Abstract
Endophenotypes are neurobiological markers cosegregating and associated with illness. These biomarkers represent a promising strategy to dissect ADHD biological causes. This study was aimed at contrasting the genetics of neuropsychological tasks for intelligence, attention, memory, visual-motor skills, and executive function in children from multigenerational and extended pedigrees that cluster ADHD in a genetic isolate. In a sample of 288 children and adolescents, 194 (67.4%) ADHD affected and 94 (32.6%) unaffected, a battery of neuropsychological tests was utilized to assess the association between genetic transmission and the ADHD phenotype. We found significant differences between affected and unaffected children in the WISC block design, PIQ and FSIQ, continuous vigilance, and visual-motor skills, and these variables exhibited a significant heritability. Given the association between these neuropsychological variables and ADHD, and also the high genetic component underlying their transmission in the studied pedigrees, we suggest that these variables be considered as potential cognitive endophenotypes suitable as quantitative trait loci (QTLs) in future studies of linkage and association.
Keywords: ADHD, Endophenotypes, Attention, Memory, Genetics, Behavior
Introduction
The neurobiological validity of attention-deficit/hyperactivity disorder (ADHD) (coded by the Online Mendelian Inheritance in Man Catalogue, OMIM, as 143465) is strongly supported by its genetic etiology and the association between structural and functional brain abnormalities with ADHD symptoms (Castellanos and Tannock 2002). The ADHD categorical classification, as defined by DSM-IV criteria, allowed us to define a fairly accurate spectrum of ADHD subtypes and also its comorbidity with other psychiatric conditions, i.e., oppositional defiant disorder (ODD), conduct disorder (CD), and substance use disorder (SUD) (Palacio et al. 2004).
In pursuing the neurophysiological basis of the ADHD phenotype, the identification of endophenotypes outlines a promising strategy to dissect ADHD biological causes. Endophenotypes were originally defined and used in psychiatry based on a concept introduced to explain insects’ evolution (Gottesman and Shields 1973). The operational definition of endophenotype was determined by looking if: (1) the endophenotype is associated with the disease in the population; (2) it is heritable; (3) it is primarily state-independent; (4) the endophenotype and the disease cosegregate within families; and (5) the endophenotype in affected family members is found in unaffected members at a higher rate than in the general population (Gottesman and Shields 1973; Gottesman and Gould 2003).
Several studies have proposed traits that might determine the operational criteria outlining endophenotypes associated with ADHD, for instance, the deregulation of executive and inhibitory brain mechanisms, stress aversion, novelty seeking, unexpected reward responses, working memory dysfunction, and personal time perception with poor fitness to real chronometry and wait aversion (Castellanos and Tannock 2002). Additionally, cognitive effort and continuous vigilance have been considered as vulnerability traits underlying ADHD symptoms (Castellanos and Tannock 2002). Following this approach, the evaluation of candidate neuropsychological ADHD endophenotypes as quantitative trait loci revealed two significant genome-wide linkage signals on chromosome 2q21.1 (LOD score: 3.944) for motor timing and on 13q12.11 (LOD score: 3.959) for Digit Span (Rommelse et al. 2008). Additional suggestive linkage signals have been reported for chromosomes 2p, 2q, 3p, 3q, 4q, 8q, 12p, 12q, 14q, 17q, and 22q (Doyle et al. 2008; Rommelse et al. 2008).
The purpose of this study was to contrast neuropsychological tasks for intelligence, attention, memory, visual-motor skills, and executive function against the operational criteria that define endophenotypes in 6–16-year-old children, boys and girls, from 141 multigenerational families clustering ADHD from a genetic isolate to test the hypothesis that some of these neuropsychological tasks accomplish with operational criteria of endophenotypes. If that is the case, this analysis will be able to define new potential neurobiological markers suitable for increasing the power to detect ADHD susceptibility loci.
Methods
Subjects and determination of eligibility procedures
The sample was selected from Paisa families inhabiting the Medellin metropolitan area of the State of Antioquia, Colombia. The families were required to have Paisa descent for more than two generations and more than two members affected with ADHD. Paisa descent was defined as having all four grandparents originating from the Paisa region of Colombia, i.e., from the former State of Viejo Caldas. Initial coded pedigrees were obtained through a fixed sampling scheme from a parent or grandparent of an index proband after having provided written informed consent, as approved by the University of Antioquia Ethics Committee. The pedigrees were individually reviewed to minimize the confounding effects of bilineal transmission of ADHD. Bilineality was defined by the presumptive diagnosis of ADHD in both parents based on the informant’s reports of childhood symptoms and/or of academic, occupational, or legal impairment, including alcoholism and related consequences. Because we assumed incomplete penetrance, particularly in women, we also imputed the presence of genotypic ADHD in parents or grandparents when two or more full siblings were reported to meet the same symptom or impairment profiles (Palacio et al. 2004). Full pedigrees identified as bilineal were excluded from further study. Pedigrees that contained bilineal branches during the selection phase were “pruned” to preserve the presumptively unilineal branches. Individuals in the selected families were then invited to participate in the current study. The first phase of the study consisted of obtaining pedigrees with provisional diagnoses and was conducted under the auspices and supervision of the University of Antioquia Ethics Committee. The Ethics Committee also approved a subsequent collaboration with investigators from the US National Institutes of Health (NIH). The proposal to conduct this study (Protocol 00-HG-0058) was jointly approved by the Institutional Review Board of the National Human Genome Research Institute (NHGRI, NIH) and the University of Antioquia Ethics Committee (Palacio et al. 2004). Informed consent documents were translated into Spanish for use in Colombia and reverse translated into English for institutional review board examination. All adult participants provided written informed consent. Parents of participating minors provided written informed consent and minors aged 6 years and older who could write also provided signed assent. Additional information regarding the process of determining eligibility for study participation can be reviewed elsewhere (Palacio et al. 2004).
