Abstract
Objectives:
Ischemia-reperfusion(IRI) is a common complication of lung transplantation(LTx). Hydrogen sulfide(H2S) is a novel agent previously shown to slow metabolism and scavenge reactive oxygen species, potentially mitigating IRI. We hypothesized that pre-treatment with inhaled H2S would improve graft function in an ex vivo model of LTx.
Methods:
Rabbits(n=10) were ventilated for 2 hours prior to heart-lung bloc procurement. The treatment group(n=5) inhaled room air(21% O2) supplemented with 150 ppm H2S while the control group(n=5) inhaled room air alone. Both groups were gradually cooled to 34 C. All heart-lung blocs were then recovered and cold-stored in low potassium dextran solution for 18 hours. Following storage, the blocs were reperfused with donor rabbit blood in an ex vivo apparatus. Serial clinical parameters were assessed and serial tissue biochemistry was examined.
Results:
Prior to heart-lung bloc procurement, rabbits pre-treated with H2S exhibited similar oxygenation(p=0.1), ventilation(p=0.7), and heart rate(p=0.5); however, treated rabbits exhibited consistently higher mean arterial blood pressures(p=0.01). During reperfusion, lungs pre-treated with H2S had better oxygenation(p<0.01) and ventilation(p=0.02) as well as lower pulmonary artery pressures(p<0.01). Reactive oxygen species levels were lower in treated lungs during reperfusion(p=0.01). Additionally, prior to reperfusion, treated lungs demonstrated more preserved mitochondrial cytochrome c oxidase activity(p=0.01).
Conclusions:
To our knowledge, this study represents the first reported therapeutic use of inhaled H2S in an experimental model of LTx. After prolonged ischemia, lungs pre-treated with inhaled H2S exhibited improved graft function during reperfusion. Donor pre-treatment with inhaled H2S represents a potentially novel adjunct to conventional preservation techniques and merits further exploration.
Keywords: Transplantation, Lung; Ischemia/reperfusion injury, Lung; Animal model; Hydrogen sulfide
INTRODUCTION
Although lung transplantation(LTx) is an effective and life-saving treatment for end-stage lung disease, 15-25% of LTx recipients experience severe post-transplant respiratory failure known as primary graft dysfunction(PGD).(1-5) PGD is associated with impaired short-term graft function, an 8-fold increase in 30-day mortality, and an increased risk of bronchiolitis obliterans syndrome.(3) A proposed cause of PGD is ischemia-reperfusion injury(IRI).(1,3,4) Despite many experimental and clinical therapies aimed at mitigating IRI, PGD is a persistent problem.
Exogenously administered hydrogen sulfide(H2S) gas has recently been shown to reduce metabolism and induce a state of suspended animation in several animal models.(6-11) This state is characterized by significantly reduced oxygen requirements, allowing animals to survive severe hypoxia without apparent detriment.(8-10,12) Therefore, H2S-induced suspended animation may represent a novel means of augmenting conventional organ preservation techniques. Theoretically, inducing a state of reduced metabolism prior to allograft procurement could decrease oxygen requirements during the ischemic phase of transplantation, thus mitigating IRI. Therefore, we undertook this study to investigate whether pre-treatment of donor lungs with H2S would improve graft function in an experimental model of LTx.
METHODS
Model
These experiments were conducted using an ex vivo model of lung reperfusion whereby rabbit lungs were pre-treated with inhaled H2S, subjected to cold ischemia, and then externally ventilated and perfused with donor rabbit blood. Our protocol has been described in detail previously.(1) The experiment utilized 4 kg, male New Zealand White rabbits(Myrtle’s Rabbitry Inc., Thompson Station, TN). This study was approved by the Animal Care and Use Committee at the Johns Hopkins University.
A total of 10 rabbits were randomly divided into 2 treatment groups and underwent en bloc heart-lung harvest followed by 18 hours of cold storage. These blocs were subsequently reperfused with donor rabbit blood for 120 minutes while physiologic data were recorded and tissue biopsies were taken.
Pre-Treatment and Heart-Lung Bloc Harvest
All rabbits were anesthetized with an intramuscular injection of ketamine(35mg/kg) and xylazine(6.5mg/kg). Additional sedation was given with acepromazine(5mg/kg) as needed. A rectal probe was placed for continuous temperature monitoring. A tracheotomy was performed to facilitate endotracheal intubation and mechanical ventilation was initiated(Harvard ventilator apparatus, model 665; Harvard Apparatus Co., Holliston, MA). The initial experimental ventilator settings were: Volume Control mode; rate, 20 breaths/minutes; tidal volume, 10mL/kg; Fractional inspired concentration of oxygen(FiO2), 100%. The left carotid artery was dissected, ligated distally, and cannulated with a 22Fr intravenous catheter for blood gas and hemodynamic monitoring. The chest was then entered through a median sternotomy.
