol. 2001;11:227–232. doi: 10.1016/s0924-977x(01)00089-x. [DOI] [PubMed] [Google Scholar]
- 49.Khan A, Brodhead AE, Kolts RL, Brown WA. Severity of depressive symptoms and response to antidepressants and placebo in antidepressant trials. J Psychiatr Res. 2005;39:145–150. doi: 10.1016/j.jpsychires.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry. 2001;178:234–241. doi: 10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- 51.Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: A review of current concepts and methods. Can J Psychiatry. 2007;52:46–54. doi: 10.1177/070674370705200108. [DOI] [PubMed] [Google Scholar]
- 52.Deisenhammer EA, Whitworth AB, Geretsegger C, Kurzthaler I, Gritsch S, Miller CH, et al. Intravenous versus oral administration of amitriptyline in patients with major depression. J Clin Psychopharmacol. 2000;20:417–422. doi: 10.1097/00004714-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Pollock BG, Perel JM, Nathan RS, Kupfer DJ. Acute antidepressant effect following pulse loading with intravenous and oral clomipramine. Arch Gen Psychiatry. 1989;46:29–35. doi: 10.1001/archpsyc.1989.01810010031005. [DOI] [PubMed] [Google Scholar]
- 54.Brunton LL. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th. New York: McGraw-Hill; 2006. [Google Scholar]
- 55.Cusack B, Nelson A, Richelson E. Binding of antidepressants to human brain receptors: Focus on newer generation compounds. Psychopharmacology (Berl) 1994;114:559–565. doi: 10.1007/BF02244985. [DOI] [PubMed] [Google Scholar]
- 56.Richelson E. Are receptor studies useful for clinical practice? J Clin Psychiatry. 1983;44:4–9. [PubMed] [Google Scholar]
- 57.Preskorn SH. Clinical Pharmacology of Selective Serotonin Reuptake Inhibitors. Caddo, OK: Professional Communications Inc.; 1996. [Google Scholar]
- 58.Anderson IM. SSRIS versus tricyclic antidepressants in depressed inpatients: a meta-analysis of efficacy and tolerability. Depress Anxiety. 1998;7(suppl 1):11–17. [PubMed] [Google Scholar]
- 59.Gillin JC, Lauriello J, Kelsoe JR, Rapaport M, Golshan S, Kenny WM, Sutton L. No antidepressant effect of biperiden compared with placebo in depression: A double-blind 6-week clinical trial. Psychiatry Res. 1995;58:99–105. doi: 10.1016/0165-1781(95)02700-7. [DOI] [PubMed] [Google Scholar]
- 60.Richelson E. Cholinergic transduction. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. [Google Scholar]
- 61.Tizabi Y, Getachew B, Rezvani AH, Hauser SR, Overstreet DH. Antidepressant-like effects of nicotine and reduced nicotinic receptor binding in the Fawn-Hooded rat, an animal model of co-morbid depression and alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:398–402. doi: 10.1016/j.pnpbp.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang DW, Zhou RB, Yao YM. Role of cholinergic anti-inflammatory pathway in regulating host response and its interventional strategy for inflammatory diseases. Chin J Traumatol. 2009;12:355–364. [PubMed] [Google Scholar]
- 63.Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders Sensitive Line rat: A selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Liu HF, Zhou WH, Xie XH, Cao JL, Gu J, Yang GD. Muscarinic receptors modulate the mRNA expression of NMDA receptors in brainstem and the release of glutamate in periaqueductal grey during morphine withdrawal in rats. Sheng Li Xue Bao. 2004;56:95–100. [PubMed] [Google Scholar]
- 65.Janowsky DS, Overstreet DH, Nurnberger JI., Jr Is cholinergic sensitivity a genetic marker for the affective disorders? Am J Med Genet. 1994;54:335–344. doi: 10.1002/ajmg.1320540412. [DOI] [PubMed] [Google Scholar]
- 66.Rami A, Ausmeir F, Winckler J, Krieglstein J. Differential effects of scopolamine on neuronal survival in ischemia and glutamate neurotoxicity: Relationships to the excessive vulnerability of the dorsoseptal hippocampus. J Chem Neuroanat. 1997;13:201–208. doi: 10.1016/s0891-0618(97)00044-6. [DOI] [PubMed] [Google Scholar]
- 67.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(suppl 1):S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 68.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li N, Liu RJ, Aghajanian G, Duman RS. Program # 903.15/KK17 Neuroscience Meeting Planner 2011. Washington, DC: Society for Neurosciences; 2011. Rapid antidepressant actions of scopolamine require mtor sig-naling and synaptogenesis. [Google Scholar]
- 70.Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rawls SM, McGinty JF. Muscarinic receptors regulate extracellular glutamate levels in the rat striatum: An in vivo microdialysis study. J Pharmacol Exp Ther. 1998;286:91–98. [PubMed] [Google Scholar]
- 73.Putcha L, Tietze KJ, Bourne DW, Parise CM, Hunter RP, Cintron NM. Bioavailability of intranasal scopolamine in normal subjects. J Pharm Sci. 1996;85:899–902. doi: 10.1021/js950327b. [DOI] [PubMed] [Google Scholar]
- 74.Voleti B, Navarria AM, Duman RS. Program# 69.28/Y1 Neuroscience Meeting Planner 2012. New Orleans, LA: Society for Neurosciences; 2012. Identification of muscarinic receptor subtype(s) that mediate the rapid antidepressant effects of scopolamine. [Google Scholar]
- 75.Nachum Z, Shahal B, Shupak A, Spitzer O, Gonen A, Beiran I, et al. Scopolamine bioavailability in combined oral and transdermal delivery. J Pharmacol Exp Ther. 2001;296:121–123. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


