Abstract
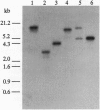
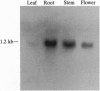
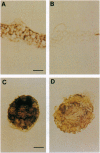
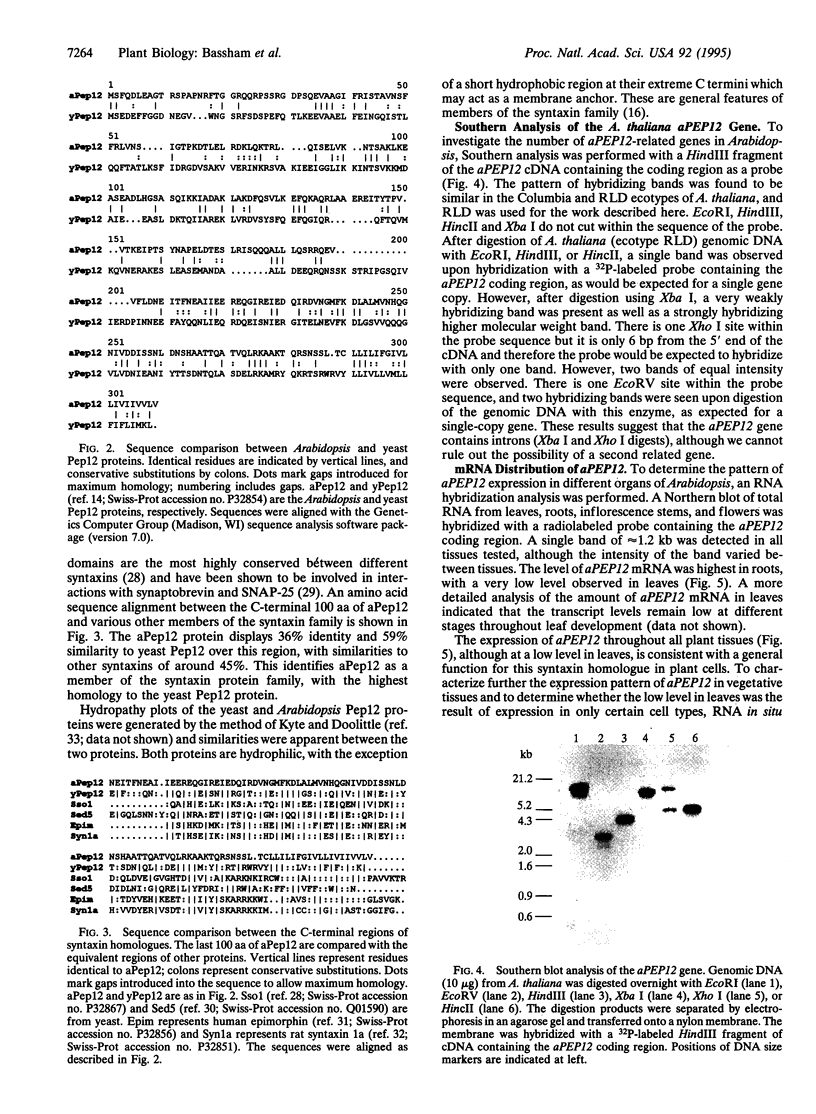
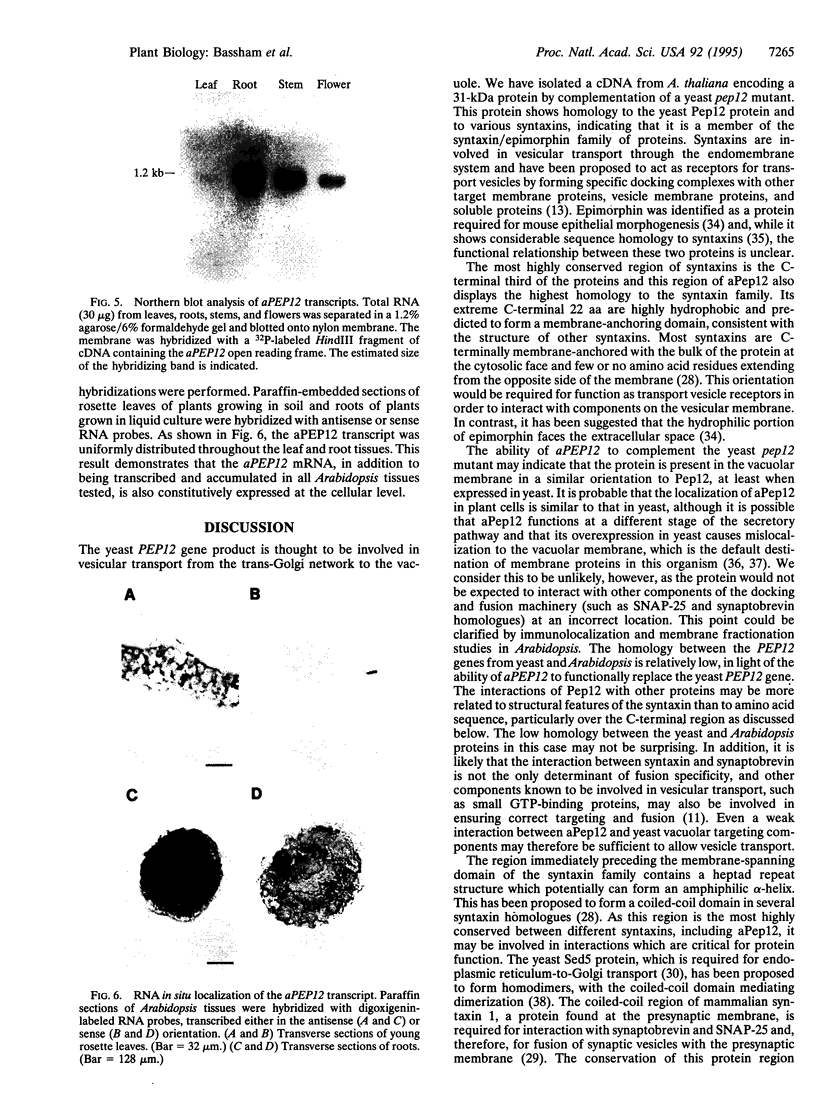
The syntaxin family of integral membrane proteins are thought to function as receptors for transport vesicles, with different isoforms of this family localized to various membranes throughout the cell. The yeast Pep12 protein is a syntaxin homologue which may function in the trafficking of vesicles from the trans-Golgi network to the vacuole. We have isolated an Arabidopsis thaliana cDNA by functional complementation of a yeast pep12 mutant. The Arabidopsis cDNA (aPEP12) potentially encodes a 31-kDa protein which is homologous to yeast Pep12 and to other members of the syntaxin family, indicating that this protein may function in the docking or fusion of transport vesicles with the vacuolar membrane in plant cells. Northern blot analysis indicates that the mRNA is expressed in all tissues examined, although at a very low level in leaves. The mRNA is found in all cell types in roots and leaves, as shown by in situ hybridization experiments. The existence of plant homologues of proteins of the syntaxin family indicates that the basic vesicle docking and fusion machinery may be conserved in plants as it is in yeast and mammals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalto M. K., Ronne H., Keränen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 1993 Nov;12(11):4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield D. K., Lewis M. J., Rabouille C., Warren G., Pelham H. R. Localization of Sed5, a putative vesicle targeting molecule, to the cis-Golgi network involves both its transmembrane and cytoplasmic domains. J Cell Biol. 1994 Oct;127(2):357–371. doi: 10.1083/jcb.127.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled M., Conceicao AdS., Frigerio L., Raikhel N. V. Expression and Regulation of aERD2, a Gene Encoding the KDEL Receptor Homolog in Plants, and Other Genes Encoding Proteins Involved in ER-Golgi Vesicular Trafficking. Plant Cell. 1995 Jun;7(6):667–676. doi: 10.1105/tpc.7.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bark I. C., Wilson M. C. Regulated vesicular fusion in neurons: snapping together the details. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4621–4624. doi: 10.1073/pnas.91.11.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D. M., Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- Bednarek S. Y., Raikhel N. V. Intracellular trafficking of secretory proteins. Plant Mol Biol. 1992 Oct;20(1):133–150. doi: 10.1007/BF00029156. [DOI] [PubMed] [Google Scholar]