After obtaining baseline arterial blood gas and lung tissue samples, pre-treatment commenced. In the experimental group(n=5), the rabbits were pre-treated with 150ppm H2S mixed with room air(21% oxygen, 78% nitrogen; Praxair Inc., Danbury, CT). In the control group(n=5), the rabbits were pre-treated with room air alone(21% oxygen, 78% nitrogen). In both groups, pre-treatment continued for 2 hours. During pre-treatment, both groups were gradually externally cooled to 34°C. Throughout pre-treatment, serial arterial blood gases were taken and physiologic measures were recorded.
Intravenous heparin(1000U/kg) was given and 30ug of prostaglandin E1 was injected directly into the PA. The PA was cannulated through a right ventriculotomy and the left atrium was cannulated directly. The lungs were flushed with 250mL of cold(4°C) low-potassium dextran(Perfadex; Vitrolife, Englewood, CO) via gravity drainage through the PA cannula. The superior and inferior vena cavae and the aorta were ligated. Topical cold ice slush was placed around the heart-lung bloc which was then excised from the chest. A lung sample was taken and the lungs were inflated and preserved in low-potassium dextran at 4°C for 18 hours.
Perfusion Pump System
Two donor rabbits were heparinized(1000U/kg) and exsanguinated through a right ventriculotomy to obtain 300mL of whole blood for each reperfusion. Following cold storage, the heart-lung bloc was suspended by the trachea and ventilated at 10mL/kg, 20 breaths/minute, with an FiO2 of 100%. All animals were reperfused for 120 minutes with the donor rabbit blood using a Sarns 5000 roller head pump(Sarns Inc., Ann Arbor, MI). Blood removed via the left atrial cannula was collected in a reservoir and deoxygenated to achieve a PO2 and PCO2 of 60 mmHg(to simulate venous blood) before being returned to the heart-lung bloc through the PA cannula.
Hemodynamic Measurements
Physiologic measurements were recorded at baseline and then every 15 minutes during pre-treatment and during reperfusion. Every 15 minutes, arterial blood gas samples were analyzed during pre-treatment, while PA and left atrial blood samples were analyzed with the same frequency during reperfusion.
Lung Samples
Lung samples were taken prior to pre-treatment, after 2 hours of pre-treatment, after cold storage but prior to reperfusion, after 1 hour of reperfusion, and after 2 hours of reperfusion. Samples were removed from similar regions of the lungs regardless of treatment for consistency. All lung samples were removed sharply after the application of hemoclips for hemostasis. Samples were flash frozen in liquid nitrogen and stored at −80°C for biochemical analysis.
Wet to Dry Ratios
Wet to dry ratios were obtained to determine levels of pulmonary edema. Left lower lobe samples were removed and weighed after 120 minutes of reperfusion. These samples were dried at 80°C for 72 hours and subsequently reweighed for ratio determination.
Reactive Oxygen Species (ROS) Levels
ROS levels were examined in lung samples using a green fluorescence assay(OxiSelect In Vitro ROS Assay Kit, Cell Biolabs, San Diego, CA). Lung samples were homogenized on ice in a phosphate-buffered solution, centrifuged, and re-suspended in assay buffer. Cell permeable 2’,7’-dichlorodihydrofluorescin diacetate fluorogenic probe was used to assay ROS levels. Fluorescence was read on a Spectramax M5 plate reader.
Cytochrome C Oxidase (COX) Activity
Mitochondria were isolated with a mitochondrial isolation kit(Mitoiso1; Sigma Aldrich, St. Louis, MO). Fresh lung tissue was washed with an extraction buffer, homogenized, centrifuged, and re-suspended in storage buffer. The isolated mitochondria were assayed for COX activity using a commercially available kit(Cytocox1; Sigma Aldrich, St. Louis, MO). The mitochondrial isolate was placed in a 10mM Tris-HCl buffer. 50uL of Ferrocytochrome c substrate solution was added and activity was read immediately with a spectrophotometer.
Cyclic Guanosine Monophosphate (cGMP) Levels
cGMP concentrations were determined by a commercially available enzyme immunoassay(Amersham cGMP Enzymeimmunoassay, GE Healthcare Life Sciences, Piscataway, NJ). Tissue samples were weighed and homogenized in 500uL of 6% trichloroacetic acid. Samples were centrifuged and supernatants recovered and washed five times in 2mL of water saturated ether. The aqueous layer was recovered and dried to recover a pellet which was re-suspended in assay buffer. The acetylation assay was performed according to the vendor’s specifications.
Statistical Analysis
Physiologic reperfusion data was evaluated by two independent statistical tests to assess the longitudinal differences between the treatment groups. First, repeated-measures analysis of variance(RM-ANOVA) was performed to evaluate the effects of H2S over time. Post hoc comparisons at specific time points were evaluated with the Tukey-Honest significant difference tests. Multilevel random effects modeling was also performed to account for interactions both between and within each animal. The model used(generalized estimating equation, GEE) estimated the variance in the physiological parameters between animals and across time.
Results of biochemical activity assays were compared using the Student’s t-test(cGMP), the RM-ANOVA test(ROS, COX), and the GEE(ROS, COX). Data are presented as means ± standard deviations. P-values<0.05 were considered statistically significant. All graphs are presented as mean values with error bars defining standard error. Statistical analysis was performed using Stata 12.0(Stata Corporation LP, College Station, TX).