Instruments
Structured psychiatric interview
The diagnostic interview for children and adolescents-revised-parent version was used to conduct the structured interview [DICA-IV-P, Spanish version translated during the development of this study with permission from Reich (2000)]. Parents of all selected children underwent a fully structured psychiatric interview regarding their offspring. This instrument has an acceptable inter-rater kappa coefficient ranging from 0.5 to 1. Internal consistency and test–retest reliability have been calculated independently for each disorder and previously reported elsewhere (Fristad et al. 1998; Rubio-Stipec et al. 1999; Palacio et al. 2004; Quintana et al. 2004, 2007; Renou et al. 2004).
Structured psychiatric interviews were conducted by one psychologist, one neuropsychologist, or two general psychiatrists, either at the Neurosciences Clinic of the University of Antioquia, Medellin, Colombia, or during home visits to the families. The interviewers, who were blind to the participants’ presumptive diagnoses, had been trained by a child and adolescent psychiatrist using a videotaped interview, theoretical discussions, and a 30-h tutorial controlled training. After this training, interviewers followed the administration of interviews by an expert psychiatrist during 60 additional hours, and their administered interviews were supervised for an additional 30 h. In addition, the psychiatric team discussed all completed protocols to define the accuracy of the responses given by the participants’ parents and teachers. Training was done until inter-rater kappa coefficients ≥0.7 were achieved for disruptive behavioral disorders, major depression, bipolar disorder, pervasive disorders, schizophrenia, obsessive compulsive diagnosis, and SUD (Palacio et al. 2004). A consistency score was estimated for each interview using a scale from 1 (unreliable) to 10 (fully reliable), in keeping with the concordance between two questions tied to each DSM-IV symptom, and also according to the agreement between the structured interview, the known participant’s behavior, and the information given by other members of the family. Only interviews with a reliability score ≥6 were considered for the final analyses.
Neuropsychological tests
The Wechsler intelligence scale for children-revised (WISC-R) (Wechsler 1974) was used to determine intelligence. The short form included: (1) similarities and vocabulary to estimate the verbal intelligence quotient (VIQ) and (2) picture completion and block design to estimate the performance intelligence quotient (PIQ). The full-scale intelligence quotient (FSIQ) was obtained using standardized tables according to the instrument’s instruction manual.
The mental control of the Wechsler memory scale (MC-WMS) (Wechsler 1949) was used to assess sustained attention and mental effort. This task has three items that must be performed as fast as possible: (1) count backward from 20 to 1; (2) recite the alphabet; and (3) count by three from 1 to 40. The Spanish version of this test has been validated by our group in both children and adult samples (Pineda et al. 1999, 2007; Ostrosky-Solis et al. 2007).
The ≪ A ≫ cancelation and vigilance test (A-CVT) is generally used to assess sustained attention (Matier et al. 1994). It consists of a set of 60 random letters distributed on paper. Letters are presented orally and subjects respond by knocking on the table with the dominant hand, when they hear the letter ≪ A ≫. Correct responses and errors (omissions and additions) are scored. It has been used as a continuous performance test for Spanish speaking adults and children (Pineda et al. 1999, 2007; Ostrosky-Solis et al. 2007).
The visual-verbal learning curve (VVLC) includes ten common objects (e.g., tree, trousers, chair, pencil, watch, etc.) that are drawn on a card and are visually and orally presented to the subject. The card is turned face down, and the participant tries to recall the name of the objects. Visual-verbal span, maximum score, number of trials to obtain the maximum score, and delayed recall 20 min later are scored (Pineda et al. 1999, 2007).
The Rey-Osterrieth complex figure test (ROCFT) (Rey 1941, 1994; Osterrieth 1944) is administered and scored by copy and by immediate memory recall (Lezak 1994). This task has been standardized for Colombian children and adolescents to evaluate visual-motor skills (Pineda et al. 1999).
Language comprehension
A short version of the Token Test with 36 items of increasing complexity (De Renzi and Faglioni 1978) was administered as a test of comprehension of instructions. Normative data are available for Spanish speakers.
Language Fluency
The Verbal Fluency test, with both phonologic (/f/,/a/,/s/) and semantic (animals and fruits) elements, measures the number of words produced in a given category during 1 min. This test has been considered either as a language denominative test, given its semantic component, or as an executive function test, due to its phonologic categorization component. Colombian norms are available (Pineda et al. 2007).
The Wisconsin Card Sorting Test-Abbreviated Version (WCST-A) is derived from the standard version (Heaton 1981). The WCST-A eliminates the ambiguous cards using only two sets of 24 non-ambiguous cards, which improves administration time. This task is used to assess executive function and has been standardized for Colombian children and adolescents (Pineda et al. 1999, 2007).
The set of neuropsychological tasks was administered to all selected children and adolescents in two sessions of approximately 45 min. As mentioned above, all tasks have been previously validated for Colombian ADHD and normal children and adolescents to assess their intelligence, attention, memory, visual-motor skills, and executive function (Pineda et al. 1999, 2007). The purpose of this cognitive assessment was to: (1) detect low intellectual capacity and (2) screen for cognitive deficiencies in ADHD children and adolescents.
Statistical procedures
Frequencies and proportions were estimated for categorical variables. Means and standard deviations were calculated for continuous variables. Categorical variables were compared using the chi-square test. Continuous variables meeting the assumptions of normality and homogeneity of variance were compared using the t test for independent samples; otherwise, they were tested using the non-parametric Mann–Whitney U test. Normality and homogeneity of variance were tested with the Shapiro–Wilk and the Bartlett tests, respectively. Cohen’s d effect size of non-overlapped data was estimated for all variables using pooled variances. To explore cognitive variables as ADHD predictors, a generalized linear model (GLM) with a binomial link was used. Fitting of the best model was conducted by a stepwise procedure. A receiver operating characteristic (ROC) curve was built to determine its accuracy. Statistical analyses were performed with R 2.8.0 patch (http://www.R-project.org). The ROC curve was drawn using the lroc function in the epicalc package (http://cran.r-project.org/web/packages/epicalc/index.html).
