Abstract
Introduction:
Although several studies have evaluated risk factors for mortality after lung transplantation (LTx), few studies have focused on the highest risk recipients. We undertook this study to evaluate the impact of high lung allocation scores (LAS), ventilator support, and extracorporeal membrane oxygenation (ECMO) support on outcomes after LTx.
Methods:
We retrospectively reviewed all LTx recipients in the United Network for Organ Sharing database. Primary stratification was by recipient acuity at the time of LTx. The three strata consisted of: (1) recipients in the highest LAS quartile (LAS≥48.4); (2) those requiring ventilator support; and (3) those requiring ECMO support. The primary outcome was 1-year mortality. Subgroup analysis focused on temporal trends.
Results:
From 05/2005-06/2011, 9,267 adult patients underwent LTx. Prior to LTx, 1,874 (20.2%) were in the highest LAS quartile, 526 (5.7%) required ventilator support, and 122 (1.3%) required ECMO support. On unadjusted analysis, ventilator and ECMO support were both associated with decreased 1-year survival compared to those in the highest LAS quartile (High LAS: 81.0% vs. Vent: 67.7% vs. ECMO: 57.6%, p<0.001 for each comparison). These differences persisted on adjusted analysis (Ventilator support, HR: 1.99, p<0.001; ECMO support, HR: 3.03, p<0.001). Increasing annual center volume was associated with decreased mortality. In patients bridged to LTx with ECMO support, 1-year survival improved over time (Coefficient: 8.03%/year, p=0.06).
Conclusions:
High acuity LTx recipients, particularly those bridged with ventilator or ECMO support, have increased short-term mortality after LTx. However, since the introduction of the LAS, high-risk patients have demonstrated improving outcomes, particularly at high volume centers.
Keywords: Transplantation, lung, Extracorporeal membrane oxygenation, ECMO
INTRODUCTION
The US Lung Allocation Score(LAS) gives recipients requiring mechanical support the highest priority for organ allocation.[1,2] Thus clinicians will be increasingly required to consider these high acuity recipients for LTx. Although several studies suggest patients can be successfully bridged to LTx with mechanical support, most of these single institutional studies suffer from relatively small sample size.[3,4] Additionally, no previous studies have evaluated the temporal trends in the outcomes of these highest acuity recipients. We undertook this study to understand the impact of recipient acuity on outcomes after LTx, with special emphasis on temporal trends in 1-year survival.
METHODS
Data Source
For this study, we utilized the UNOS database from the UNOS registry, an open cohort of all patients undergoing LTx in the United States. The Johns Hopkins Medicine institutional review board approved this study.
Study Design
We conducted a retrospective cohort study of all adults(≥18 years) who underwent LTx from 5/2005-6/2011. Patients undergoing combined heart-lung or multi-visceral organ transplantation were excluded.
Primary stratification was by recipient acuity at the time of LTx. The cohort was subdivided into three high risk groups: (1) recipients in the highest LAS quartile that did not require ventilatory or ECMO support; (2) recipients requiring ventilator support; and (3) patients requiring ECMO support. Temporal trends in survival were evaluated. Subgroup analysis focused on recipients bridged to LTx with ECMO.
Variables Examined and Outcomes Measured
We examined pertinent covariates in the data set, including: recipient demographics and co-morbidities; recipient hemodynamics, pulmonary function, measures of acuity, and need for support; donor demographics and co-morbidities; and transplant variables. Variables with >15% missing data were excluded from analysis. The primary end-points included 30-day, 1-year, and 2-year mortality. Temporal trends in 1-year mortality were also examined.
Statistical Analysis
Baseline characteristics of the entire cohort were stratified by acuity. Baseline characteristics of the ECMO cohort were then stratified by the year of LTx. Continuous variables were compared using analysis of variance(ANOVA) or the Wilcoxon rank-sum test and categorical variables using the chi-square or Fisher’s exact test. For associations found to be significant on preliminary testing, post hoc pair-wise comparisons were performed using the Tukey-Kramer method for continuous variables and by univariate logistic regression for categorical variables.
Survival was estimated by the Kaplan-Meier method and survival comparisons were performed using the log-rank test. Trends in 1-year survival over time were analyzed by linear regression and Cox proportional hazard regression modeling. Multivariable Cox proportional hazards regression models were constructed to estimate the risk of death with censoring for loss to follow up and death. To construct multivariable models, independent covariates were initially tested in univariate fashion. Variables with significant associations on exploratory analysis(p<0.20), those with biological plausibility, and those with previous literature support, were incorporated in a forward stepwise fashion into the multivariable model. The likelihood ratio test and Akaike’s information criterion were utilized in a nested model approach to identify the most parsimonious model with the greatest explanatory power. Patients missing data were excluded from the model using case-wise deletion.
To test for age and annual center volume cut-offs that would optimally predict 1-year survival in patients bridged to LTx with ECMO, receiver operative characteristic curves(ROC) were utilized. The outcome measure was 1-year mortality.
For all analyses, p<0.05(2-tailed) was considered statistically significant. Mean values are displayed with their standard deviations and median values are displayed with their interquartile ranges(IQR). Hazard ratios(HR) are presented with their 95% confidence intervals(CI). Statistical analysis was performed using Stata12.1SE(StataCorp LP, College Station, Texas).
RESULTS
Cohort Statistics
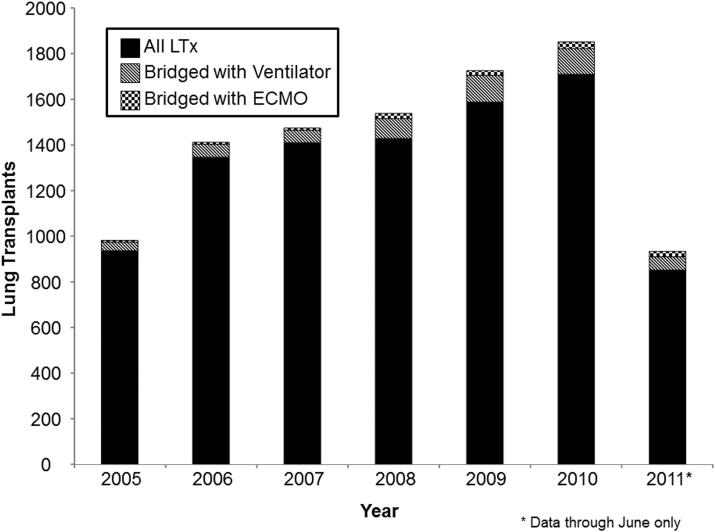
In the study period, 9,650 patients underwent LTx. After excluding pediatric patients(n=344) and multi-visceral organ transplants(n=39), our preliminary cohort comprised 9,267 patients including 442(4.6%) redo-LTx. The distribution of patients requiring ventilatory or ECMO support per year compared to the annual national volume is displayed in Figure 1. In any given year, the percent of patients bridged to LTx with ventilator support ranged from 3.8-7.5% while those bridged with ECMO support ranged from 0.6-2.8%.