RESULTS
Pre-Treatment Physiology
During pre-treatment, both experimental and control lungs exhibited similar levels of oxygenation(GEE, p=0.1) and ventilation(GEE, p=0.7). More specifically, lungs pretreated with H2S had similar baseline oxygenation with an FiO2 of 100%(460±48 vs. 416±70mmHg, p=0.2) and similar oxygenation after pre-treatment with an FiO2 of 21% just prior to procurement(88±50 vs. 87±3 mmHg, p=0.9; Table 1). The pattern of cooling was also similar between the two groups(GEE: p=0.9). Although the groups had similar heart rates during pre-treatment(GEE: p=0.5), rabbits pre-treated with H2S exhibited higher mean arterial blood pressures during cooling(GEE: p=0.01).
Table 1.
Physiologic Parameters during Pre-Treatment Stratified by Pre-Treatment Strategy
| Variable | Control (n=5) |
Hydrogen Sulfide (n=5) |
P-value |
|---|---|---|---|
| At Baseline | |||
| Temperature (°C) | 38.9 ± 0.5 | 39.0 ± 0.3 | 0.7 |
| Initial PaO2 (FiO2 100%, mmHg) | 460 ± 48 | 416 ± 70 | 0.2 |
| PaCO2 (mmHg) | 46 ± 18 | 41 ± 7 | 0.5 |
| Heart rate (beats/min) | 184 ± 52 | 179 ± 52 | 0.9 |
| Mean arterial pressure (mmHg) | 52 ± 8 | 61 ± 12 | 0.3 |
| At the Conclusion of Pre-Treatment | |||
| Temperature (°C) | 33.2 ± 0.9 | 33.9 ± 1.5 | 0.5 |
| Heart rate (beats/min) | 148 ± 21 | 168 ± 20 | 0.3 |
| Mean arterial pressure (mmHg) | 45 ± 4 | 40 ± 7 | 0.3 |
| PaO2 (FiO2 21%, mmHg) | 88 ± 50 | 87 ± 3 | 0.9 |
| PaCO2 (mmHg) | 32 ± 14 | 40 ± 12 | 0.4 |
Reperfusion Physiology
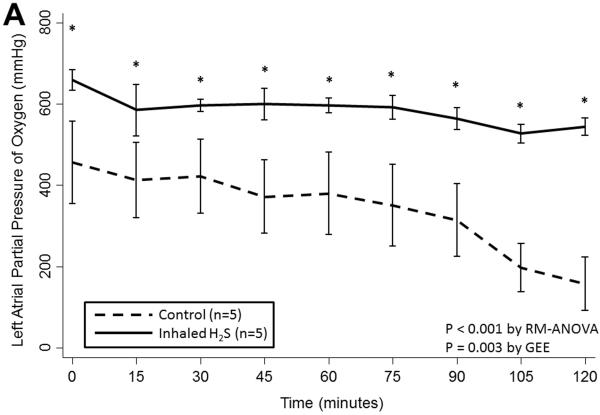
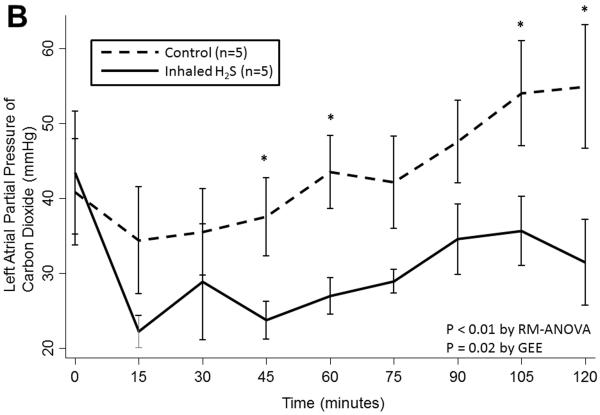
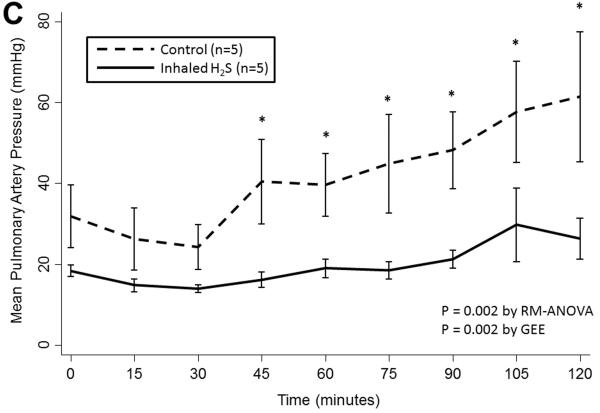
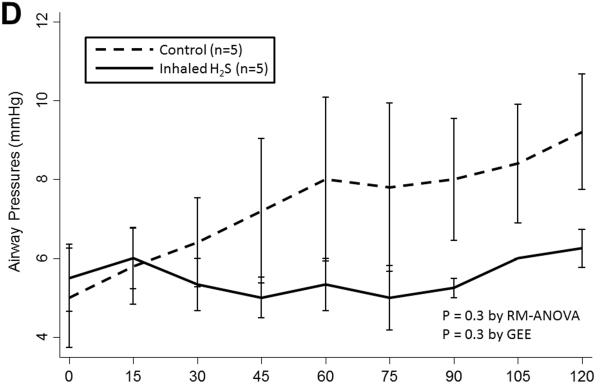
Prolonged cold storage produced a significant deleterious effect on lung performance in the control group; however, lungs pre-treated with inhaled H2S were relatively spared (Table 2). Lungs pre-treated with inhaled H2S exhibited better oxygenation(RM-ANOVA: p< 0.001; GEE: p=0.003; Figure 1A) and ventilation(RM-ANOVA: p=0.005; GEE: p=0.02; Figure 1B) than controls. The superior oxygenation of lungs pre-treated with H2S was apparent both at the beginning of reperfusion(659±64 vs. 456±226mmHg, p=0.06) and at the conclusion of reperfusion(544±37 vs. 158±146mmHg, p=0.005). Additionally, lungs pre-treated with H2S exhibited lower pulmonary artery pressures(RM-ANOVA: p=0.002; GEE: p=0.002; Figure 1C) but similar airway pressures(RM-ANOVA: p=0.3; GEE: p=0.3; Figure 1D). Although treated lungs had lower wet:dry ratios, this difference did not reach statistical significance(7.1±1.9 vs. 8.7±2.0, p=0.3).
