Abstract
The neonatal proximal tubule has a lower rate of bicarbonate absorption than that of adults. This is due, in part, to a lower rate of apical membrane Na+/H+ antiporter activity. The purpose of these studies was to examine if thyroid hormone could be a factor in the maturational increase in Na+/H+ antiporter activity. Hypothyroid (0.01% propylthiouracil in drinking water starting at day 14 gestation and throughout the postnatal period), euthyroid, and hyperthyroid (intraperitoneal triiodothyronine, 10 μg/100 g body wt, once daily on days 17 to 20 of postnatal life) rats were all studied at 21 days of life. Renal cortical brush border Na+/H+ antiporter activity was 453 ± 24, 527 ± 30 and 608 ± 25 pmol/mg protein in the hypothyroid, euthyroid and hyperthyroid groups, respectively (P < 0.001). Hyperthyroid neonates had ~twofold greater renal cortical NHE-3 mRNA abundance than euthyroid and hypothyroid neonates (P < 0.05). Brush border membrane NHE-3 protein abundance in hypothyroid and hyperthyroid neonates was one-third and twofold that of euthyroid 21-day-old rats, respectively (P < 0.001). These data are consistent with a potential role of thyroid hormone in the postnatal increase in Na+/H+ antiporter activity.
Keywords: NHE-3, proximal tubule, renal development, brush border membrane vesicles
The threshold for bicarbonaturia is significantly less in neonates than adults [1]. This is due, in large part, to the lower rate of bicarbonate reabsorption by the neonatal proximal tubule [2–4]; the nephron segment responsible for 80% of bicarbonate reabsorption. Most of proximal tubule apical proton secretion is mediated by the Na+/H+ antiporter [5]. In addition to its role in bicarbonate reabsorption, the Na+/H+ antiporter in conjunction with Cl−/base exchange mediates active NaCl transport in this nephron segment [6]. There is concordance between the postnatal maturation of neonatal rabbit proximal tubule bicarbonate transport, volume absorption and Na+/H+ antiporter activity [4, 7].
Several isoforms of the Na+/H+ antiporter have been cloned [8, 9]. NHE-1 and NHE-3 have been localized to the proximal tubule [10–12]. NHE-1 has a wide distribution in mammalian tissues [8], and is found on the basolateral membrane of the proximal tubule [12]. NHE-3 has been localized to the apical membrane of the proximal tubule and is likely the isoform responsible for Na+/H+ exchange activity in this segment [10, 11]. While renal cortical NHE-1 abundance does not change significantly with postnatal maturation, there is an increase in NHE-3 mRNA and protein abundance [13].
The factors responsible for the maturational increase in Na+/H+ antiporter activity are unknown. Thyroid hormone levels are significantly lower in neonatal rats in the first week or two of life compared to that measured in adults [14, 15]. Thyroid hormone is known to be an important factor in central nervous system development [15, 16]. The postnatal rise in thyroid hormone has been implicated as a potential factor in mediating developmental changes in intestinal maturation [17–20]. The purpose of the present study was to examine if thyroid hormone was a potential factor in the maturational increase in renal cortical Na+/H+ antiporter activity.
METHODS
Animals
Pregnant Sprague-Dawley rats were received on the fourteenth day of gestation. All rats were given free access to food and water until time of study. All neonates were cared for by their mothers. There is a maturational increase in serum thyroid hormone levels in the rat [14, 15]. Serum thyroxine levels were 0.5 ± 0.1 μg/dl in four-day-old neonates compared to 5.8 ± 0.6 μg/dl in adult rats (P < 0.001). Rats were studied at 21 days of age when serum thyroid levels are comparable to that of adults [14, 15]. Some rat litters were made hypothyroid by the addition of 0.01% propylthiouracil to the drinking water starting at the fourteenth day of gestation and throughout the postnatal period until the time of study at day 21 of life [15, 21]. This protocol has been shown to maintain the serum thyroid hormone concentration at levels comparable to those of neonates less than one week of age and prevent the maturational increase in thyroid hormone concentration which occurs around two to three weeks of age in the rat [14, 15]. At the time of sacrifice these hypothyroid neonates had a serum thyroxine level of 0.7 ± 0.1 μg/dl, which was significantly less than euthyroid controls (3.8 ± 0.2 μg/dl; P < 0.01). Some of the control neonates were made hyperthyroid by daily intraperitoneal injections of triiodothyronine (10 μg/100 g body wt) on days 17 to 20 of life [21]. Hypothyroid and euthyroid neonates received equivalent intraperitoneal injections of vehicle. The weights of the three groups of animals on the twenty-first day of life were different (P < 0.001). Euthyroid neonates weighed 51 ± 1 g compared to the hyperthyroid and hypothyroid groups which weighed 42 ± 1 and 27 ± 1 g (both P < 0.01 vs. control), respectively. The kidneys of hypothyroid rats have also been shown to weigh less than that of euthyroid rats at 21 days of age [21].