Figure 1.
Bar graph depicting the number of annual lung transplants in the United States. Each bar represents the total lung transplants in a given year. Patients bridged to lung transplantation with ECMO support are depicted with checkered bars, patients bridged to lung transplantation with a ventilator are depicted with striped bars, and all other patients are depicted with solid black bars. Numbers for the year 2011 represent all lung transplants through June.
Patients in the highest LAS quartile had scores ranging from 48.4-95.5. When stratified into 3 high acuity quartiles, 1,874(74.3%) were in the highest LAS quartile but did not require mechanical support, 526(20.9%) were bridged to LTx with ventilator support, and 122(4.8%) were bridged to LTx with ECMO support, comprising our final cohort of 2,522 patients. In our final cohort, 212(8.4%) patients underwent redo-LTx.
Baseline Characteristics
When stratified by recipient acuity, several baseline characteristics differed markedly between the three cohorts(Table 1). Patients transplanted with either ventilatory or ECMO support tended to be younger, of white ethnicity, received organs with longer ischemic times, were more likely to undergo bilateral LTx, had higher LAS scores, and had their LTx performed at high volume centers. Although the numbers are small, an examination of the baseline characteristics of those bridged with ECMO support over time demonstrates these recipients have tended to become younger and tended to receive lungs with longer ischemic times(Table 2). Regardless of stratification, although many other characteristics were statistically different, the absolute differences were small and unlikely clinically relevant.
Table 1.
Baseline Characteristics Stratified by Recipient Acuity
| Variable | Highest LAS Quartile (n=1,874) |
Ventilatory Support (n=526) |
ECMO Support (n=122) |
P-value |
|---|---|---|---|---|
| Recipient Demographics and Co-Morbidities | ||||
| Age, years | 55 (±13) | 51 (±16) | 48 (±16) | <0.001a,b |
| Male gender, n (%) | 1151/1874 (61.4%) | 304/526 (57.8%) | 74/122 (60.7%) | 0.3 |
| White ethnicity, n (%) | 1456/1874 (77.7%) | 452/526 (85.9%) | 100/122 (82.0%) | <0.001a |
| Black ethnicity, n (%) | 189/1874 (10.1%) | 38/526 (7.2%) | 10/122 (8.2%) | 0.1 |
| Hispanic ethnicity, n (%) |
183/1874 (9.8%) | 27/526 (5.1%) | 7/122 (5.7%) | 0.002a |
| Other race, n (%) | 46/1874 (2.5%) | 9/526 (1.7%) | 5/122 (4.1%) | 0.3 |
| Creatinine, mg/dL | 0.9 (±0.5) | 0.9 (±0.7) | 1.0 (±0.8) | 0.005b |
| Total bilirubin, mg/dL | 0.5 [0.3-0.8] | 0.5 [0.3-0.7] | 0.7 [0.5-1.2] | 0.1 |
| Diabetes, n (%) | 474/1862 (25.5%) | 142/518 (27.4%) | 32/121 (26.5%) | 0.7 |
| Hypertension, n (%) | 71/275 (25.8%) | 26/91 (28.6%) | 3/15 (20.0%) | 0.7 |
| Body mass index, kg/m2 |
25 (±8) | 24 (±6) | 25 (±5) | 0.001a |
| CMV positive, n (%) | 1051/1759 (59.8%) | 226/407 (55.5%) | 54/95 (56.8%) | 0.3 |
| IPF, n (%) | 1085/1874 (57.9%) | 162/526 (30.8%) | 36/122 (29.5%) | <0.001a,b |
| COPD, n (%) | 72/1874 (3.8%) | 80/526 (15.2%) | 13/122 (10.7%) | <0.001a,b |
| Cystic fibrosis, n (%) | 181/1874 (9.7%) | 92/526 (17.5%) | 14/122 (11.5%) | <0.001a |
| PPHN, n (%) | 15/1874 (0.8%) | 3/526 (0.6%) | 3/122 (2.5%) | 0.1 |
| Recipient Acuity | ||||
| Cardiac output, L/min | 5.4 (±1.5) | 5.4 (±1.5) | 5.4 (±1.9) | 0.5 |
| Mean PA pressure, mmHg |
30 (±12) | 29 (±11) | 30 (±13) | 0.3 |
| FEV1, % predicted | 42 (±18) | 37 (±21) | 44 (±23) | <0.001 |
| 6MWT, feet | 545 [100-1000] | 710 [50-1112] | 150 [0-420] | 0.002b,c |
| O2 requirement, L | 6 [4-8] | 6 [4-13] | 15 [8-20] | <0.001a,b,c |
| LAS score | 65.4 (±14.5) | 64.9 (±22.9) | 73.9 (±21.4) | <0.001b,c |
| Donor variables | ||||
| Age, years | 34 (±14) | 35 (±14) | 34 (±15) | 0.3 |
| Male gender, n (%) | 952/1874 (50.8%) | 290/526 (55.1%) | 66/122 (54.1%) | 0.2 |
| Cigarette use, n (%) | 218/1864 (11.7%) | 84/524 (16.0%) | 16/121 (13.2%) | 0.03a |
| CMV positive, n (%) | 1184/1869 (63.4%) | 328/523 (62.7%) | 71/122 (58.2%) | 0.5 |
| Transplant Variables | ||||
| Bilateral LTx, n (%) | 1265/1874 (67.5%) | 419/526 (79.7%) | 98/122 (80.3%) | <0.001a,b |
| Ischemic time, hours | 5.3 (±1.6) | 5.5 (±1.7) | 5.9 (±2.0) | <0.001a,b |
| Waitlist time, days | 41 [12-142] | 30 [8-112] | 19.5 [6-68] | 0.2 |
| Same gender | 1203/1874 (64.2%) | 338/526 (64.3%) | 80/122 (65.6%) | 0.9 |
| matching, n (%) | ||||
| Same race matching, n (%) |
979/1874 (52.2%) | 308/526 (58.6%) | 78/122 (63.9%) | 0.003a,b |
| CMV mismatch, n (%) | 806/1754 (46.0%) | 184/406 (45.3%) | 45/95 (47.4%) | 0.9 |
| HLA mismatch, n (%) | 1003/1664 (60.3%) | 283/482 (58.7%) | 65/109 (59.6%) | 0.8 |
| Annual Center Volume | ||||
| 1-21 LTx/year | 453/1874 (24.2%) | 98/526 (18.6%) | 32/122 (26.2%) | 0.06 |
| 22-35 LTx/year | 451/1874 (24.1%) | 116/526 (22.1%) | 18/122 (14.8%) | 0.048b |
| 36-53 LTx/year | 478/1874 (25.5%) | 116/526 (22.1%) | 26/122 (21.3%) | 0.2 |
| ≥ 54 LTx/year | 492/1874 (26.3%) | 196/526 (37.3%) | 46/122 (37.7%) | <0.001a,b |
Abbreviations: LAS, lung allocation score; ECMO, extracorporeal membrane oxygenation; CMV, cytomegalovirus; IPF, idiopathic pulmonary fibrosis; COPD, chronic obstructive pulmonary disease, PPHN, primary pulmonary hypertension; PA, pulmonary artery; FEV1, forced expiratory volume in 1 second; 6MWT, six minute walk test; O2, oxygen; LAS, lung allocation score; HLA, human leukocyte antigen; LTx, lung transplantation.