Table 2.
Comparison of Physiologic Parameters during Reperfusion Stratified by Pre-Treatment
| Variable | 0 Minutes | 30 Minutes | 60 Minutes | 90 Minutes | 120 Minutes |
|---|---|---|---|---|---|
| PaO2 (mmHg) | |||||
| Control (n=5) | 456 ± 226 | 422 ± 205 | 379 ± 226 | 315 ± 200 | 158 ± 146 |
| Treatment (n=5) | 659 ± 64‡ | 596 ± 38‡ | 596 ± 46‡ | 564 ± 54‡ | 544 ± 37‡ |
| PaCO2 (mmHg) | |||||
| Control (n=5) | 41 ± 16 | 36 ± 13 | 44 ± 11 | 48 ± 12 | 55 ± 19 |
| Treatment (n=5) | 43 ± 21 | 29 ± 19 | 27 ± 6 ‡ | 35 ± 9 ‡ | 31 ± 11 ‡ |
| Mean PA Pressure (mmHg) | |||||
| Control (n=5) | 32 ± 18 | 24.2 ± 12 | 40 ± 17 | 48 ± 21 | 61 ± 36 |
| Treatment (n=5) | 18 ± 1 | 14 ± 2 | 19 ± 6‡ | 21 ± 5‡ | 26 ± 10‡ |
*P<0.001 by RM-ANOVA;
*P<0.005 by RM-ANOVA;
*P<0.002 by RM-ANOVA;
P<0.05 for comparison between control and experimental groups
Abbreviations: RM-ANOVA, repeated-measures analysis of variance.
Figure 1.
(A) Partial pressure of oxygen and (B) carbon dioxide and (C) mean pulmonary artery and (D) airway pressures over time during reperfusion. Lungs pre-treated with room air are displayed with a dashed line. Lungs pre-treated with hydrogen sulfide are displayed with a solid line. Asterisks indicate a post hoc statistical difference at given time points as determined by the Tukey-Honest significance difference test. Abbreviations: n, number; RM-ANOVA, repeated measures analysis of variance; GEE, generalized estimating equation; H2S, hydrogen sulfide.
Biochemistry
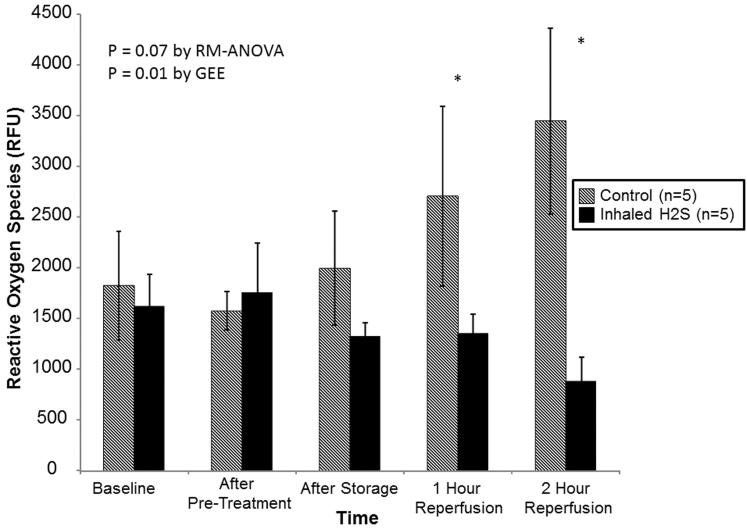
Although ROS levels were similar at all points prior to reperfusion, lungs pre-treated with room air alone exhibited progressively higher levels of ROS during reperfusion(RM-ANOVA: p=0.07; GEE: p=0.01; Figure 2). By the end of reperfusion, ROS levels were significantly lower in lungs pre-treated with H2S(3449±1832 vs. 885±574 relative fluorescence units, p<0.05).