To estimate heritability of continuous variables, we used the ASSOC module in the software package S.A.G.E. (Elston and Gray-McGuire 2004). ASSOC evaluates the association between a continuous trait and one or more covariates from extended pedigree data in the presence of familial correlations, simultaneously estimating familial variance components (and hence familial correlations and heritability) (Elston and Gray-McGuire 2004). Heritability is defined as the intraclass correlation in the case of the polygenic variance. Because of the multigenerational and extended structure of these pedigrees, an individual may belong to several different nuclear families. In these situations, the person will also have more than one distinct family effect. ASSOC estimates parameters by maximum likelihood, assuming that parameters follow multivariate normality (Elston and Gray-McGuire 2004).
Results
The original sample consisted of 1,077 family members, 725 (67.3%) adults (17-year-old and older) and 352 (32.7%) children and adolescents (6–16-years-old), from 141 nuclear and multigenerational families (126 nuclear and 15 extended and multigenerational families) of the Paisa genetic isolate (Arcos-Burgos and Muenke 2002). From the children and adolescents, 336 young subjects were sampled, 228 affected and 108 unaffected by ADHD. From the 352 individuals, 16 were excluded; 10 of them had a diagnosis of probably affected by ADHD as defined in Palacio et al. (2004) and 6 of them because their clinical information was incomplete. A FSIQ was administered in the sample of 336 young subjects remaining in the study. Only children and adolescents with FSIQ ≥81 and with regular school grades corresponding to their age were included. Even though the DSM-IV sets the IQ for mental retardation at 70, we selected stringent criteria to exclude participants potentially affected by generalized learning disabilities. After applying exclusion criteria, a final sample of 288 children and adolescents, 194 (67.4%) ADHD affected and 94 (32.6%) ADHD unaffected, were accepted into the study. The proportion of excluded children and adolescents with a low FSIQ (≤80) and academic problems was not statistically different between affected and unaffected children, 34/228 (14.9%) and 14/108 (13.0%), respectively (OR = 1.17, 95% CI: 0.6–2.3, chi-square = 0.2274, P = 0.633). We found significant differences between ADHD affected and unaffected individuals on demographic covariates: sex (P < 0.00001), age (P < 0.0001), and school grade (P < 0.0001) (see Table 1).
Table 1.
Demographic characteristics of 288 children and adolescents from 141 extended pedigrees clustering ADHD
| Unaffected (n = 94) Frequency (%) |
Affected (n = 194) Frequency (%) |
Statistic index Chi-square |
P value | Effect size | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 42 (14.58) | 150 (52.08) | 30.35 | < 0.00001 | – |
| Female | 52 (18.06) | 44 (15.28) | |||
| Mean (SD) | Mean (SD) | Mann–Whitney U | |||
|---|---|---|---|---|---|
| Age | 11.47 (3.03) | 9.63 (2.74) | 5,964 | < 0.0001 | 0.637 |
| School grade | 5.65 (3.04) | 3.98 (2.61) | 6,174.5 | < 0.0001 | 0.589 |
In order to control for age, sex, and school grade effects, both the t test and Mann–Whitney’s U test were applied to residuals of the linear model yi = β0i + β1isex + β2iage+ β2ischgra + εi, εi ~ N(0, σ2), where yi is the cognitive variable i, schgra is the school grade, and εi is the random error. Significance level for all tests was set at α = 0.05.
Significant differences between ADHD affected and unaffected children were found for cognitive variables of intelligence, e.g., WISC-R block design (P < 0.01), PIQ (P < 0.05), and FSIQ (P < 0.05); variables measuring attention, e.g., A-CVT correct responses (P < 0.05), A-CVT omissions (P < 0.05), and A-CVT total errors (P < 0.05); and a variable measuring visual-motor abilities, e.g., ROCFT copy (P < 0.05) (see Table 2).
Table 2.