P-value represents ANOVA, Wilcoxon rank-sum, chi-square, or Fisher’s exact test as appropriate
Cohort 1 significantly different than cohort 2 by post hoc test
Cohort 1 significantly different than cohort 3 by post hoc test
Cohort 2 significantly different than cohort 3 by post hoc test
Table 2.
Baseline Characteristics of Patients Bridged to Lung Transplantation with ECMO Stratified by Year
| Variable | 2005 (n=8) |
2006 (n=8) |
2007 (n=10) |
2008 (n=23) |
2009 (n=20) |
2010 (n=29) |
2011 (n=24) |
P- value* |
|---|---|---|---|---|---|---|---|---|
| Recipient Demographics and Co-Morbidities | ||||||||
| Age, years | 54 (±10) |
50 (±17) |
42 (±18) |
49 (±15) |
46 (±14) | 49 (±17) | 49 (±17) |
<0.001 |
| Male | 6/8 | 4/8 | 6/10 | 16/23 | 11/20 | 15/29 | 16/24 | 0.8 |
| gender, n (%) |
(75.0%) | (50.0%) | (60.0%) | (69.6%) | (55.0%) | (51.7%) | (66.7%) | |
| White | 8/8 | 6/8 | 7/10 | 21/23 | 15/20 | 23/29 | 20/24 | 0.5 |
| ethnicity, n (%) |
(100%) | (75.0%) | (70.0%) | (91.3%) | (75.0%) | (79.3%) | (83.3%) | |
| Black | 0/8 | 1/8 | 0/10 | 1/23 | 3/20 | 3/29 | 2/24 | 0.7 |
| ethnicity, n (%) |
(0%) | (12.5%) | (0%) | (4.4%) | (15.0%) | (10.3%) | (8.3%) | |
| Hispanic | 0/8 | 0/8 | 3/10 | 0/23 | 1/20 | 2/29 | 1/24 | 0.04 |
| ethnicity, n (%) |
(0%) | (0%) | (30.0%) | (0%0 | (5.0%) | (6.9%) | (4.2%) | |
| Other race, n (%) |
0/8 (0%) |
1/8 (6.6%) |
0/10 (0%) |
1/23 (4.4%) |
1/20 (5.0%) |
1/29 (3.5%) |
1/24 (4.2%) |
0.9 |
| Creatinine, mg/dL |
1.4 (±0.6) |
1.0 (±0.5) |
0.9 (±0.3) |
1.3 (±1.6) |
0.8 (±0.3) |
0.9 (±0.5) |
1.0 (±0.5) |
0.005 |
| Total bilirubin, mg/dL |
1.65 [0.8- 4.5] |
1.05 [0.65- 1.9] |
0.9 [0.8- 2.1] |
0.8 [0.4- 1.3] |
0.5 [0.3- 0.7] |
0.65 [0.5-1.1] |
0.75 [0.55- 1.05] |
0.7 |
| Diabetes, n (%) |
0/8 (0%) |
1/8 (12.5%) |
3/10 (30.0%) |
6/23 (26.1%) |
5/20 (25.0%) |
6/28 (21.4%) |
11/24 (45.8%) |
0.2 |
| Body mass index, kg/m2 |
28 (±4) | 24 (±4) | 22 (±4) | 25 (±5) | 27 (±6) | 24 (±5) | 25 (±5) | <0.001 |
| CMV positive, n (%) |
5/7 (71.4%) |
3/6 (50.0%) |
8/10 (80.0%) |
11/20 (55.0%) |
9/14 (64.3%) |
5/19 (26.3%) |
13/19 (68.4%) |
0.1 |
| IPF, n (%) | 1/8 (12.5%) |
0/8 (0%) |
3/10 (30.0%) |
10/23 (43.5%) |
5/20 (25.0%) |
11/29 (37.9%) |
6/24 (25.0%) |
0.2 |
| COPD, n (%) |
2/8 (25.0%) |
2/8 (25.0%) |
0/10 (0%0 |
0/23 (0%) |
2/20 (10.0%) |
6/29 (20.7%) |
1/24 (4.2%) |
0.041 |
| Cystic fibrosis, n (%) |
0/8 (0%) |
1/8 (12.5%) |
0/10 (0%) |
3/23 (13.0%) |
2/20 (10.0%) |
3/29 (10.3%) |
5/24 (20.8%) |
0.7 |
| PPHN, n (%) |
0/8 (0%) |
1/8 (12.5%) |
0/10 (0%) |
1/23 (4.4%) |
0/20 (0%) |
1/29 (3.5%) |
0/24 (0%) |
0.5 |
| Recipient Acuity | ||||||||
| Cardiac output, L/min |
5.7 (±2.5) |
5.1 (±1.8) |
4.9 (±0.9) |
6.0 (±1.8) |
4.9 (±1.4) |
5.8 (±2.5) |
5.1 (±1.5) |
0.048 |
| Mean PA pressure, mmHg |
36 (±17) |
32 (±11) |
30 (±16) |
28 (±9) | 30 (±12) | 33 (±15) | 29 (±14) |
0.1 |
| FEV1, % predicted |
43.8 (±27.1) |
41.8 (±25.5) |
42.8 (±23.4) |
47.9 (±23.7) |
42.4 (±16.6) |
42.0 (±24.9) |
42.6 (±27.2) |
<0.001 |
| LAS score | 77.5 [43.5- 89.8] |
57.8 [35.1- 77.2] |
72.3 [41.0- 90.8] |
64.1 [47.0- 90.4] |
89.9 [72.8- 91.4] |
88.6 [53.3- 90.9] |
92.0 [88.3- 93.2] |
<0.001 |
| Donor variables | ||||||||
| Age, years | 39 (±18) |
33 (±15) |
34.5 (±11) |
36 (±16) |
34 (±12) | 35 (±16) | 33 (±16) |
0.02 |
| Male gender, n (%) |
5/8 (62.5%) |
5/8 (62.5%) |