Figure 2.
Reactive oxygen species levels stratified by treatment. Lungs pre-treated with room air are displayed with shaded bars. Lungs pre-treated with hydrogen sulfide are displayed with solid bars. Asterisks indicate a post hoc statistical difference at given time points as determined by the Tukey-Honest significance difference test. Abbreviations: n, number; RM-ANOVA, repeated measures analysis of variance; GEE, generalized estimating equation; RFU, relative fluorescence units; H2S, hydrogen sulfide.
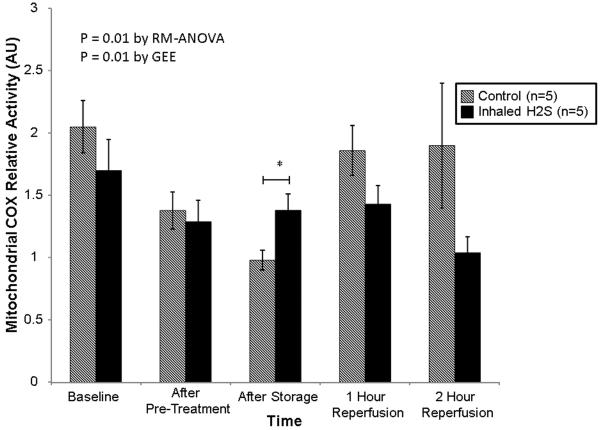
The pattern of mitochondrial COX activity was different between the groups(RM-ANOVA: p=0.01; GEE: p=0.01; Figure 3). Specifically, the relative activity of COX was higher in the group pre-treated with H2S after cold storage(1.38±0.28 vs. 0.98±0.18 arbitrary units, p<0.05). The activity was not different at other time points.
Figure 3.
Relative mitochondrial cytochrome c oxidase activity stratified by treatment. Lungs pre-treated with room air are displayed with shaded bars. Lungs pre-treated with hydrogen sulfide are displayed with solid bars. Asterisks indicate a post hoc statistical difference at given time points as determined by the Tukey-Honest significance difference test. Abbreviations: n, number; COX, cytochrome c oxidase; RM-ANOVA, repeated measures analysis of variance; AU, arbitrary units; H2S, hydrogen sulfide.
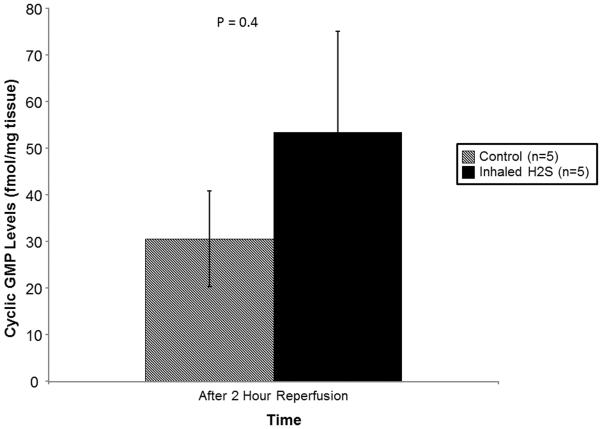
Although there were increased cGMP levels in lungs pre-treated with H2S, this difference did not reach statistical significance(53.44±53.26 vs. 30.60±22.92 fmol/mg tissue, p=0.4; Figure 4).
Figure 4.
Cyclic GMP levels at the conclusion of reperfusion stratified by treatment. Lungs pre-treated with room air are displayed with shaded bars. Lungs pre-treated with hydrogen sulfide are displayed with solid bars. P-value determined by Student’s t-test. Abbreviations: n, number; GMP, guanosine monophosphate; H2S, hydrogen sulfide.
DISCUSSION
In our experimental model of LTx, pre-treating donor rabbits with inhaled H2S resulted in improved oxygenation and ventilation as well as decreased PA pressures during reperfusion. Despite having similar levels of ROS during pre-treatment and after ischemic storage, lungs pre-treated with H2S demonstrated lower levels of ROS during reperfusion. Finally, pre-treated lungs exhibited higher levels of mitochondrial COX activity after ischemic storage.