Performance on neuropsychological tasks of 288 children and adolescents from 141 extended pedigrees clustering ADHD
| ADHD status [mean (SD)]
|
Effect size
|
Difference of means***
|
Heritability
|
|||||
|---|---|---|---|---|---|---|---|---|
| Unaffected | Affected | Difference* | Pooled** | Statistic | P value | h2 | P | |
| Mental control | 5.52 (2.09) | 4.07 (2.49) | 0.292 | 0.633 | −1.890 | 0.0603 | 0.24 | 0.363 |
| WISC similarities | 11.83 (3.07) | 10.85 (2.99) | 0.154 | 0.324 | −1.092 | 0.2764 | 0.18 | 0.125 |
| WISC vocabulary | 9.52 (2.83) | 9.84 (3.15) | 0.05 | 0.108 | −0.512 | 0.6094 | 0.42 | 0.004 |
| WISC verbal IQ | 105.09 (17.12) | 101.77 (17.06) | 0.091 | 0.194 | −1.520 | 0.1302 | 0.47 | 0.002 |
| WISC picture completion | 10.63 (2.26) | 11.18 (2.47) | 0.109 | 0.235 | 0.794 | 0.4279 | 0.29 | 0.037 |
| WISC block design | 10.16 (2.72) | 9.26 (2.82) | 0.153 | 0.326 | 6,811.5 | 0.0014 | 0.45 | 0.002 |
| WISC performance IQ | 103.82 (14.56) | 100.71 (14.89) | 0.099 | 0.211 | −2.405 | 0.0172 | 0.67 | 0.000 |
| WISC FSIQ | 104.99 (14.66) | 101.83 (13.86) | 0.105 | 0.222 | 7,716.5 | 0.0402 | 0.60 | 0.000 |
| A-CVT correct response | 14.91 (1.33) | 13.61 (2.29) | 0.365 | 0.695 | 7,245.5 | 0.0323 | 0.35 | 0.033 |
| A-CVT omissions | 1.09 (1.33) | 2.36 (2.29) | 0.357 | 0.681 | 9,919.5 | 0.0376 | 0.39 | 0.019 |
| A-CVT commissions | 1.28 (2.01) | 1.41 (1.91) | 0.031 | 0.066 | 8,416.5 | 0.8256 | 0.52 | NA |
| A-CVT errors | 2.37 (2.53) | 3.77 (3.05) | 0.276 | 0.498 | 9,996.5 | 0.0278 | NA | NA |
| Memory verbal span | 7.1 (1.89) | 6.53 (1.83) | 0.145 | 0.306 | −0.448 | 0.6549 | 0.18 | 0.144 |
| Memory number of trials | 2.88 (1.86) | 3.29 (1.89) | 0.102 | 0.216 | 8,840.0 | 0.8215 | 0.38 | 0.02 |
| Memory delay recalls | 8.59 (1.45) | 8.61 (1.58) | 0.006 | 0.014 | 9,236.5 | 0.2154 | 0.24 | 0.10 |
| Memory organization index | 0.61 (0.33) | 0.68 (0.33) | 0.094 | 0.2 | 9,492.5 | 0.1596 | 0.16 | 0.188 |
| ROCFT copy time | 193.84 (85.98) | 221.33 (90.68) | 0.146 | 0.311 | 9,175.5 | 0.5481 | 0.48 | 0.001 |
| ROCFT copy | 27.58 (6.75) | 21.92 (8.68) | 0.396 | 0.728 | 7,373.0 | 0.0383 | 0.39 | 0.021 |
| ROCFT memory time | 129.87 (55.74) | 121.43 (54.55) | 0.073 | 0.153 | 8,014.0 | 0.3898 | 0.03 | 0.453 |
| ROCFT memory | 16.83 (7.86) | 12.48 (7.78) | 0.263 | 0.556 | 7,597.5 | 0.1122 | 0.41 | 0.018 |
| Verbal fluency letter F | 6.86 (3.42) | 5.25 (3.02) | 0.242 | 0.501 | 0.086 | 0.9317 | 0.05 | 0.449 |
| Verbal fluency letter A | 7.84 (3.9) | 5.87 (3.05) | 0.348 | 0.563 | 8,248.5 | 0.4410 | 0.003 | 0.490 |
| Verbal fluency letter S | 7.19 (3.46) | 5.78 (3.39) | 0.197 | 0.414 | 8,819.5 | 0.9042 | 0.19 | 0.147 |
| Phonological total verbal fluency | 21.89 (9.61) | 16.95 (8.38) | 0.265 | 0.549 | −0.399 | 0.6906 | 0.18 | 0.148 |
| Phonological verbal fluency mean | 7.33 (3.23) | 5.63 (2.8) | 0.273 | 0.562 | −0.516 | 0.6068 | 0.17 | 0.154 |
| Verbal fluency animals | 15.86 (4.71) | 14.2 (4.92) | 0.162 | 0.345 | 8,999.0 | 0.7445 | 0.38 | 0.012 |
| Verbal fluency fruits | 11.53 (3.62) | 9.9 (3.43) | 0.221 | 0.463 | 0.435 | 0.6640 | 0.11 | 0.258 |
| Semantic total verbal fluency | 27.39 (7.55) | 24.08 (7.42) | 0.209 | 0.442 | 0.635 | 0.5262 | 0.27 | 0.053 |
| Semantic verbal fluency means | 13.72 (3.81) | 12.03 (3.79) | 0.211 | 0.446 | 0.509 | 0.6110 | 0.29 | 0.043 |
| Token test | 33.07 (2.65) | 31.15 (3.66) | 0.325 | 0.604 | 8,568.5 | 0.4886 | 0.11 | 0.438 |
| WCST correct responses | 23.18 (8.88) | 22.13 (12.7) | 0.051 | 0.096 | 8,297.5 | 0.3670 | 0.58 | 0.0003 |
| WCST categories achievement | 2.82 (1.33) | 2.65 (1.45) | 0.056 | 0.12 | 8,584.5 | 0.6458 | 0.33 | 0.037 |
| WCST errors | 25.66 (9.6) | 27.74 (10.41) | 0.097 | 0.207 | 9,261.5 | 0.5599 | 0.30 | 0.073 |
| WCST perseverative errors | 17.44 (8.2) | 18.87 (9.97) | 0.087 | 0.157 | 8,512.5 | 0.5683 | 0.24 | 0.116 |
| WCST non-perseverative errors | 8.21 (4.5) | 8.81 (5.11) | 0.057 | 0.123 | 9,293.0 | 0.5277 | 0.24 | 0.344 |
| WCST percent perseverative errors | 35.66 (15.73) | 38.1 (19.26) | 0.076 | 0.139 | 8,488.5 | 0.5909 | 0.418 | 0.019 |
| WCST number of trial to first category | 12.77 (9.44) | 10.36 (8.71) | 0.128 | 0.265 | 7,155.0 | 0.2532 | 0.098 | 0.311 |
| WCST conceptual responses | 20.51 (8.63) | 18.54 (11.37) | 0.104 | 0.195 | 7,657.0 | 0.1745 | 0.58 | 0.0003 |
| WCST percent of conceptual responses | 42.23 (17.84) | 36.79 (19.01) | 0.139 | 0.296 | −0.871 | 0.3849 | 0.49 | 0.005 |
| WCST failure to maintain set | 0.74 (0.95) | 0.59 (0.82) | 0.079 | 0.163 | 9,114.5 | 0.2984 | NA | NA |
Significance P values of <0.05 are shown in bold
NA parameter cannot be maximized
Computed from the value of the t test of the differences between cases and controls
Average variance was used in this case
Tests for the difference after verification of both normality and homogeneity of variance. If both criteria were met, the t statistic was used; otherwise we used the non-parametric Mann–Whitney U test
Genetic effects and hereditary transmission, as measured by the heritability parameter, were significant for variables related to intellect performance (intelligence measures), e.g., WISC-R. Only the verbal subtest of similarities had no significant heritability (0.18, P > 0.05). As a trend, most of the cognitive measures had significant heritability with the exception of: the verbal memory span (0.18) (a variable related to immediate memory or verbal working memory), the memory organization index (0.16), the ROCFT memory time (a variable related to visual-motor information recall speed), the phonological verbal fluency (a variable related to word controlled performance), and the token test (a variable related to verbal comprehension) (see Table 2).