3/10 (30.0%) |
13/23 (56.5%) |
9/20 (45.0%) |
15/29 (51.7%) |
16/24 (66.7%) |
0.5 |
| Cigarette use, n (%) |
0/8 (0%) |
1/8 (12.5%) |
1/9 (11.1%) |
5/23 (21.7%) |
4/20 (20.0%) |
3/29 (10.3%) |
2/24 (8.3%) |
0.6 |
| CMV positive, n (%) |
6/8 (75.0%) |
8/8 (100%) |
6/10 (60.0%) |
12/23 (52.2%) |
10/20 (50.0%) |
15/29 (51.7%0 |
14/24 (58.3%) |
0.2 |
| Transplant Variables | ||||||||
| Bilateral LTx, n (%) |
5/8 (62.5%) |
6/8 (75.0%) |
6/10 (60.0%) |
19/23 (82.6%) |
18/20 (90.0%) |
25/29 (86.2%) |
19/24 (79.2%) |
0.4 |
| Ischemic time, hours |
4.9 (±2.3) |
4.0 (±2.5) |
5.3 (±2.2) |
6.1 (±2.0) |
6.0 (±1.6) |
5.9 (±1.1) |
6.6 (±2.4) |
0.002 |
| Waitlist time, days |
50.5 [3- 153] |
23 [6- 49] |
8 [4-39] | 18 [6- 90] |
37 [8.5- 51.5] |
47 [8- 85] |
9 [3.5- 35.5] |
<0.001 |
| Same gender matching, n (%) |
5/8 (62.5%) |
5/8 (62.5%) |
5/10 (50.0%) |
16/23 (69.6%) |
14/20 (70.0%) |
19/29 (65.5%) |
16/24 (66.7%) |
0.9 |
| Same race matching, n (%) |
5/8 (62.5%) |
6/8 (75.0%) |
7/10 (70.0%) |
15/23 (65.2%) |
14/20 (70.0%0 |
18/29 (62.1%) |
13/24 (54.2%) |
0.9 |
| CMV mismatch, n (%) |
2/7 (28.6%) |
3/6 (50.0%) |
4/10 (40.0%) |
12/20 (60.0%) |
5/14 (35.7%) |
11/19 (57.9%) |
8/19 (42.1%) |
0.6 |
| HLA mismatch, n (%) |
1/3 (33.3%) |
5/6 (83.3%) |
5/10 (50.0%) |
14/22 (63.6%) |
11/20 (55.0%) |
17/29 (58.6%) |
12/19 (63.2%) |
0.8 |
| Annual Center Volume | ||||||||
| 1-21 LTx/year |
5/8 (62.5%) |
2/8 (25.0%) |
5/10 (50.0%) |
4/23 (17.4%) |
3/20 (15.0%) |
2/29 (6.9%) |
11/24 (45.8%) |
0.002 |
| 22-35 LTx/year |
2/8 (25.0%) |
3/8 (37.5%) |
1/10 (10.0%) |
1/23 (4.4%) |
5/20 (25.0%) |
4/29 (13.8%) |
1/24 (4.2%) |
0.1 |
| 36-53 LTx/year |
1/8 (12.5%) |
2/8 (25.0%) |
2/10 (20.0%) |
4/23 (17.4%) |
0/20 (0%0 |
8/29 (27.6%) |
10/24 (41.7%) |
0.056 |
| ≥ 54 LTx/year |
0/8 (0%) |
1/8 (12.5%) |
2/10 (20.0%) |
14/23 (60.9%) |
12/20 (60.0%) |
15/29 (51.7%0 |
2/24 (8.3%) |
<0.001 |
Abbreviations: LAS, lung allocation score; ECMO, extracorporeal membrane oxygenation; CMV, cytomegalovirus; IPF, idiopathic pulmonary fibrosis; COPD, chronic obstructive pulmonary disease, PPHN, primary pulmonary hypertension; PA, pulmonary artery; FEV1, forced expiratory volume in 1 second; LAS, lung allocation score HLA, human leukocyte antigen; LTx, lung transplantation.
P-value represents ANOVA, Wilcoxon rank-sum, chi-square, or Fisher’s exact test as appropriate.
Outcomes
Impact of Recipient High Acuity
On unadjusted analysis, ECMO and ventilatory support were associated with decreased 30-day(High LAS: 95.2% vs. Vent: 91.1% vs. ECMO: 76.4%, p<0.001), 1-year(81.0% vs. 67.7% vs. 57.6%, p<0.001), and 2-year(71.9% vs. 60.7% vs. 42.3%, p<0.001; Figure 2a) survival compared to patients in the highest LAS quartile. Although much of the decreased long-term survival appears to be mediated by early mortality, among patients who survived to 90-days, patients bridged to LTx with ventilator support still had significantly worse 1-year(p=0.001) and 2-year(p=0.004; Figure 2b) survival. Patients bridged with ECMO support who survived to 90 days had similar 1-year(p=0.7) survival but worse 2-year(p=0.01) survival compared to those in the highest LAS quartile. Among those who survived to 6 months, there were no differences in survival at 2-years(p=0.2). Excluding redo LTx did not significantly alter these outcomes.