- Bednarek S. Y., Raikhel N. V. The barley lectin carboxyl-terminal propeptide is a vacuolar protein sorting determinant in plants. Plant Cell. 1991 Nov;3(11):1195–1206. doi: 10.1105/tpc.3.11.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Calakos N., Scheller R. H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992 Jul 10;257(5067):255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., García-Arrarás J. E., Elferink L. A., Peterson K., Fleming A. M., Hazuka C. D., Scheller R. H. The syntaxin family of vesicular transport receptors. Cell. 1993 Sep 10;74(5):863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. A molecular description of synaptic vesicle membrane trafficking. Annu Rev Biochem. 1994;63:63–100. doi: 10.1146/annurev.bi.63.070194.000431. [DOI] [PubMed] [Google Scholar]
- Chapman E. R., An S., Barton N., Jahn R. SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem. 1994 Nov 4;269(44):27427–27432. [PubMed] [Google Scholar]
- Conceiço A. da S., Krebbers E. A cotyledon regulatory region is responsible for the different spatial expression patterns of Arabidopsis 2S albumin genes. Plant J. 1994 Apr;5(4):493–505. doi: 10.1046/j.1365-313x.1994.05040493.x. [DOI] [PubMed] [Google Scholar]
- De Block M., Debrouwer D. RNA-RNA in situ hybridization using digoxigenin-labeled probes: the use of high-molecular-weight polyvinyl alcohol in the alkaline phosphatase indoxyl-nitroblue tetrazolium reaction. Anal Biochem. 1993 Nov 15;215(1):86–89. doi: 10.1006/abio.1993.1558. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Mulligan J. T., Ramer S. W., Spottswood M., Davis R. W. Lambda YES: a multifunctional cDNA expression vector for the isolation of genes by complementation of yeast and Escherichia coli mutations. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1731–1735. doi: 10.1073/pnas.88.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal S., Raikhel N. V. Protein sorting in the endomembrane system of plant cells. Curr Opin Cell Biol. 1993 Aug;5(4):636–640. doi: 10.1016/0955-0674(93)90133-b. [DOI] [PubMed] [Google Scholar]
- Hardwick K. G., Pelham H. R. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol. 1992 Nov;119(3):513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y. Molecular cloning of human epimorphin: identification of isoforms and their unique properties. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1332–1337. doi: 10.1006/bbrc.1993.1363. [DOI] [PubMed] [Google Scholar]
- Hirai Y., Takebe K., Takashina M., Kobayashi S., Takeichi M. Epimorphin: a mesenchymal protein essential for epithelial morphogenesis. Cell. 1992 May 1;69(3):471–481. doi: 10.1016/0092-8674(92)90448-l. [DOI] [PubMed] [Google Scholar]
- Holwerda B. C., Padgett H. S., Rogers J. C. Proaleurain vacuolar targeting is mediated by short contiguous peptide interactions. Plant Cell. 1992 Mar;4(3):307–318. doi: 10.1105/tpc.4.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977 Jan;85(1):23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W. Tackling the protease problem in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:428–453. doi: 10.1016/0076-6879(91)94034-a. [DOI] [PubMed] [Google Scholar]