Simultaneous associations with the diagnosis of ADHD and significant genetic effects and hereditary transmission (significant heritability) were observed for the WISC-R block design, PIQ and FSIQ, A-CVT correct responses, A-CVT omissions, and ROCFT copy (see Table 2).
Generalized linear model
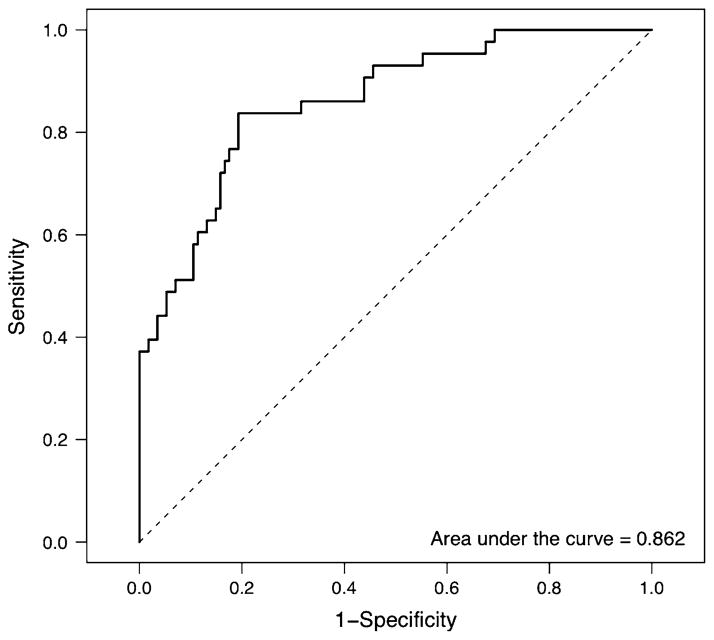
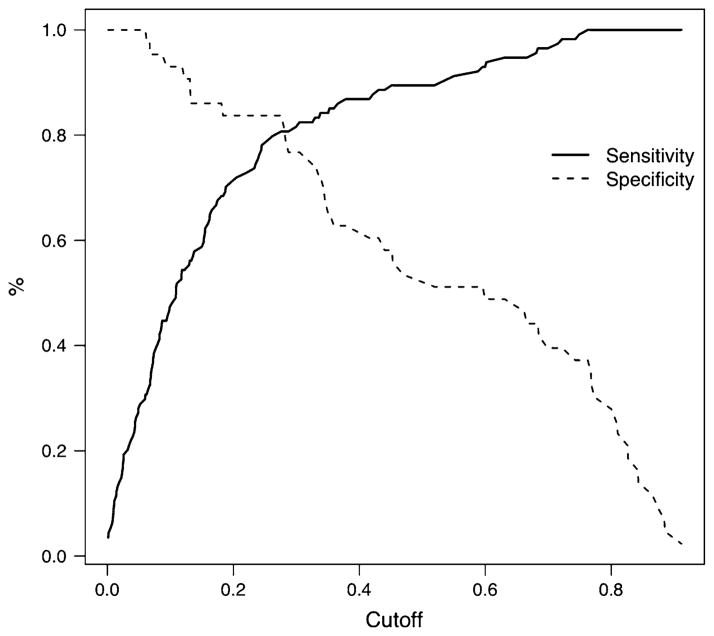
Coefficients for the GLM are presented in Table 3, and its AUC is 86.2%. Figures 1 and 2 present the ROC curve and cutoff/sensitivity/specificity analyses, respectively. The estimated cutoff is 0.2759. At this value, the sensitivity is 80.7% and the specificity 81.4%. The prediction model is given by the equations:
where β̂ 0 is the intercept term and β̂i, i = 1,…8, is the estimated regression coefficient for every variable included in the model (see Table 3). Two of the variables associated with ADHD and exhibiting significant genetic transmission, e.g., the WISC-R block design and the ROCFT copy, had significant weight in this model.
Fig. 1.
ROC curve for the binomial GLM to predict ADHD status using cognitive variables as covariates
Fig. 2.
Sensitivity and specificity versus cutoff for the fitted GLM predicting ADHD status using age, sex, school grade, and cognitive variables as covariates
Table 3.