In LTx, lung allografts are necessarily subjected to a period of ischemia in order to facilitate explantation, transport, and implantation, thus exposing lung allografts to IRI.(1,3,4) The ischemic period is characterized by oxidative stress which precipitates an inflammatory response, leading to the production of ROS by endothelial cells, macrophages, and other immune cells.(2-5) At the cellular level, the combination of ROS and inflammation results in mitochondrial injury, cell wall damage, and the induction of apoptosis.(2) This injury produces diffuse alveolar and vascular damage, causing increased microvascular permeability and pulmonary edema, leading to increased pulmonary vascular pressures and limited gas exchange.(2-4) The ischemic injury is then exacerbated by reperfusion. Cells primed for the low oxygen environment of cold storage are inundated with oxygen resulting in the generation of more ROS that overwhelm the lung’s anti-oxidant defenses and trigger an inflammatory response which further damages the pulmonary alveoli and vasculature.(2,4) Clinically, this combined IRI can result in severe pulmonary hypertension and progressive hypoxia.(2-4)
Hypothermic flush and storage of organs has been shown to lower the metabolic rate of allografts, decreasing their oxygen requirement, and thus ameliorating the oxygen supply and demand imbalance of ischemia.(13) Although hypothermia is highly effective at slowing metabolism, the prevalence and severity of IRI after LTx demonstrates a remaining need to augment the protective effects of cold storage.(3)
Several studies have demonstrated that exogenous administration of H2S can dramatically lower metabolism, inducing a state the authors refer to as suspended animation.(8-11) This state is characterized by a decreased metabolic rate, lower oxygen consumption, and reduced carbon dioxide production.(9) Moreover, when placed in lower metabolic state, animals are able to survive severely hypoxic conditions without obvious detriment.(9) Since IRI is initiated by an ischemic phase in which oxygen demand exceeds supply, we pre-treated our rabbit lung donors with H2S in an effort to induce a state of suspended animation and thus lower oxygen demand and avoid ischemic injury.
Whether our experiment achieved animation state of reduced metabolism is unclear. During pre-treatment, oxygen consumption and carbon dioxide production were not measured. Moreover, core body temperature and heart rate were similar between the two groups. Thus, there is no empiric evidence that a state of suspended animation, as described by previous researchers, was achieved.(7)
However, pre-treatment with H2S prevented ischemic injury and improved graft function during reperfusion. Although the numerous cytoprotective and physiologic mechanisms of H2S are still being explored, an examination of our biochemical results suggests some possible protective mechanisms.(6) First, prior to reperfusion, ROS levels were similar between the two groups; however, during reperfusion, ROS levels increased significantly and progressively in the control group while they remained stable in the treatment group. These findings suggest that ischemic injury may have been mitigated by H2S, decreasing any subsequent inflammatory response and consequent damage during reperfusion. That ischemic injury may have been partially averted is also supported by the improved oxygenation and lower pulmonary artery pressures in the pre-treated group at the beginning of reperfusion. Whether this reflects the directly cytoprotective and anti-apoptotic properties of H2S or resulted from a reduction in the oxygen requirement of the lungs during cold storage is unclear. Future studies will focus on identifying which cell types are injured in this model and how H2S ameliorates this injury.
Second, a number of studies have demonstrated H2S to be an effective scavenger of free radicals.(6,14-16) While this may have been a contributing factor to our improved outcomes, this mechanism alone does not fully explain our observations. There is no difference in ROS levels during H2S treatment; rather, ROS levels do not diverge until reperfusion, when there was no further H2S therapy. Although it is possible that during reperfusion, some small amount of H2S was present in the lungs from the pre-treatment phase, we feel it is unlikely that this small quantity could fully account for the observed differences.
Third, several studies have demonstrated that in addition to functioning as a free radical scavenger, H2S also results in the up-regulation and potentiation of other antioxidant compounds including glutathione, N-acetylcysteine, catalase, and superoxide dismutase.(6,17-19) Although we did not measure tissue levels of these compounds, it is possible that the 2 hours of H2S pre-treatment increased lung anti-oxidant levels. Then when reperfusion triggered further release of ROS, the pre-treated lungs had an ample supply of anti-oxidants to scavenge these free radicals and thus prevent damage. This would also explain the rising level of ROS observed in the control lungs as the anti-oxidants were consumed, and the stable levels in the treated lungs which had significant anti-oxidant reserves.
Fourth, previous research suggests that H2S slows metabolism by directly inhibiting mitochondrial COX activity, preventing the transfer of electrons from complex II to COX(complex III) in the electron transport chain, and thus inhibiting oxidative phosphorylation.(14,20) Moreover, H2S has also been shown to decrease COX levels by down-regulating the production of its constituent subunits.(21) Decreased COX activity resulting in reduced metabolism and oxygen consumption could help explain the differences in ROS levels in our experiment; however, we observed increased COX activity in pre-treated lungs just prior to reperfusion. This could be partly explained by the complex relationship between H2S and COX. Although at high concentrations, H2S inhibits COX, at lower concentrations it acts as an electron donor for the electron transport chain, thus increasing oxidative phosphorylation.(20,22) Perhaps in the low oxygen environment of ischemia, H2S acted as an electron donor, maintaining COX activity levels, increasing ATP production, and thus preventing cellular injury. Further complicating this relationship is the fact that COX can be released from mitochondria during apoptosis.(23,24) Thus the badly damaged control lungs in our experiment may have been experiencing significant apoptosis prior to reperfusion and thus have extruded significant quantities of COX from mitochondria. Finally, given the relatively small size of our experiment and the isolated time point of significance, we cannot exclude the possibility that this finding is spurious.