Brush border membrane vesicle isolation
Neonatal rat kidneys were rapidly removed and placed in an ice-cold isolation buffer containing 300 mM mannitol, 16 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 5 mM ethylene glycol-bis (β-aminoethyl ether) N,N,N′N′-tetraacetic acid (EGTA), titrated to pH 7.4 with Tris. The isolation buffer contained aprotinin (2 μg/ml), leupeptin (2 μg/ml) and phenyl-methylsulfonyl fluoride (10 μg/ml). The cortex was dissected and then homogenized with 20 strokes with a Teflon-glass homogenizer at 4°C. Brush border membrane vesicles (BBMV) were then isolated by differential contrifugation and magnesium precipitation as previously described [22–24]. The final BBMV fraction was resuspended in isolation buffer. Brush border enzyme activity measurements for alkaline phosphatase were performed as previously described in our laboratory and were ~20-fold greater than homogenate in all groups [24]. Protein was assayed using the bicinchoninic acid assay (BCA; Pierce Chemical Company) using bovine serum albumin as the standard.
Na+/H+ antiporter activity
Na+/H+ antiporter activity was measured in BBMV as the pH dependent uptake of 22Na as previously described [22]. Briefly, vesicles were loaded with intravesicular buffer (300 mM mannitol and 20 mM 2-(N-morpholino) ethanesulfonic acid (MES)-Tris, pH 5.5, by homogenization with 10 strokes of a glass-teflon homogenizer at 4°C. The vesicles were incubated for 30 minutes and then centrifuged at 20,000 rpm for 30 minutes at 4°C. The vesicles were resuspended in intravesicular solution at a final protein concentration of ~7 μg/μl.
Transport was initiated by addition of 150 μl of extravesicular buffer, containing 300 mM mannitol, 20 mM Tris (hydroxymethyl) aminomethane (Tris), 0.1 mM NaCl and 22NaCl, titrated to a pH of 7.5, to 10 μl of vesicles. After incubation for 10 seconds at room temperature, the reaction was terminated by rapid dilution, and filtration through, 0.65 μm filters (Millipore). All studies were performed in triplicate and the mean was used as the uptake for that sample. Radioactivity on the filters were determined using liquid scintillation counting. Background was subtracted from all measurements. Some experiments were performed in the presence of 100 μM 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) to inhibit the Na+/H+ antiporter.
SDS-PAGE and immunoblotting
Brush border membranes (10 μg/lane) were denatured and separated on a 7.5% polyacrylamide gel as previously described [13, 24]. The proteins were transferred to polyvinylidiene difluoride membrane overnight at 140 mA at 4°C. The blot was blocked with fresh Blotto (5% nonfat milk, 0.1% Tween 20, and PBS, pH 7.4) for one hour, and then a primary antibody to NHE-3 was added at a 1:250 dilution and incubated for one hour at room temperature. The blot was washed with Blotto, and then the secondary horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin was added for one hour at 1/10,000 dilution. The blot was washed with Blotto, and enhanced chemiluminescence was used to detect bound antibody (Amersham Life Science). Protein samples from hypothyroid, euthyroid, and hyperthyroid rat kidney cortex were run simultaneously on the same blot. The NHE-3 protein abundance was quantitated using densitometry.