Figure 2.
(A) 2-year Kaplan-Meier survival curves stratified by recipient acuity. (B) 2-year Kaplan-Meier survival curves conditional on 90-day survival stratified by recipient acuity. Recipients in the highest LAS quartile are depicted with a solid line, those requiring ventilatory support with a dashed line, and those requiring ECMO support with a dash-dot line. P-values were determined by the log-rank test.
On adjusted analysis, both ventilatory and ECMO support were associated with increased 30-day(Ventilatory support HR: 1.90 [1.26-2.86], p=0.002; ECMO support HR: 4.38 [2.44-7.87], p<0.001), 1-year(Ventilatory support HR: 1.99 [1.58-2.51], p<0.001; ECMO support HR: 3.03 [2.00-4.59], p<0.001; Table 3), and 2-year(Ventilatory support HR: 1.76 [1.43-2.16], p<0.001; ECMO support HR: 2.76 [1.87-4.08], p<0.001) mortality. In these models, hyperbilirubinemia and renal dysfunction were also associated with increased mortality. Bilateral LTx, increasing annual center volume, and increasing time since the implementation of the LAS system were all associated with decreased mortality.
Table 3.
Multivariable Cox Proportional Hazards Regression Model of 1-Year Mortality
| Variable | Hazard Ratio | 95% Confidence Interval |
P-Value |
|---|---|---|---|
| Acuity | |||
| Highest LAS Quartile | 1 (Reference) | -- | |
| Ventilator Support | 1.99 | [1.59-2.50] | <0.001 |
| ECMO Support | 3.03 | [2.00-4.59] | <0.001 |
| Recipient Variables | |||
| Age > 60 years | 1.23 | [0.97-1.55] | 0.1 |
| Total bilirubin (per mg/dL) | 1.07 | [1.04-1.10] | <0.001 |
| GFR > 90 | 1 (Reference) | -- | |
| GFR 60-90 | 1.29 | [1.03-1.61] | 0.02 |
| GFR < 60 | 1.55 | [1.16-2.07] | 0.003 |
| FEV1 (per % predicted) | 1.01 | [0.99-1.01] | 0.1 |
| Inhaled NO | 1.74 | [0.97-3.11] | 0.1 |
| COPD | 1 (Reference) | -- | |
| IPF | 0.86 | [0.57-1.28] | 0.4 |
| CF | 0.94 | [0.58-1.53] | 0.8 |
| PPHN | 0.60 | [0.18-2.04] | 0.4 |
| Other diagnosis | 1.01 | [0.67-1.54] | 0.9 |
| Donor Variables | |||
| Age (per year) | 1.01 | [0.99-1.01] | 0.051 |
| Male gender | 0.94 | [0.77-1.15] | 0.6 |
| Cigarette use | 1.12 | [0.86-1.47] | 0.4 |
| Transplant Variables | |||
| Redo LTx | 1.13 | [0.79-1.63] | 0.5 |
| Bilateral LTx | 0.73 | [0.58-0.94] | 0.01 |
| Ischemic time (per hour) | 1.05 | [0.99-1.13] | 0.5 |
| Same race matching | 0.98 | [0.80-1.19] | 0.8 |
| Transplant year | 0.93 | [0.88-0.99] | 0.02 |
| (per year since LAS) | |||
| Annual Center Volume | |||
| 1-21 LTx/year | 1 (Reference) | -- | |
| 22-35 LTx/year | 0.74 | [0.56-0.98] | 0.04 |
| 36-53 LTx/year | 0.59 | [0.44-0.78] | <0.001 |
| ≥ 54 LTx/year | 0.56 | [0.43-0.73] | <0.001 |
Abbreviations: LAS, lung allocation score; ECMO, extracorporeal membrane oxygenation; GFR, glomerular filtration rate; FEV1, forced expiratory volume in 1 second; NO, nitric oxide; COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis; CF, cystic fibrosis; PPHN, primary pulmonary hypertension; LTx, lung transplantation.
Complications
Patients in the highest LAS quartile had a significantly shorter length of stay compared to recipients bridged to LTx with either ventilator or ECMO support(Table 4). This cohort also had a lower incidence of drug-treated infection in the 1st year. As recipient acuity increased, there was also a progressive increase in the incidence of acute kidney injury requiring renal replacement therapy. The incidence of stroke and drug-treated rejection were similar.
Table 4.
Post-LTx Complications Stratified by Recipient Acuity
| Complication | Highest LAS Quartile (n=1,874) |
Ventilatory Support (n=526) |
ECMO Support (n=122) |
P-value* |
|---|---|---|---|---|
| Length of stay (days) | 17 [11-30] | 30 [19-50] | 32 [16.5-60] | <0.001a,b |
| Drug-treated infection, n (%) |
144/279 (51.6%) | 64/92 (69.6%) | 9/14 (64.3%) | 0.01a |
| Renal replacement therapy, n (%) |
137/1841 (7.4%) | 72/526 (13.7%) | 42/118 (35.6%) | <0.001a,b,c |
| Stroke, n (%) | 41/1830 (2.2%) | 19/523 (3.6%) | 3/144 (2.6%) | 0.2 |
| Biopsy-proven rejection, n (%) |
31/1858 (1.7%) | 7/526 (1.3%) | 1/122 (0.8%) | 0.8 |
P-value represents ANOVA, Wilcoxon rank-sum, chi-square, or Fisher’s exact test as appropriate.