Coefficients for the generalized linear model (GLM)—stage II
| Coefficient | Estimate | Standard error | z value | Pr( >| z|) |
|---|---|---|---|---|
| (Intercept) | −14.678 | 4.220 | −3.478 | 0.000505*** |
| Sex (female) | 2.272 | 0.512 | 4.436 | 9.17 × 10−6*** |
| WISC | ||||
| Block design | 0.214 | 0.083 | 2.568 | 0.010219* |
| ACVT correct response | 0.251 | 0.162 | 1.549 | 0.121401 |
| ROCFT | ||||
| Copy time | −0.008 | 0.004 | −1.854 | 0.06376 |
| ROCFT | ||||
| Copy | 0.091 | 0.041 | 2.234 | 0.025483* |
| ROCFT | ||||
| Memory time | 0.007 | 0.004 | 1.616 | 0.106129 |
| Semantic total | ||||
| Verbal fluency | −0.081 | 0.044 | −1.847 | 0.064698 |
| Token test | 0.227 | 0.127 | 1.787 | 0.073991 |
Significance codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘’ 1; Null deviance: 184.34 on 156 degrees of freedom; Residual deviance: 124.65 on 148 degrees of freedom; AIC: 142.65; Number of Fisher Scoring iterations: 6
Discussion
Psychometric procedures have adopted dimension and factor theories, as the strongest explanation of behavioral and cognitive skills. This model assumes that behavior and cognition are complex constructs of several integrated independent activities immersed into independent dimensions (Clark et al. 2000, 2002; Rosselli et al. 2001). In other words, cognition depends on specific activation patterns of different brain areas (“modules” associated with specific “dimensions”), each one making its own contribution to the whole system. Impairment of these modules would be responsible for specific neuropsychological disorders (Tannock 1998; Ardila and Bernal 2007). Functional brain images provided evidence for this type of modular structure, i.e., the same brain area may be potentially involved in different types of cognition (Cabeza and Nyberg 2000).
While looking for cognitive measures associated with ADHD deficiencies, several authors reported that children with ADHD perform lower than controls on tasks assessing continuous vigilance, processing speed, verbal learning and memory, working memory, phonological awareness, and executive function (Riccio et al. 1994; Denckla 1996a, b; Pineda et al. 2007). A meta-analysis in this field of research found that PIQ, as a measure of fluid intelligence, could be an important cognitive deficiency underlying the ADHD phenotype in children and adolescents (Frazier et al. 2004). Other authors found that inhibitory control had substantial sensitivity and specificity when predicting ADHD (Fischer et al. 2005; Lijffijt et al. 2005), while executive function measured by the WCST, the stop signal test, and working memory tasks strongly correlate with cognitive characteristics of ADHD patients (Seidman et al. 2005; Willcutt et al. 2005).
In this study, we report significant differences between ADHD affected and unaffected children in measures of WISC block design (as measures of conceptual, categorizational, and flexibility skills related to executive function), PIQ and FSIQ (related to intellectual capacity and fluid intelligence), continuous vigilance (A-CVT), and visual-motor skills (ROCFT copy). These variables also exhibit significant heritability, making them suitable measures to be considered as potential cognitive endophenotypes. Effect sizes of these significant and highly heritable variables oscillated between small and medium suggesting that bigger samples sizes will be needed in future genetic studies.
By comparing ADHD affected with unaffected siblings, others have reported significantly lower scores of ADHD children on tasks measuring dimensions of attention (continuous vigilance and cognitive effort), visual-motor skills, executive function (categorization, flexibility, and inhibitory control), and processing speed (reaction times) (Faraone et al. 1993; Seidman et al. 2000). These findings are consistent with some of the results of our study, e.g., the ADHD group had lower scores on variables measuring cognitive effort, continuous auditory vigilance, visual-motor skills, and performance speed.
In our families, we find significant statistical differences on PIQ and FSIQ, when comparing ADHD affected with unaffected children. These differences could be caused by the lower score on the block design task obtained by ADHD children, even though the effect size over a wide range of related variables is moderate, which might be indicating overlapping of both groups. Similar results were reported by other authors, who found that ADHD children had significantly lower FSIQ than unaffected siblings and that unaffected siblings of ADHD children had significantly lower FSIQ than unrelated healthy controls (Faraone et al. 1993; Seidman et al. 2000).
Significant coefficients of heritability (0.42–0.58) were found for categorization, planning, and flexibility abilities, as measured by the WCST. These cognitive functions have been reported by recent studies of linkage as efficient QTLs during genetic mapping (Doyle et al. 2008; Rommelse et al. 2008). It is worth mentioning that our study found that the same set of variables exhibited significant genetic effects, but we were unable to discriminate between ADHD children and controls.
Finally, independent of the discussion regarding cognitive impairment as related to: (1) intellectual capacity, (2) continuous vigilance, (3) cognitive effort, (4) planning and flexible abilities, and (5) inhibitory control skills, it is necessary to discuss fundamental aspects of these tasks that were conceived to assess brain damage and not to discriminate mild disabilities, mostly present in ADHD children (Willcutt et al. 2005). This could explain why the WISC, PIQ and FSIQ, and block design tests, originally conceived to evaluate a normally distributed parameter (intellectual performance), exhibit significant power to discriminate between ADHD affected and unaffected individuals.
Future studies involving this set of families will include screening of cognitive endophenotypes for parents and other adult siblings. Our next plan is to use these continuous variables with some of the operational criteria to be considered as potential cognitive endophenotypes in future genetic analyses of association and linkage.
Limitations
The results must be interpreted with caution as provisional findings since they are only screening gross information about the cognitive characteristics of ADHD family members with linkage to several ADHD vulnerability loci.
Acknowledgments
This research was funded by COLCIENCIAS, University of Antioquia, University of San Buenaventura and the NHGRI-NIH, who granted the Project: “Genética del Trastorno de Atención-Hiperactividad: los fenotipos complejos, los endofenotipos y la asociación con genes mayores y de susceptibilidad”. Code: 1115-04-18083, Contract: 459-2005.
Footnotes
Conflicts of interest None to declare.
Contributor Information
David A. Pineda, Group of Neurosciences of Antioquia, University of Antioquia, Medellin, Colombia. Group of Neuropsychology and Conduct Disorder, University of San Buenaventura, Medellin, Colombia
Francisco Lopera, Group of Neurosciences of Antioquia, University of Antioquia, Medellin, Colombia.