RNA isolation and analysis
The kidneys were decapsulated, and renal cortex was homogenized in RNAzol [1:1, phenol-RNAzol stock (4 M guanidinium thiocyanate, 25 mM disodium-citrate, pH 7.0), 0.5% sarcosyl] containing 3.6 μl/ml β-mercaptoethanol. RNA was extracted using 3 M NaOAc (pH 4.0) and chloroform, purified using isopropanol precipitation, and washed twice with 75% ethanol [25]. Poly (A)+ RNA was purified using oligo (dT) column chromatography. Five micrograms of poly(A)+ RNA were fractionated by agarose-formaldehyde gel electrophoresis and transferred to a nylon filter (GeneScreen Plus; New England Nuclear, Boston, MA, USA). The filter was prehybridized at 42°C for four hours with 5× standard saline citrate (SSC), 5× Denhardt’s (Ficoll, bovine serum albumin and polyvinylpyrrolidone, each at 1 mg/ml), 0.5% sodium dodecyl sulfate (SDS), and 0.5 mg/ml of sheared salmon sperm DNA, then hybridized to double-stranded uniformly 32P-labeled cDNA probes (> 106 counts/min/ml) in the above hybridization solution at 42°C for 16 hours. The probes were synthesized by the random hexamer method using 50 to 100 ng of the following cDNAs. NHE3 was the rat 1.2-kilobase PstI fragment [8], and β-actin was a human 404-base pair EcoRI fragment [26]. The filter was then washed twice with 2 × SSC and 0.1% SDS for five minutes at room temperature and then with 0.1 × SSC and 1% SDS at 55°C for 40 minutes one or two additional times. Message abundance was quantitated by autoradiography and densitometry.
Statistical analysis
Each experiment was performed at least four times. Data are expressed as mean ± SEM. Student’s t-test and analysis of variance were used to determine statistical significance.
RESULTS
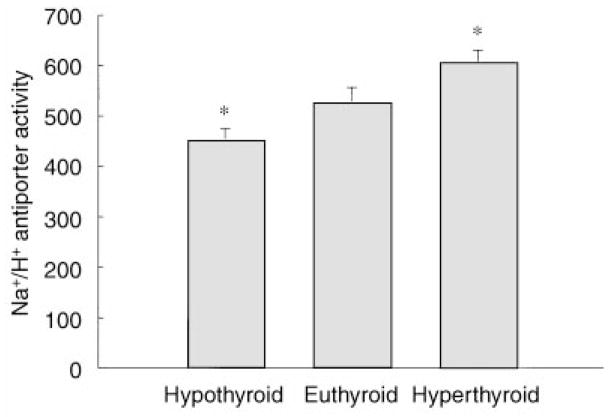
The first series of experiments examined the effect of thyroid hormone on Na+/H+ antiporter activity in 21-day-old hypothyroid (N = 24), euthyroid (N = 20) and hyperthyroid (N = 19) rats. The results of brush border membrane vesicle Na+/H+ antiporter activity, measured as the pH dependent 22Na uptake after incubation for 10 seconds, are shown in Figure 1. The rate of Na+/H+ antiporter activity was significantly less in brush border membrane vesicles from hypothyroid 21-day-old rats compared to vehicle treated 21-day-old euthyroid rats. Twenty-one-day-old rats that received four injections of triiodothyronine had a higher rate of Na+/H+ antiporter activity than did the euthyroid controls. There was no difference in sodium uptake in brush border membrane vesicles in the presence of 100 μM EIPA. 22Na uptake was 120 ± 16, 131 ± 21 and 116 ± 13 pmol/mg protein in the hypothyroid, euthyroid and hyperthyroid groups, respectively. These data show that neonatal rat renal Na+/H+ antiporter activity is affected by thyroid hormone.
Fig. 1. Na+/H+ antiporter activity (pmol/mg protein) in renal brush border membrane vesicles from hypothyroid, euthyroid and hyperthyroid 21-day-old rats.
Na+/H+ antiporter activity is the pH dependent uptake of 22Na after a 10 seconds incubation at room temperature. Data are mean ± SEM. *P < 0.05 versus euthyroid.