Although the impact of H2S on cGMP levels is unknown, previous research suggests that higher cGMP levels are associated with improved lung function.(1) Thus, we measured cGMP levels as a surrogate for a favorable biochemical profile during reperfusion. In our experiment, there were higher levels of cGMP in treated lungs after reperfusion but this difference did not reach statistical significant. Further investigation is warranted.
Previous Literature
Although studies have shown H2S to exert a cytoprotective effect in models of IRI, most of these models have focused on post-injury treatment.(6) Organ transplantation presents a unique opportunity to anticipate the ischemic insult and administer therapies to mitigate or prevent ischemic injury. Along these lines, Fu et al. pre-treated rat lungs with intravenous H2S prior to inducing IRI.(19) They found H2S to be associated with improved histology, decreased edema, and preserved compliance. Similar beneficial effects of pre-treatment with H2S have been demonstrated in models of myocardial infarction, stroke, and heart, liver, and kidney transplantation.(16,25-28) Post-injury treatment with H2S has also been associated with beneficial results in lung models of ventilator-induced and smoke-induced lung injury.(29,30) Despite the potential advantages of H2S in LTx, caution is warranted. Very high levels of H2S can be directly toxic to the lungs.(7,31)
Limitations
In this study, we utilized an ex vivo model of LTx. We did not induce brain death in the donors nor did we utilize immunosuppression. Therefore, we did not completely replicate the in vivo LTx procedure. Moreover, since we only conducted 2 hours of reperfusion, we cannot evaluate long-term effects. Furthermore, although pre-treatment with H2S resulted in improved graft function and decreased ROS, the underlying mechanism of this improved function remains unclear. Future experiments will focus on identifying which cell types suffered the most severe injury, which cells are responsible for the increased ROS production, and the way in which H2S ameliorates this injury. Finally, although we planned to induce suspended animation, whether we achieved this state is uncertain.
CONCLUSION
To our knowledge, this study represents the first reported therapeutic use of inhaled H2S in an experimental model of LTx. Pre-treatment of donor rabbits with inhaled H2S is associated with significantly improved graft function during reperfusion. Donor pre-treatment with hydrogen sulfide appears to mitigate IRI and may represent a novel adjunct to conventional preservative techniques.
ACKNOWLEDGEMENTS
The authors would like to thank Mr. Jeffrey Brawn and Mrs. Melissa Jones for their technical assistance.
FUNDING
This research was supported by grant 90038390 from the Thoracic Surgery Foundation for Research and Education and by grant T32 2T32DK007713-12 from the National Institutes of Health. Dr. George is the Hugh R. Sharp Cardiac Surgery Research Fellow. Drs. Arnaoutakis and Beaty are the Irene Piccinini Investigators in Cardiac Surgery.
ABBREVIATIONS
- cGMP
Cyclic guanosine monophosphate
- COX
Cytochrome c oxidase
- FiO2
Fractional inspired concentration of oxygen
- GEE
Generalized estimating equation
- H2S
Hydrogen sulfide
- IRI
Ischemia-reperfusion injury
- LTx
Lung transplantation
- PA
Pulmonary artery
- PGD
Primary graft dysfunction
- RM-ANOVA
Repeated-measures analysis of variance
- ROS
Reactive oxygen species
Footnotes
Presentation: The contents of this manuscript were presented at the 48th Annual Meeting of the STS.
Conflicts: No conflicts of interest.
REFERENCES
- 1.Weiss ES, Champion HC, Williams JA, Baumgartner WA, Shah AS. Long-xacting oral phosphodiesterase inhibition preconditions against reperfusion injury in an experimental lung transplantation model. J Thorac Cardiovasc Surg. 2009;137(5):1249–57. doi: 10.1016/j.jtcvs.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 2.den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010;299(5):H1283–99. doi: 10.1152/ajpheart.00251.2010. [DOI] [PubMed] [Google Scholar]
- 3.Lee JC, Christie JD. Primary graft dysfunction. Clin Chest Med. 2011;32(2):279–93. doi: 10.1016/j.ccm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 4.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167(4):490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 5.Ng CS, Wan S, Arifi AA, Yim AP. Inflammatory response to pulmonary ischemia-reperfusion injury. Surg Today. 2006;36(3):205–14. doi: 10.1007/s00595-005-3124-2. [DOI] [PubMed] [Google Scholar]
- 6.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6(11):917–35. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 7.Aslami H, Schultz MJ, Juffermans NP. Potential applications of hydrogen sulfide-induced suspended animation. Curr Med Chem. 2009;16(10):1295–303. doi: 10.2174/092986709787846631. [DOI] [PubMed] [Google Scholar]