- Kirsch T., Paris N., Butler J. M., Beevers L., Rogers J. C. Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3403–3407. doi: 10.1073/pnas.91.8.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lee H. I., Gal S., Newman T. C., Raikhel N. V. The Arabidopsis endoplasmic reticulum retention receptor functions in yeast. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11433–11437. doi: 10.1073/pnas.90.23.11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner D. R., Raikhel N. V. The gene for stinging nettle lectin (Urtica dioica agglutinin) encodes both a lectin and a chitinase. J Biol Chem. 1992 Jun 5;267(16):11085–11091. [PubMed] [Google Scholar]
- Matsuoka K., Nakamura K. Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):834–838. doi: 10.1073/pnas.88.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J. M., Sticher L., Meins F., Jr, Boller T. A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10362–10366. doi: 10.1073/pnas.88.22.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman T., de Bruijn F. J., Green P., Keegstra K., Kende H., McIntosh L., Ohlrogge J., Raikhel N., Somerville S., Thomashow M. Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 1994 Dec;106(4):1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Nothwehr S. F., Stevens T. H. Membrane protein sorting in the yeast secretory pathway: evidence that the vacuole may be the default compartment. J Cell Biol. 1992 Oct;119(1):69–83. doi: 10.1083/jcb.119.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rothman J. H., Howald I., Stevens T. H. Characterization of genes required for protein sorting and vacuolar function in the yeast Saccharomyces cerevisiae. EMBO J. 1989 Jul;8(7):2057–2065. doi: 10.1002/j.1460-2075.1989.tb03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach G., Jung R., Kunze G., Saalbach I., Adler K., Müntz K. Different legumin protein domains act as vacuolar targeting signals. Plant Cell. 1991 Jul;3(7):695–708. doi: 10.1105/tpc.3.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder M. R., Borkhsenious O. N., Matsuoka K., Nakamura K., Raikhel N. V. Colocalization of barley lectin and sporamin in vacuoles of transgenic tobacco plants. Plant Physiol. 1993 Feb;101(2):451–458. doi: 10.1104/pp.101.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Spring J., Kato M., Bernfield M. Epimorphin is related to a new class of neuronal and yeast vesicle targeting proteins. Trends Biochem Sci. 1993 Apr;18(4):124–125. doi: 10.1016/0968-0004(93)90018-i. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Wilcox C. A., Redding K., Wright R., Fuller R. S. Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol Biol Cell. 1992 Dec;3(12):1353–1371. doi: 10.1091/mbc.3.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]