In the next series of experiments we examined the effect of thyroid hormone on renal cortical NHE-3 mRNA abundance. These results are shown in Figure 2. The ratio of NHE-3 mRNA abundance to β-actin mRNA abundance (in arbitrary densitometric units) was 0.49 ± 0.11 in the hypothyroid group, which was not different than that of the euthyroid control group (0.39 ± 0.08). The ratio of NHE-3 to β-actin mRNA abundance was higher in the hyperthyroid group (0.77 ± 0.09) than in the hypothyroid and euthyroid group (P < 0.05).
Fig. 2.
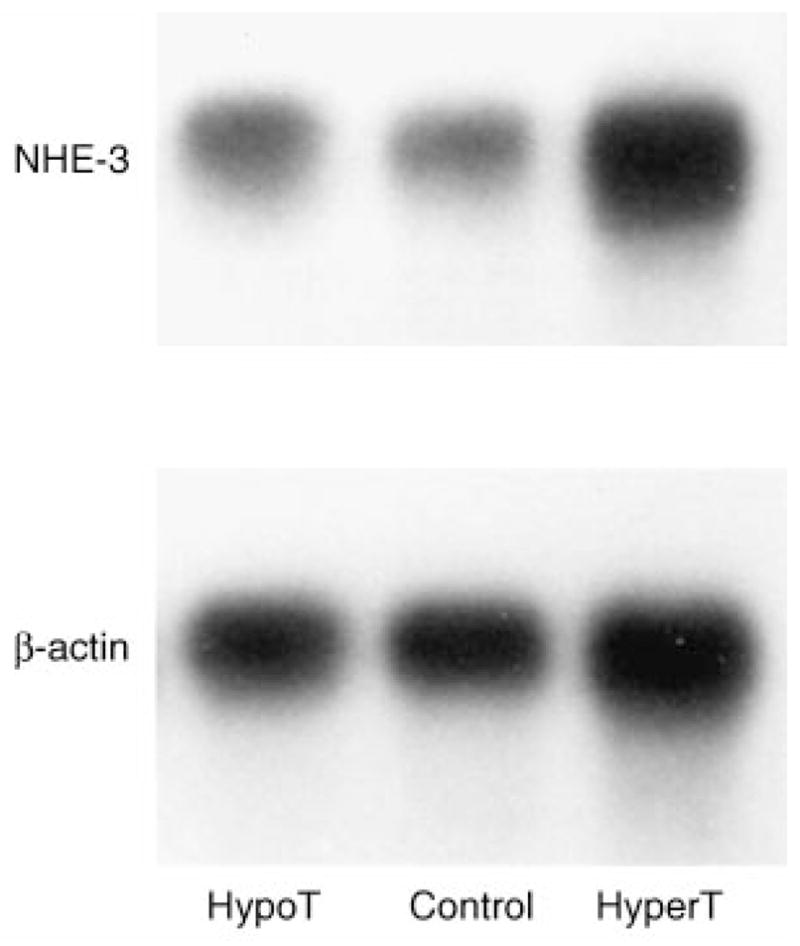
Renal cortical NHE-3 and β-actin in mRNA abundance in hypothyroid, euthyroid and hyperthyroid 21-day-old rats.
In the final series of experiments we examined the abundance of NHE-3 in brush border membranes using Western blot analysis. These results are shown in the representative blot in Figure 3. In four experiments there was 39,000 ± 4000 arbitrary densitometric units in the hypothyroid group compared to 117,000 ± 3000 units in the control (P < 0.05). Thyroid hormone caused a significant increase in brush border membrane NHE-3 protein abundance (225,000 ± 5,000 units, P < 0.01 vs. control).
Fig. 3.
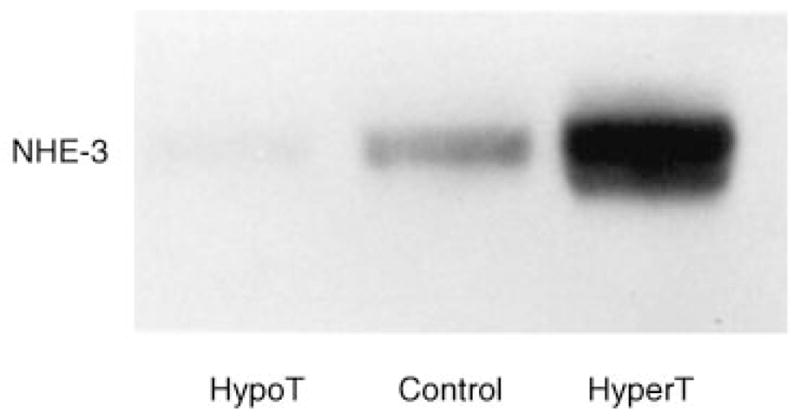
Brush border membrane NHE-3 protein abundance in hypothyroid, euthyroid and hyperthyroid rats.