- 8.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308(5721):518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 9.Blackstone E, Roth MB. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;27(4):370–2. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- 10.Volpato GP, Searles R, Yu B, Scherrer-Crosbie M, Bloch KD, Ichinose F, et al. Inhaled hydrogen sulfide: a rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology. 2008;108(4):659–68. doi: 10.1097/ALN.0b013e318167af0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth MB, Nystul T. Buying time in suspended animation. Sci Am. 2005;292(6):48–55. doi: 10.1038/scientificamerican0605-48. [DOI] [PubMed] [Google Scholar]
- 12.Morrison ML, Blackwood JE, Lockett SL, Iwata A, Winn RK, Roth MB. Surviving blood loss using hydrogen sulfide. J Trauma. 2008;65(1):183–8. doi: 10.1097/TA.0b013e3181507579. [DOI] [PubMed] [Google Scholar]
- 13.Pegg DE. Organ preservation. Surg Clin North Am. 1986;66(3):617–32. doi: 10.1016/s0039-6109(16)43944-7. [DOI] [PubMed] [Google Scholar]
- 14.Pun PB, Lu J, Kan EM, Moochhala S. Gases in the mitochondria. Mitochondrion. 2009;10(2):83–93. doi: 10.1016/j.mito.2009.12.142. [DOI] [PubMed] [Google Scholar]
- 15.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, et al. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2008;33(5):906–13. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sivarajah A, Collino M, Yasin M, Benetti E, Gallicchio M, Mazzon E, et al. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. 2009;31(3):267–74. doi: 10.1097/SHK.0b013e318180ff89. [DOI] [PubMed] [Google Scholar]
- 17.Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010;12(1):1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 18.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. Faseb J. 2004;18(10):1165–7. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 19.Fu Z, Liu X, Geng B, Fang L, Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci. 2008;82(23-24):1196–202. doi: 10.1016/j.lfs.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Volkel S, Grieshaber MK. Mitochondrial sulfide oxidation in Arenicola marina. Evidence for alternative electron pathways. Eur J Biochem. 1996;235(1-2):231–7. doi: 10.1111/j.1432-1033.1996.00231.x. [DOI] [PubMed] [Google Scholar]
- 21.Leschelle X, Goubern M, Andriamihaja M, Blottiere HM, Couplan E, Gonzalez-Barroso MD, et al. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim Biophys Acta. 2005;1725(2):201–12. doi: 10.1016/j.bbagen.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Yamane M, Liu M, Kaneda H, Uhlig S, Waddell TK, Keshavjee S. Reperfusion-induced gene expression profiles in rat lung transplantation. Am J Transplant. 2005;5(9):2160–9. doi: 10.1111/j.1600-6143.2005.01017.x. [DOI] [PubMed] [Google Scholar]
- 23.Baskar R, Li L, Moore PK. Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. Faseb J. 2007;21(1):247–55. doi: 10.1096/fj.06-6255com. [DOI] [PubMed] [Google Scholar]
- 24.Adhikari S, Bhatia M. H2S-induced pancreatic acinar cell apoptosis is mediated via JNK and p38 MAP kinase. J Cell Mol Med. 2008;12(4):1374–83. doi: 10.1111/j.1582-4934.2008.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosgood SA, Nicholson ML. Hydrogen sulphide ameliorates ischaemia-reperfusion injury in an experimental model of non-heart-beating donor kidney transplantation. Br J Surg. 2009;97(2):202–9. doi: 10.1002/bjs.6856. [DOI] [PubMed] [Google Scholar]
- 26.Ren C, Du A, Li D, Sui J, Mayhan WG, Zhao H. Dynamic change of hydrogen sulfide during global cerebral ischemia-reperfusion and its effect in rats. Brain Res. 2010;1345:197–205. doi: 10.1016/j.brainres.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Kang K, Zhao M, Jiang H, Tan G, Pan S, Sun X. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transpl. 2009;15(10):1306–14. doi: 10.1002/lt.21810. [DOI] [PubMed] [Google Scholar]
- 28.Hu X, Li T, Bi S, Jin Z, Zhou G, Bai C, et al. Possible role of hydrogen sulfide on the preservation of donor rat hearts. Transplant Proc. 2007;39(10):3024–9. doi: 10.1016/j.transproceed.2007.05.086. [DOI] [PubMed] [Google Scholar]
- 29.Aslami H, Heinen A, Roelofs JJ, Zuurbier CJ, Schultz MJ, Juffermans NP. Suspended animation inducer hydrogen sulfide is protective in an in vivo model of ventilator-induced lung injury. Intensive Care Med. 2010;36(11):1946–52. doi: 10.1007/s00134-010-2022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esechie A, Enkhbaatar P, Traber DL, Jonkam C, Lange M, Hamahata A, et al. Beneficial effect of a hydrogen sulphide donor (sodium sulphide) in an ovine model of burn- and smoke-induced acute lung injury. Br J Pharmacol. 2009;158(6):1442–53. doi: 10.1111/j.1476-5381.2009.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klentz RD, Fedde MR. Hydrogen sulfide: effects on avian respiratory control and intrapulmonary CO2 receptors. Respir Physiol. 1978;32(3):355–67. doi: 10.1016/0034-5687(78)90123-8. [DOI] [PubMed] [Google Scholar]