Cohort 1 significantly different than cohort 2 by post hoc test
Cohort 1 significantly different than cohort 3 by post hoc test
Cohort 2 significantly different than cohort 3 by post hoc test
Temporal Trends
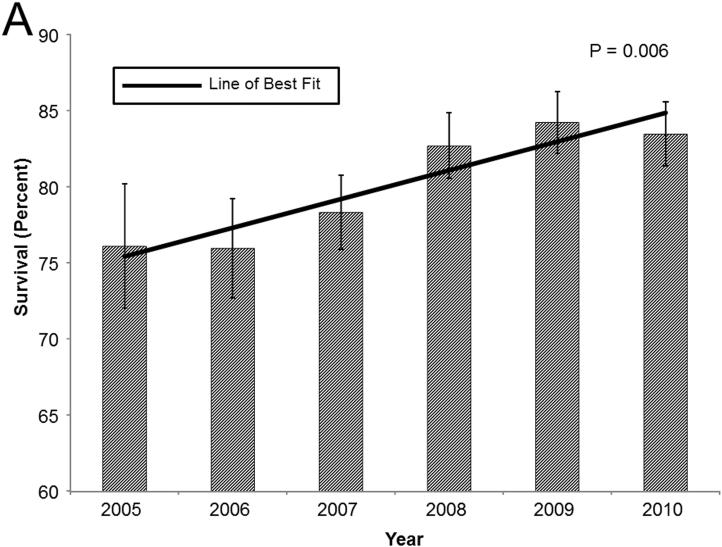
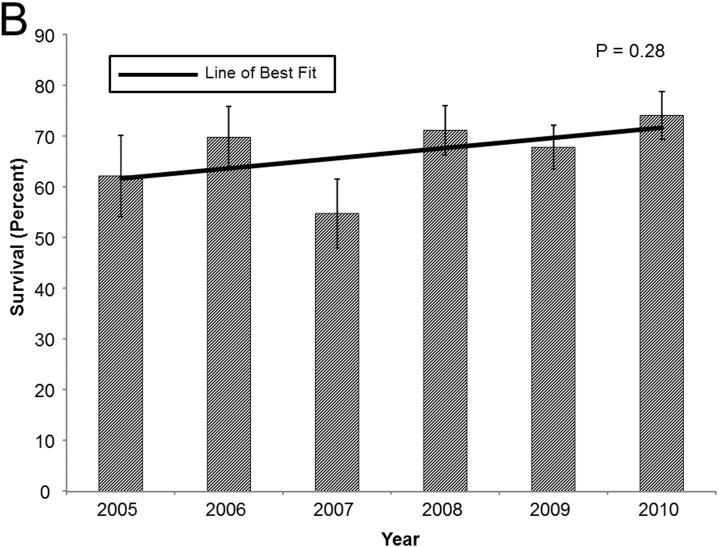
In general, in these high acuity recipients, 1-year survival has improved over time. In patients in the upper LAS quartile, 1-year survival improved from 76.1% in 2005 to 83.5% in 2010. Cox proportional hazards modeling and linear regression demonstrated a positive correlation between the year of LTx and 1-year survival(Coefficient: 1.89, p=0.006; HR: 0.92, p=0.01; Figure 3a). A similar, though less impressive, trend in 1-year survival was evident in patients bridged to LTx with ventilatory support(Coefficient: 2.00, p=0.28; HR: 0.94, p=0.2; Figure 3b). The most dramatic improvement in survival is in patients bridged to LTx with ECMO support. In this cohort, 1-year survival increased from 29.3% in 2005 to 74.7% in 2010(Coefficient: 8.03, p=0.06; HR: 0.76, p=0.001; Figure 3c).
Figure 3.
Bar graphs of 1-year survival stratified by year for (A) recipients in the highest LAS quartile, (B) recipients requiring ventilatory support, and (C) recipients requiring ECMO support. Survival was determined by the Kaplan-Meier method. Error bars denote standard error. Solid line represents the line of best fit. P-values were determined by linear regression.
ECMO
In patients bridged to LTx with ECMO support, a comparison of baseline characteristics of 1-year survivors and non-survivors suggests recipient age, diagnosis, annual center volume, and type of transplant may be important predictors of survival. When stratified by age, older patients exhibited worse 1-year survival. ROC analysis suggested that an age of 62 years was the optimal cutoff for predicting 1-year mortality, though the low sensitivity accounted for the overall low accuracy(sensitivity: 37%, specificity: 88%, AUC 0.58). When stratified relative to an age of 62 years, younger patients did have improved survival(64.7% vs. 38.3%, p=0.02). When stratified by diagnosis, patients with COPD tended to have the best 1-year survival while patients undergoing redo LTx had the worst(83.9% vs. 40.0%, p=0.1; Figure 4a). When stratified by annual center volume, patients undergoing LTx at high volume centers had improved survival compared to those at low volume centers(Figure 4b). ROC analysis suggests an optimal cutoff of 30 LTx/year(sensitivity: 48%, specificity: 83%, AUC: 0.63). Centers with ≥30 LTx/year did have improved survival(66.5% vs. 39.2%, p=0.003). Finally, patients bridged to LTx with ECMO support undergoing bilateral LTx had significantly improved survival compared to those undergoing single LTx(65.2% vs. 27.2%, p<0.001; Figure 4c). After adjusting for recipient age, diagnosis, donor age, and annual center volume, only the association of bilateral LTx and improved survival persisted(HR: 0.40 [0.20-0.80], p=0.01).
Figure 4.
Kaplan-Meier curves of 1-year survival stratified by (A) diagnosis, (B) annual center volume strata, and (F) type of lung transplant. P-value determined by the log-rank test. Abbreviations: LTx, lung transplantation; SLT, single lung transplant; BLT, bilateral lung transplant.
DISCUSSION
In this study, patients bridged to LTx with either ventilator or ECMO support had significantly decreased short and long-term survival compared to other high risk recipients. Patients requiring mechanical support also had a higher incidence of postoperative complications. Despite the increased risk of mortality, temporal analysis suggests that outcomes in high risk recipients have improved over time. Finally, in patients bridged to LTx with ECMO support, those who are younger, have COPD, those who undergo bilateral LTx, and those undergoing LTx at higher volume centers all have more favorable outcomes.
Historically, the need for ventilator or ECMO support has been considered a contraindication to LTx.[5,6] Ventilator support prior to LTx is thought to increase the risk of microbial airway colonization and lead to respiratory muscle deconditioning resulting in increased post-LTx ventilation time and pneumonia.[7,8] Thus, when many patients are intubated they are removed from the transplant waitlist.[8]
Similarly, pre-LTx ECMO support has been associated with poor outcomes since its inception.[9-11] Early efforts at bridging patients to LTx with ECMO focused on salvaging LTx recipients suffering from primary graft dysfunction necessitating emergent re-transplantation.[3] Because these patients had generally poor outcomes, the use of ECMO to bridge patients to their initial LTx has been limited. These patients tend to be the sickest recipients, often with either concomitant or consequent multi-system organ dysfunction, leading to poorer outcomes. Additionally, these patients are at increased risk for multiple complications during support, particularly bleeding and infection.