DISCUSSION
The present study examined the effect of thyroid hormone on neonatal rat renal cortical Na+/H+ antiporter activity, NHE-3 mRNA and NHE-3 protein abundance. We found that hypothyroid neonates had comparable NHE-3 mRNA abundance as that of euthyroid neonates, but had lower levels of Na+/H+ antiporter activity and brush border membrane NHE-3 protein abundance. Hyperthyroid neonates had higher rates of Na+/H+ antiporter activity, and greater NHE-3 mRNA and NHE-3 protein abundance than euthyroid controls.
Thyroid hormone has been shown to affect proximal tubule transport. In split droplet microperfusion studies, hypothyroid rats had a lower rate of volume absorption than euthyroid controls [27, 28]. Administration of thyroid hormone to hypothyroid rats increased the rate of volume absorption [27, 28]. In this study, administration of thyroid hormone to hypothyroid rats resulted in an increase in Na+,K+-ATPase activity. However, there was a discordance between the dose and time required for the increase in volume absorption and the thyroid hormone-induced increase in Na+,K+-ATPase activity [28]. A higher dose of thyroid hormone and a longer time of administration were required for a stimulation in Na+,K+-ATPase activity than that required for an increase in volume absorption [28]. The reason for this is unclear.
A number of studies have found that thyroid hormone regulates proximal tubule Na+,K+-ATPase [29–33]. In primary cultures of rabbit proximal tubule cells, thyroid hormone was shown to have a direct epithelial action to increase activity as well as α and β subunit protein and mRNA abundance [32]. Of interest and pertinent to our findings, α- and β-Na+,K+-ATPase mRNA abundance was the same in hypothyroid and euthyroid rats, but mRNA for both subunits increased after injection of triiodothyronine [33].
Previous studies have examined the effect of thyroid hormone on renal brush border membrane Na+/H+ antiporter activity in adult rats [34]. Consistent with our findings in neonates, brush border membrane Na+/H+ antiporter activity was significantly higher in hyperthyroid rats and lower in hypothyroid rats compared to euthyroid rats. The effect of thyroid hormone on Na+/H+ antiporter activity could, in part, be due to the well described effect on glomerular filtration rate. However, a direct epithelial action of thyroid hormone to stimulate Na+/H+ antiporter activity has been demonstrated in opossum kidney (OK) and OKP cells [35, 36]. In OKP cells our laboratory has shown that thyroid hormone increase NHE-3 mRNA by increasing transcription [35]. This study also shows a greater effect of thyroid hormone on NHE-3 protein abundance than on Na+/H+ antiporter activity. The reason for this discrepancy is unclear.
A recent study has examined the effect of thyroid hormone on NHE-3 mRNA and protein abundance in adult rats [37]. As with our study, they found that NHE-3 mRNA abundance was the same in hypothyroid and euthyroid rats, but significantly higher in hyperthyroid rats. It is clear from these studies that while elevated levels of thyroid hormone can activate NHE-3 transcription, the basal mRNA level is not influenced by low levels of thyroid hormone. At variance with other studies, the level of renal cortical membrane NHE-3 protein abundance in adult hypothyroid and hyperthyroid rats was not different [37]. The reason for this difference is unclear.
The causes for the increase in perinatal Na+/H+ antiporter activity and proximal tubule volume absorption are unknown. Both glucocorticoid and thyroid hormone levels are lower in rats at birth than adult levels and increase near the time of weaning [14, 15, 38]. Prenatal glucocorticoids can accelerate the maturational increase in proximal tubule bicarbonate absorption and Na+/H+ antiporter activity [5, 7, 39]. We found a parallel increase in Na+/H+ antiporter activity, NHE-3 mRNA abundance and NHE-3 protein abundance by glucocorticoids [13]. Whether glucocorticoid deficiency delays the maturational increase in proximal tubule maturation has not been examined. This study shows that hypothyroid neonatal rats have a lower rate of Na+/H+ antiporter activity and NHE-3 protein abundance than euthyroid neonatal rats. It is possible that these data show that aberrant thyroid status can affect neonatal Na+/H+ antiporter activity. However, our data are consistent with the potential role for thyroid hormone as well as glucocorticoids playing a role in maturation of proximal tubule acidification.