Isabel C. Puerta, Group of Neurosciences of Antioquia, University of Antioquia, Medellin, Colombia. Group of Neuropsychology and Conduct Disorder, University of San Buenaventura, Medellin, Colombia
Natalia Trujillo-Orrego, Group of Neurosciences of Antioquia, University of Antioquia, Medellin, Colombia. Group of Neuropsychology and Conduct Disorder, University of San Buenaventura, Medellin, Colombia.
Daniel C. Aguirre-Acevedo, Group of Neurosciences of Antioquia, University of Antioquia, Medellin, Colombia. Group of Neuropsychology and Conduct Disorder, University of San Buenaventura, Medellin, Colombia
Liliana Hincapié-Henao, Group of Neurosciences of Antioquia, University of Antioquia, Medellin, Colombia.
Clara P. Arango, Group of Neurosciences of Antioquia, University of Antioquia, Medellin, Colombia. Group of Neuropsychology and Conduct Disorder, University of San Buenaventura, Medellin, Colombia
Maria T. Acosta, National Human Genome Research Institute, National Institutes of Health, 35 Convent Dr., MSC 3717, Building 35, Room 1B209, Bethesda, MD 20892, USA
Sandra I. Holzinger, Department of Psychiatry and Behavioral Sciences, Leonard M. Miller School of Medicine, University of Miami, Miami, FL, USA
Juan David Palacio, Group of Neurosciences of Antioquia, University of Antioquia, Medellin, Colombia.
Daniel E. Pineda-Alvarez, Group of Neurosciences of Antioquia, University of Antioquia, Medellin, Colombia. National Human Genome Research Institute, National Institutes of Health, 35 Convent Dr., MSC 3717, Building 35, Room 1B209, Bethesda, MD 20892, USA
Jorge I. Velez, National Human Genome Research Institute, National Institutes of Health, 35 Convent Dr., MSC 3717, Building 35, Room 1B209, Bethesda, MD 20892, USA
Ariel F. Martinez, National Human Genome Research Institute, National Institutes of Health, 35 Convent Dr., MSC 3717, Building 35, Room 1B209, Bethesda, MD 20892, USA
John E. Lewis, Department of Psychiatry and Behavioral Sciences, Leonard M. Miller School of Medicine, University of Miami, Miami, FL, USA
Maximilian Muenke, Email: mamuenke@mail.nih.gov, National Human Genome Research Institute, National Institutes of Health, 35 Convent Dr., MSC 3717, Building 35, Room 1B209, Bethesda, MD 20892, USA.
Mauricio Arcos-Burgos, Email: arcosburgosm@mail.nih.gov, National Human Genome Research Institute, National Institutes of Health, 35 Convent Dr., MSC 3717, Building 35, Room 1B209, Bethesda, MD 20892, USA.
References
- Arcos-Burgos M, Muenke M. Genetics of population isolates. Clin Genet. 2002;61:233–247. doi: 10.1034/j.1399-0004.2002.610401.x. [DOI] [PubMed] [Google Scholar]
- Ardila A, Bernal B. What can be localized in the brain? Toward a “factor” theory on brain organization of cognition. Int J Neurosci. 2007;117:935–969. doi: 10.1080/00207450600912222. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Clark C, Prior M, Kinsella GJ. Do executive function deficits differentiate between adolescents with ADHD and oppositional defiant/conduct disorder? A neuropsychological study using the six elements test and Hayling sentence completion test. J Abnorm Child Psychol. 2000;28:403–414. doi: 10.1023/a:1005176320912. [DOI] [PubMed] [Google Scholar]
- Clark C, Prior M, Kinsella G. The relationship between executive function abilities, adaptive behaviour, and academic achievement in children with externalising behaviour problems. J Child Psychol Psychiat Allied Discipl. 2002;43:785–796. doi: 10.1111/1469-7610.00084. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Faglioni P. Normative data and screening power of a shortened version of the token test. Cortex. 1978;14:41–49. doi: 10.1016/s0010-9452(78)80006-9. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Biological correlates of learning and attention: what is relevant to learning disability and attention-deficit hyperactivity disorder? J Dev Behav Pediatr. 1996a;17:114–119. [PubMed] [Google Scholar]
- Denckla MB. A theory and model of executive function. A neuropsychological perspective. In: Lyon GR, Krasnegor NA, editors. Attention, memory, and executive function. Baltimore, MD: Paul H. Brookes Publishing Co; 1996b. pp. 263–278. [Google Scholar]
- Doyle AE, Ferreira MA, Sklar PB, Lasky-Su J, Petty C, Fusillo SJ, Seidman LJ, Willcutt EG, Smoller JW, Purcell S, Biederman J, Faraone SV. Multivariate genomewide linkage scan of neurocognitive traits and ADHD symptoms: suggestive linkage to 3q13. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1399–1411. doi: 10.1002/ajmg.b.30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston RC, Gray-McGuire C. A review of the ‘statistical analysis for genetic epidemiology’ (SAGE) software package. Hum Genomics. 2004;1:456–459. doi: 10.1186/1479-7364-1-6-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Lehman BK, Spencer T, Norman D, Seidman LJ, Kraus I, Perrin J, Chen WJ, Tsuang MT. Intellectual performance and school failure in children with attention deficit hyperactivity disorder and in their siblings. J Abnorm Psychol. 1993;102:616–623. doi: 10.1037/0021-843X.102.4.616. [DOI] [PubMed] [Google Scholar]