The inception of the LAS has changed the pattern of organ allocation in the United States.[1] Despite the poor historical outcomes associated with mechanical support of pre-LTx recipients, the LAS accords patients requiring ventilator or ECMO support the highest priority in organ allocation.[1,2] Although the LAS has improved both waitlist and post-transplant mortality, it has also forced clinicians to consider LTx in these high risk recipients.[3] Therefore, we undertook this study to examine outcomes in these high acuity recipients since the inception of the LAS.
Previous studies have compared patients requiring ventilator and ECMO support to all other recipients.[2,12] In order to more specifically assess the impact of these supportive therapies, we elected to compare recipients bridged with these therapies to another high risk cohort. Since we have previously demonstrated that the LAS is predictive of post-LTx mortality, we chose to compare recipients requiring mechanical support to the other recipients in the highest LAS quartile.[13,14]
In the post-LAS era, we found the 1-year survival of patients in the highest LAS quartiles, those requiring ventilator support, and those requiring ECMO support to be 81%, 68%, and 58%, respectively. After adjusting for other recipient, donor, and transplant factors, ventilator and ECMO support were associated with a 2 and 3-fold increase in the hazard of mortality, respectively. These findings mirror those previously published by Mason et al.[2] Additionally, we found that both annual center volume and years since the implementation of the LAS were associated with decreased mortality. Our finding that high volume centers have improved outcomes in these high risk recipients is not surprising; however, our finding that centers with an annual volume greater than 30 optimizes outcomes in patients bridged with ECMO is novel and represents one third of LTx centers in the US. Several studies have demonstrated that high volume heart and lung transplant centers have improved outcomes, particularly in high risk recipients.[15,16] However, the hazard ratio associated with time suggests that outcomes in these high acuity recipients are improving. To further analyze these temporal trends, we examined 1-year survival in each of the high risk cohorts over time. Recipients in the highest LAS quartile, those requiring ventilator support, and those requiring ECMO support have all demonstrated consistently improving 1-year survival since the implementation of the LAS.
Although several reports suggest contemporary outcomes compare favorably with historic outcomes, to our knowledge, we are the first to report that 1-year survival in patients bridged with ECMO appears to be improving over time.[2,3,10,11,17] Several explanations of this trend are possible. First, ECMO technology has and continues to improve over time. Technical improvements including the replacement of silicone oxygenators and the introduction of heparin-bonded circuits and centrifugal pumps have all improved outcomes during ECMO support.[9,11] These newer circuits decrease platelet, complement, and granulocyte activation resulting in less systemic inflammation.[11,18,19] These improvements may mitigate pre-LTx organ injury and systemic inflammation, decreasing systemic adverse events and metabolic derangements, thus preventing the accumulation of organ dysfunction prior to transplantation. However, while these improvements have been significant since the inception of ECMO, it seems unlikely that they would be the controlling factor over this brief 5 year survey. Second, the improving outcomes may reflect better patient selection. We would speculate that the initial enthusiasm over the ability to bridge these patients to LTx was quickly tempered by the poor results. Thus, over time, clinicians have become more cautious with regard to patient selection, leading to improved outcomes. Again, the sample size is too small to demonstrate annual differences but in general, the recipients have tended to be younger over time and more likely to undergo a bilateral LTx, a factor we have previously demonstrated to be associated with decreased mortality in certain patients.[20,21] However, ECMO patients also tend to receive organs with longer ischemic times. Nevertheless, even granting these limitations, a positive temporal trend seems evident. Given the small sample size, we can only speculate on the reasons for the improving outcomes. Most likely, the improved outcomes represent some combination of improving technology, better operative technique, superior patient selection, and more optimal recipient-donor matching; however, this study cannot answer this question and these proposed explanations are speculative and further investigation of these trends is warranted.
Finally, although our study suggests that patients bridged to LTx with ECMO have significantly decreased survival, LTx in this population does not appear to be futile, as it had been considered in the past. While only 58% survive to 1-year, it is unlikely that any would survive without LTx. However, given the relative scarcity of donor lungs and the increasing number of potential recipients, it is incumbent on all clinicians involved in LTx to carefully allocate these scarce resources. Therefore we compared 1-year survivors and non-survivors in an effort to identify subgroups of patients who are likely to benefit from LTx despite the need for pre-transplant ECMO support. Unfortunately, few factors proved to be discriminating. Although the numbers are small, patients younger than 62 years, those who underwent bilateral LTx, and those whose transplants were performed at centers with an annual volume equal or greater than 30 had greater than 60% 1-year survival. The few patients with a diagnosis of COPD had greater than 80% survival. Given the degree to which pre-LTx ECMO support negatively impacts post-transplant survival, it is possible that the best predictors of survival may be intangible. Therefore, while patients bridged to LTx had relatively increased mortality compared to other high risk recipients, LTx in this population is not futile with select groups achieving acceptable survival, and certainly higher survival than they would achieve without transplantation. Further investigation of factors that may predict favorable outcomes in these high acuity recipients is warranted.
Limitations
First, the data concerning ventilator and ECMO support in the UNOS database are limited. We do not know the duration of mechanical support, the modes of ventilation utilized, whether veno-arterial or veno-venous cannulation was employed, or the type of ECMO circuit or pump used. All of these factors could affect pre and post-transplant outcomes.
Second, we only examined patients who were successfully bridged to transplantation. Other patients supported with these therapies may have died on the waitlist or prior to listing, or may have been removed from the waitlist because they progressed to mechanical support. While not directly affecting our findings, these patients do impact the overall utility of mechanical support as a bridge to LTx.
Third, the data on post-LTx complications are limited. In particular, we do not have data on the use of post-LTx ECMO or other measures of primary graft dysfunction. Thus we cannot comment on the use of intraoperative or postoperative ECMO.
Fourth, because of the limited national experience with patients bridged to LTx with mechanical support, our sample size is limited. Thus our study is at risk for a type II error, particularly when analyzing subgroups of those bridged to LTx with ECMO. Thus, conclusions should be drawn cautiously.