The developmental increase in proximal tubule transport must be paralleled by an increase in oxidative metabolism. A maturational increase in proximal tubule 3-ketoacid-CoA transferase, acetoacetyl-CoA thiolase, carnitine acetyltransferase activity and a modest increase in citrate synthase, have been demonstrated [21]. Juxtamedullary proximal convoluted tubules from 21-day-old hypothyroid rats failed to demonstrate the maturational increase in 3-ketoacid-CoA transferase, citrate synthase and carnitine acetyl transferase activities [21]. This could be reversed by thyroid hormone replacement. Propylthiouracyl induced hypothyroidism did not affect actoacetyl-CoA thiolase activity. Injection of triiodothyronine to eight-day-old euthyroid pups resulted in a precocious rise in juxtamedullary proximal convoluted tubule 3-ketoacid-CoA transferase, carnitine acetyltransferase and citrate synthase activities [21]. These data are consistent with thyroid hormone playing a role in the maturation of several mitochondrial oxidative enzymes in the proximal tubule.
Acknowledgments
This work was supported by National Institute of Health grant DK41612 to 06A1 (M. Baum), DK-48482 (O. Moe), and DK39398 (R.J. Alpern). We thank Janell McQuinn for her secretarial assistance.
References
- 1.Edelman CM, Jr, Soriano JR, Boichis H, Gruskin AB, Acosta MI. Renal bicarbonate reabsorption and hydrogen ion excretion in normal infants. J Clin Invest. 1967;46:1309–1317. doi: 10.1172/JCI105623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum M, Quigley R. Ontogeny of proximal tubule acidification. Kidney Int. 1995;48:1697–1704. doi: 10.1038/ki.1995.467. [DOI] [PubMed] [Google Scholar]
- 3.Baum M, Quigley R. Prenatal glucocorticoids stimulate neonatal juxtamedullary proximal convoluted tubule acidification. Am J Physiol. 1991;261:F746–F752. doi: 10.1152/ajprenal.1991.261.5.F746. Renal Fluid Electrolyte Physiol 30. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GJ, Evan AP. Development of solute transport in rabbit proximal tubule. I. HCO3− and glucose absorption. Am J Physiol. 1983;245:F382–F390. doi: 10.1152/ajprenal.1983.245.3.F382. Renal Fluid Electrolyte Physiol 14. [DOI] [PubMed] [Google Scholar]
- 5.Baum M. Developmental changes in rabbit juxtamedullary proximal convoluted tubule acidification. Pediatr Res. 1992;31:411–414. doi: 10.1203/00006450-199204000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Aronson PS, Giebisch G. Mechanisms of chloride transport in the proximal tubule. Am J Physiol. 1997;273:F179–F182. doi: 10.1152/ajprenal.1997.273.2.F179. Renal Physiol 42. [DOI] [PubMed] [Google Scholar]
- 7.Baum M. Neonatal rabbit juxtamedullary proximal convoluted tubule acidification. J Clin Invest. 1990;85:499–506. doi: 10.1172/JCI114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na/H exchanger gene family. J Biol Chem. 1992;267:9331–9339. [PubMed] [Google Scholar]
- 9.Tse M, Levine S, Yun C, Brant S, Counillon LT, Pouyssegur J, Donowitz M. Structure/function studies of the epithelial isoforms of the mammalian Na+/H+ exchanger gene family. J Membr Biol. 1993;135:93–108. doi: 10.1007/BF00231435. [DOI] [PubMed] [Google Scholar]
- 10.Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int. 1995;48:1206–1215. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 11.Biemesderfer D, Pizzonia JH, Exner M, Reilly RF, Igarashi P, Aronson PS. NHE3: A Na+/H+ exchanger isoform of the renal brush border. Am J Physiol. 1993;34:F736–F742. doi: 10.1152/ajprenal.1993.265.5.F736. Renal Fluid Electrolyte Physiol 5. [DOI] [PubMed] [Google Scholar]