- Fischer M, Barkley RA, Smallish L, Fletcher K. Executive functioning in hyperactive children as young adults: attention, inhibition, response perseveration, and the impact of comorbidity. Dev Neuropsychol. 2005;27:107–133. doi: 10.1207/s15326942dn2701_5. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychol. 2004;18:543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Fristad MA, Cummins J, Verducci JS, Teare M, Weller EB, Weller RA. Study IV: concurrent validity of the DSM-IV revised Children’s Interview for Psychiatric Syndromes (ChIPS) J Child Adolesc Psychopharmacol. 1998;8:227–236. doi: 10.1089/cap.1998.8.227. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin card sorting test (WCST) Manual Psychological Assessment Resources; Odessa, FL: 1981. [Google Scholar]
- Lezak MD. Domains of behavior from a neuropsychological perspective: the whole story. Neb Symp Motiv. 1994;41:23–55. [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Matier K, Wolf L, Halperin J. The psychometric properties and clinical utility of a cancellation test in children. Dev Neuropsychol. 1994;10:165–177. [Google Scholar]
- Osterrieth PA. Le teste de copie d’une figure complexe [The complex figure copy test] Arch Psychol. 1944;30:206–256. [Google Scholar]
- Ostrosky-Solis F, Esther Gomez-Perez M, Matute E, Rosselli M, Ardila A, Pineda D. NEUROPSI attention and memory: a neuropsychological test battery in Spanish with norms by age and educational level. Appl Neuropsychol. 2007;14:156–170. doi: 10.1080/09084280701508655. [DOI] [PubMed] [Google Scholar]
- Palacio JD, Castellanos FX, Pineda DA, Lopera F, Arcos-Burgos M, Quiroz YT, Henao GC, Puerta IC, Ramirez DL, Rapoport JL, Bailey-Wilson J, Berg K, Muenke M. Attention-deficit/hyperactivity disorder and comorbidities in 18 Paisa Colombian multigenerational families. J Am Acad Child Adolesc Psychiatry. 2004;43:1506–1515. doi: 10.1097/01.chi.0000142279.79805.dc. [DOI] [PubMed] [Google Scholar]
- Pineda D, Ardila A, Rosselli M. Neuropsychological and behavioral assessment of ADHD in seven- to twelve-year-old children: a discriminant analysis. J Learn Disabil. 1999;32:159–173. doi: 10.1177/002221949903200206. [DOI] [PubMed] [Google Scholar]
- Pineda DA, Puerta IC, Aguirre DC, Garcia-Barrera MA, Kamphaus RW. The role of neuropsychologic tests in the diagnosis of attention deficit hyperactivity disorder. Pediatr Neurol. 2007;36:373–381. doi: 10.1016/j.pediatrneurol.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Quintana MI, Andreoli SB, Jorge MR, Gastal FL, Miranda CT. The reliability of the Brazilian version of the composite international diagnostic interview (CIDI 2.1) Braz J Med Biol Res. 2004;37:1739–1745. doi: 10.1590/s0100-879x2004001100020. [DOI] [PubMed] [Google Scholar]
- Quintana MI, Gastal FL, Jorge MR, Miranda CT, Andreoli SB. Validity and limitations of the Brazilian version of the composite international diagnostic interview (CIDI 2.1) Rev Bras Psiquiatr. 2007;29:18–22. [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Renou S, Hergueta T, Flament M, Mouren-Simeoni MC, Lecrubier Y. Diagnostic structured interviews in child and adolescent’s psychiatry. Encephale. 2004;30:122–134. doi: 10.1016/s0013-7006(04)95422-x. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans le cas d’encephalopathie traumatique. Arch Psychol. 1941;28:286–340. [Google Scholar]
- Rey A. Test de copia de una figura compleja. TEA Ediciones; Madrid: 1994. [Google Scholar]
- Riccio CA, Hynd GW, Cohen MJ, Hall J, Molt L. Comorbidity of central auditory processing disorder and attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1994;33:849–857. doi: 10.1097/00004583-199407000-00011. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Arias-Vasquez A, Altink ME, Buschgens CJ, Fliers E, Asherson P, Faraone SV, Buitelaar JK, Sergeant JA, Oosterlaan J, Franke B. Neuropsychological endophenotype approach to genome-wide linkage analysis identifies susceptibility loci for ADHD on 2q21.1 and 13q12.11. Am J Hum Genet. 2008;83:99–105. doi: 10.1016/j.ajhg.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselli M, Ardila A, Bateman JR, Guzman M. Neuropsychological test scores, academic performance, and developmental disorders in Spanish-speaking children. Dev Neuropsychol. 2001;20:355–373. doi: 10.1207/S15326942DN2001_3. [DOI] [PubMed] [Google Scholar]
- Rubio-Stipec M, Peters L, Andrews G. Test-retest reliability of the computerized CIDI (CIDI-Auto): substance abuse modules. Subst Abus. 1999;20:263–272. doi: 10.1080/08897079909511411. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Monuteaux MC, Weber W, Faraone SV. Neuropsychological functioning in nonreferred siblings of children with attention deficit/hyperactivity disorder. J Abnorm Psychol. 2000;109:252–265. doi: 10.1037/0021-843X.109.2.252. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Monuteaux MC, Valera E, Doyle AE, Faraone SV. Impact of gender and age on executive functioning: do girls and boys with and without attention deficit hyperactivity disorder differ neuropsychologically in preteen and teenage years? Dev Neuropsychol. 2005;27:79–105. doi: 10.1207/s15326942dn2701_4. [DOI] [PubMed] [Google Scholar]
- Tannock R. Etiology/risk factors for ADHD—cognitive and behavioral correlates. In: William H, editor. NIH consensus development conference on diagnosis and treatment of attention deficit hyperactivity disorder. Natcher Conference Center, National Institutes of Health; Bethesda, MD: 1998. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. The Psychological Corporation; New York: 1949. [Google Scholar]
- Wechsler D. Manual for the Wechsler intelligence scale for children—revised. The Psychological Corporation; New York: 1974. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]