Finally, in an effort to explore the increased hazard of mortality specifically associated with bridging patients to LTx with mechanical support, we utilized the highest LAS quartile as our reference group. Although this approach helps prevent overestimating the risk of mechanical support, it potentially increases the risk of underestimating its risk. Although the reported impact of mechanical support in this report is similar to others suggesting our approach was valid, this choice of reference group is a potential limitation.[2,12]
CONCLUSIONS
In conclusion, high acuity LTx recipients, particularly those bridged with ventilator or ECMO support have increased short-term mortality. Despite the increased risk of mortality, several subgroups have marginally improved survival and outcomes appear to be improving over time. In these high risk recipients, LTx is not futile. In order to optimize lung allocation, careful patient selection is critical and referral to high volume centers should be considered.
ACKNOWLEDGEMENTS
This work was supported in part by Health Resources and Services Administration contract 231-00-0115. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported by grant T32 2T32DK007713-12 from the National Institutes of Health(Dr. George). Dr. George is the Hugh R. Sharp Cardiac Surgery Research Fellow. Dr. Beaty is the Irene Piccinini Investigators in Cardiac Surgery.
Footnotes
Presentation: The contents of this manuscript will be presented at the 32nd Annual Meeting and Scientific Sessions of the International Society for Heart and Lung Transplantation in Prague, Czech Republic.
REFERENCES
- 1.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5):1212–27. doi: 10.1111/j.1600-6143.2006.01276.x. Pt 2. [DOI] [PubMed] [Google Scholar]
- 2.Mason DP, Thuita L, Nowicki ER, et al. Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg. 2010;139(3):765–73. doi: 10.1016/j.jtcvs.2009.09.031. e1. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez CA, Rocha RV, Zaldonis D, et al. Extracorporeal membrane oxygenation as a bridge to lung transplant: midterm outcomes. Ann Thorac Surg. 2011;92(4):1226–31. doi: 10.1016/j.athoracsur.2011.04.122. discussion 31-2. [DOI] [PubMed] [Google Scholar]
- 4.Vermeijden JW, Zijlstra JG, Erasmus ME, van der Bij W, Verschuuren EA. Lung transplantation for ventilator-dependent respiratory failure. J Heart Lung Transplant. 2009;28(4):347–51. doi: 10.1016/j.healun.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Bartz RR, Love RB, Leverson GE, et al. Pre-transplant mechanical ventilation and outcome in patients with cystic fibrosis. J Heart Lung Transplant. 2003;22(4):433–8. doi: 10.1016/s1053-2498(02)00667-8. [DOI] [PubMed] [Google Scholar]
- 6.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25(7):745–55. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Baz MA, Palmer SM, Staples ED, et al. Lung transplantation after long-term mechanical ventilation : results and 1-year follow-up. Chest. 2001;119(1):224–7. doi: 10.1378/chest.119.1.224. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien G, Criner GJ. Mechanical ventilation as a bridge to lung transplantation. J Heart Lung Transplant. 1999;18(3):255–65. doi: 10.1016/s1053-2498(98)00010-2. [DOI] [PubMed] [Google Scholar]
- 9.Cypel M, Keshavjee S. Extracorporeal life support as a bridge to lung transplantation. Clin Chest Med. 2011;32(2):245–51. doi: 10.1016/j.ccm.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 10.de Perrot M, Granton JT, McRae K, et al. Impact of extracorporeal life support on outcome in patients with idiopathic pulmonary arterial hypertension awaiting lung transplantation. J Heart Lung Transplant. 2011;30(9):997–1002. doi: 10.1016/j.healun.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Jackson A, Cropper J, Pye R, et al. Use of extracorporeal membrane oxygenation as a bridge to primary lung transplant: 3 consecutive, successful cases and a review of the literature. J Heart Lung Transplant. 2008;27(3):348–52. doi: 10.1016/j.healun.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Singer JP, Blanc PD, Hoopes C, et al. The impact of pretransplant mechanical ventilation on short- and long-term survival after lung transplantation. Am J Transplant. 2011;11(10):2197–204. doi: 10.1111/j.1600-6143.2011.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss ES, Allen JG, Merlo CA, Conte JV, Shah AS. Lung allocation score predicts survival in lung transplantation patients with pulmonary fibrosis. Ann Thorac Surg. 2009;88(6):1757–64. doi: 10.1016/j.athoracsur.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Merlo CA, Weiss ES, Orens JB, et al. Impact of U.S. Lung Allocation Score on survival after lung transplantation. J Heart Lung Transplant. 2009;28(8):769–75. doi: 10.1016/j.healun.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Arnaoutakis GJ, George TJ, Allen JG, et al. Institutional volume and the effect of recipient risk on short-term mortality after orthotopic heart transplant. J Thorac Cardiovasc Surg. 2012;143(1):157–67. doi: 10.1016/j.jtcvs.2011.09.040. 67 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss ES, Allen JG, Meguid RA, et al. The impact of center volume on survival in lung transplantation: an analysis of more than 10,000 cases. Ann Thorac Surg. 2009;88(4):1062–70. doi: 10.1016/j.athoracsur.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Hammainen P, Schersten H, Lemstrom K, et al. Usefulness of extracorporeal membrane oxygenation as a bridge to lung transplantation: a descriptive study. J Heart Lung Transplant. 2011;30(1):103–7. doi: 10.1016/j.healun.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Moen O, Fosse E, Dregelid E, et al. Centrifugal pump and heparin coating improves cardiopulmonary bypass biocompatibility. Ann Thorac Surg. 1996;62(4):1134–40. doi: 10.1016/0003-4975(96)00492-4. [DOI] [PubMed] [Google Scholar]
- 19.Fosse E, Moen O, Johnson E, et al. Reduced complement and granulocyte activation with heparin-coated cardiopulmonary bypass. Ann Thorac Surg. 1994;58(2):472–7. doi: 10.1016/0003-4975(94)92231-4. [DOI] [PubMed] [Google Scholar]
- 20.Weiss ES, Allen JG, Merlo CA, Conte JV, Shah AS. Factors indicative of long-term survival after lung transplantation: a review of 836 10-year survivors. J Heart Lung Transplant. 2010;29(3):240–6. doi: 10.1016/j.healun.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Weiss ES, Allen JG, Merlo CA, Conte JV, Shah AS. Survival after single versus bilateral lung transplantation for high-risk patients with pulmonary fibrosis. Ann Thorac Surg. 2009;88(5):1616–25. doi: 10.1016/j.athoracsur.2009.06.044. discussion 25-6. [DOI] [PubMed] [Google Scholar]