- 12.Biemesderfer D, Reilly RF, Exner M, Igarashi P, Aronson PS. Immunocytochemical characterization of Na+-H+ exchanger isoform NHE-1 in rabbit kidney. Am J Physiol. 1992;263:F833–F840. doi: 10.1152/ajprenal.1992.263.5.F833. Renal Fluid Electrolyte Physiol. [DOI] [PubMed] [Google Scholar]
- 13.Baum M, Biemesderfer D, Gentry D, Aronson PS. Ontogeny of rabbit renal cortical NHE3 and NHE1: Effect of glucocorticoids. Am J Physiol. 1994;268:F815–F820. doi: 10.1152/ajprenal.1995.268.5.F815. Renal Fluid Electrolyte Physiol 37. [DOI] [PubMed] [Google Scholar]
- 14.Walker P, Dubois JD, Dussault JH. Free thyroid hormone concentrations during postnatal development in the rat. Pediatr Res. 1980;14:247–249. doi: 10.1203/00006450-198003000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Wysocki SJ, Segal W. Influence of thyroid hormones on enzyme activities of myelinating rat central-nervous tissues. Eur J Biochem. 1972;28:183–189. doi: 10.1111/j.1432-1033.1972.tb01901.x. [DOI] [PubMed] [Google Scholar]
- 16.Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;31:1072–1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- 17.Israel EJ, Pang KY, Harmatz PR, Walker WA. Structural and functional maturation of rat gastrointestinal barrier with thyroxine. Am J Physiol. 1987;252:G762–G767. doi: 10.1152/ajpgi.1987.252.6.G762. Gastrointest Liver Physiol 15. [DOI] [PubMed] [Google Scholar]
- 18.McDonald MC, Henning SJ. Synergistic effects of thyroxine and dexamethasone on enzyme ontogeny in rat small intestine. Pediatr Res. 1992;32:306–311. doi: 10.1203/00006450-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Yeh K-Y, Moog F. Influence of the thyroid and adrenal glands on the growth of the intestine of the suckling rat, and on the development of intestinal alkaline phosphatase and disaccharidase activities. J Exp Zool. 1977;200:337–348. doi: 10.1002/jez.1402000304. [DOI] [PubMed] [Google Scholar]
- 20.Yeh K-Y, Yeh M, Holt PR. Thyroxine and cortisone cooperate to modulate postnatal intestinal enzyme differentiation in the rat. Am J Physiol. 1991;260:G371–G378. doi: 10.1152/ajpgi.1991.260.3.G371. Gastrointest Liver Physiol 23. [DOI] [PubMed] [Google Scholar]
- 21.Wijkhuisen A, Djouadi F, Vilar J, Merlet-Benichou C, Bastin J. Thyroid hormones regulate development of energy metabolism enzymes in rat proximal convoluted tubule. Am J Physiol. 1995;268:F634–F642. doi: 10.1152/ajprenal.1995.268.4.F634. Renal Fluid Electrolyte Physiol 37. [DOI] [PubMed] [Google Scholar]
- 22.Levi M, Henrich WL. Dietary calcium modulates renal BBM angiotensin II binding and Na+-H+ antiporter activity in SHR. Am J Physiol. 1991;260:F657–F662. doi: 10.1152/ajprenal.1991.260.5.F657. Renal Fluid Electrolyte Physiol 29. [DOI] [PubMed] [Google Scholar]
- 23.Moe OW, Tejedor A, Levi M, Seldin DW, Preisig PA, Alpern RJ. Dietary NaCl modulates Na+-H+ antiporter activity in renal cortical apical membrane vesicles. Am J Physiol. 1991;260:F130–F137. doi: 10.1152/ajprenal.1991.260.1.F130. Renal Fluid Electrolyte Physiol 29. [DOI] [PubMed] [Google Scholar]
- 24.Prabhu S, Levi M, Dwarakanath V, Arar M, Biber J, Murer H, Baum M. Effect of glucocorticoids on neonatal rabbit renal cortical sodium-inorganic phosphate messenger RNA and protein abundance. Pediatr Res. 1997;41:20–24. doi: 10.1203/00006450-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynmski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 26.Erba HP, Gunning P, Kedes L. Nucleotide sequence of the human τ 12 cytoskeletal actin mRNA: Anomalous evolution of vertebrate non-muscle actin genes. Nucleic Acids Res. 1986;14:5275–5294. doi: 10.1093/nar/14.13.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Santo NG, Capasso G, Paduano C, Carella C, Giordano C. Tubular transport processes in proximal tubules of hypothyroid rats. Pflügers Arch. 1980;384:117–122. doi: 10.1007/BF00584426. [DOI] [PubMed] [Google Scholar]
- 28.De Santo NG, Capasso G, Kinne R, Moewes B, Carella C, Anastasio P, Giordano C. Tubular transport processes in proximal tubules of hypothyroid rats. Lack of relationship between thyroidal dependent rise of isotonic fluid reabsorption and Na+-K+-ATPase activity. Pflügers Arch. 1982;394:294–301. doi: 10.1007/BF00583693. [DOI] [PubMed] [Google Scholar]
- 29.Barlet C, Doucet A. Kinetics of triiodothyronine action on Na-K-ATPase in single segments of rabbit nephron. Pflügers Arch. 1986;407:27–32. doi: 10.1007/BF00580716. [DOI] [PubMed] [Google Scholar]
- 30.Garg LC, Tisher CC. Effects of thyroid hormone on Na-K-adenosine triphosphatase activity along the rat nephron. J Lab Clin Med. 1985;106:568–572. [PubMed] [Google Scholar]
- 31.Deleted in the proof.
- 32.Lin H-H, Tang M-J. Thyroid hormone upregulates Na,K-ATPase α and β mRNa in primary cultures of proximal tubule cells. Life Sci. 1997;60:375–382. doi: 10.1016/s0024-3205(96)00661-3. [DOI] [PubMed] [Google Scholar]
- 33.McDonough AA, Brown RA, Horowitz B, Chiu R, Schlotterbeck J, Bowen J, Schmitt CA. Thyroid hormone coordinately regulates Na+-K+-ATPase α- and β-subunit mRNA levels in kidney. Am J Physiol. 1988;254:C323–C329. doi: 10.1152/ajpcell.1988.254.2.C323. Cell Physiol 23. [DOI] [PubMed] [Google Scholar]
- 34.Kinsella J, Sacktor B. Thyroid hormones increase Na+-H+ exchange activity in renal brush border membranes. Proc Natl Acad Sci USA. 1985;82:3606–3610. doi: 10.1073/pnas.82.11.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cano A, Baum M. T3 stimulates the activity of NHE3 by transcriptional activation (abstract) J Am Soc Nephrol. 1996;7:1253. [Google Scholar]
- 36.Yonemura K, Cheng L, Sacktor B, Kinsella JL. Stimulation by thyroid hormone of Na+-H+ exchange activity in cultured opossum kidney cells. Am J Physiol. 1990;258:F333–F338. doi: 10.1152/ajprenal.1990.258.2.F333. Renal Fluid Electrolyte Physiol 27. [DOI] [PubMed] [Google Scholar]
- 37.Azuma KK, Balkovetz DF, Magyar DE, Lescale-Matys L, Zhang Y, Chambrey R, Warnock DG, McDonough AA. Renal Na+/H+ exchanger isoforms and their regulation by thyroid hormone. Am J Physiol. 1996;270:C585–C592. doi: 10.1152/ajpcell.1996.270.2.C585. Cell Physiol 39. [DOI] [PubMed] [Google Scholar]
- 38.Henning SJ. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol. 1978;235:E451–E456. doi: 10.1152/ajpendo.1978.235.5.E451. Endocril Metab Gastrointest Physiol 4. [DOI] [PubMed] [Google Scholar]
- 39.Beck JC, Lipkowitz MS, Abramson RG. Ontogeny of Na/H aniporter activity in rabbit renal brush border membrane vesicles. J Clin Invest. 1991;87:2067–2076. doi: 10.1172/JCI115237. [DOI] [PMC free article] [PubMed] [Google Scholar]