Abstract
Objective:
The industrial aluminum production process is addressed. The purpose is to give a short but comprehensive description of the electrolysis cell technology, the raw materials used, and the health and safety relevance of the process.
Methods:
This article is based on a study of the extensive chemical and medical literature on primary aluminum production.
Results:
At present, there are two main technological challenges for the process—to reduce energy consumption and to mitigate greenhouse gas emissions. A future step may be carbon dioxide gas capture and sequestration related to the electric power generation from fossil sources.
Conclusions:
Workers' health and safety have now become an integrated part of the aluminum business. Work-related injuries and illnesses are preventable, and the ultimate goal to eliminate accidents with lost-time injuries may hopefully be approached in the future.
Industrial production of primary aluminum is carried out by the Hall–Héroult process, named after its inventors, who independently of each other, in 1886, developed and patented an electrolytic process in which aluminum oxide (or alumina, Al2O3) is dissolved in an electrolyte consisting mainly of molten cryolite (Na3AlF6) and aluminum fluoride (AlF3). In modern aluminum electrolysis cells, several prebaked carbon anodes are dipped into the electrolyte, and oxide ions from the dissolved alumina are discharged electrolytically onto the anodes as an intermediate product. Nevertheless, the oxide immediately reacts further with the carbon anodes and gradually consumes them by formation of gaseous carbon dioxide (CO2). Below the electrolyte, there is a pool of molten aluminum, which is the cathode in the cell. Fresh aluminum is formed from aluminum-containing anions that are reduced at the electrolyte–aluminum interface.
AN ALUMINUM PRODUCTION PLANT (SMELTER)
The buildings where the electrolysis cells are located (the potrooms) are huge. They can be more than 1 km long, in some cases about 50 m wide, and perhaps 20 m high. In a potroom, between 100 and 400 electrolysis cells are arranged in series, with the cathode of one cell electrically connected to the anode of the next cell, to form a cell line (which in the industry jargon is called a potline). Series connection allows the use of high-voltage rectifiers; and for modern potlines, the maximum voltage now may be well above 1500 V. Although the current of the potlines are kept constant, the cells have individual voltage adjustments to satisfy special technological requirements, such as heat balance, cell-operating conditions, and the age and condition of the cathode. Figure 1 shows a modern potline.
FIGURE 1.

A modern potline with high-amperage side-by-side prebake cells.
Modern potlines now typically have amperages from about 300 kA and up to about 600 kA, which are the largest cells in operation at present. These cells are placed side by side as shown in Figure 1, to reduce the adverse magnetic effects of the high electrical current and also to reduce the heat loss from the cells. Older cells, which can have amperages less than 200 kA, are often placed end to end, but not always.
Day and night, each of these cells produces this valuable metal in large amounts, maybe 100 kg or more every hour. Added together, the aluminum production in the plant can be huge, and the largest aluminum plants in the world now report an annual production close to or even more than 1 million metric tons.
The process is still far from fully automated. Cranes are moved back and forth for transportation and changing of anodes and for removal of aluminum from the cells. Large vehicles transport the metal out of the potline building. They bring the metal to the cast house for further treatment and casting of aluminum products.
The upper part of the cell is called the cell superstructure. Aluminum hoods are there to facilitate collection of the anode gases and fluoride vapors from the electrolyte, and these are sent to the fume-treatment plant. Large vertical aluminum bars (called anode risers) conduct the current from the negative cathode of the neighbor cell to the positive anode of the present cell.
A layer of alumina plus solid electrolyte cover the top of the anodes. There should preferably be open holes in the crust along the center line between the two rows of anodes, where the alumina is added automatically to the electrolyte. Underneath the crust, there is a 15- to 20-cm deep layer of electrolyte, and with 10 to 20 cm of molten aluminum beneath. These two melts have different densities, and as such, they do not mix with each other. Alumina is dissolved in the electrolyte and is electrolyzed at the cathode to form molten aluminum.
There is a high ambient temperature in the potrooms, due to the heat emitted from the cells. Ambient temperature in potlines is poor if there is no designed natural ventilation system, as may be the case for Søderberg potlines. In hot regions, heat exposure is a serious problem in the potrooms, and extensive programs for information, acclimatization, and preventive measures are set up. It is, therefore, important to have strict rules for fluid intake, rest areas, and measures to be taken when the operators show signs of heat stress and heat exhaustion. Some individuals are more at risk than others; for example, high body mass index is a well-known risk factor for reduced tolerance to heat exposure.
The high electric current flowing through each cell creates strong static magnetic fields, and because these cannot be felt by the human body, the fields can cause damage to watches and credit cards. People will not be allowed to enter the potroom if they have a pacemaker, as these also can be affected.
In these huge systems, there are lots of joints that used to be packed with asbestos material, usually chrysotile. Previously, asbestos was also used to cover metal that had leaked from the cells. Asbestos is a known carcinogen; however, a study supported by the Norwegian Cancer Institute could not find any asbestos-related lung cancer among former and present operators in the Norwegian aluminum industry.1
THE ALUMINUM PRODUCTION PROCESS—FROM ART TO SCIENCE
Throughout the years since its invention in 1886, the industrial aluminum production has developed from art to science. Steadily increased understanding of the process has been achieved as a result of extensive research and development work, particularly in the latter half of the twentieth century, both in aluminum plants and in several universities and academic institutions. During monitoring and intervention of the process, the cell operators are constantly faced with decision-making situations. Theoretical and practical training of the operators and their supervisors and superintendents give them the skills and knowledge needed to improve steadily cell operation and work practices.
The overall electrochemical reaction for industrial production of molten aluminum may be written as follows:
| [1] |
This reaction is simple and shows that the two main raw materials are alumina and carbon and that there are two chemical products, molten aluminum, which we want, and gaseous CO2, which we really do not want.
The amounts of raw materials used in the process are illustrated in Fig. 2. Alumina is consumed according to the stoichiometric ratio predicted from Equation 1. The consumption of alumina theoretically amounts to 1.89 kg per kilogram of aluminum produced. Nevertheless, in practice, the real value for the specific alumina consumption in the industry is a little higher, typically 1.93 kg, because the alumina supplied is not 100% pure. It always contains small amounts of impurity oxides like Na2O, CaO, Fe2O3, and SiO2. Furthermore, from the aforementioned chemical equation, we see that we produce three-fourth moles of CO2 per mole of aluminum. One-half mole of alumina should then theoretically react with 0.33 kg of carbon and produce 1 kg of aluminum and 1.22 kg of CO2. Nevertheless, because of other reactions of carbon with both oxygen and CO2, between 0.40 and 0.45 kg carbon are consumed per kilogram of aluminum in practice. This is called the net anode consumption, and this in turn produces about 1.5 kg of CO2 per kilogram of aluminum.
FIGURE 2.
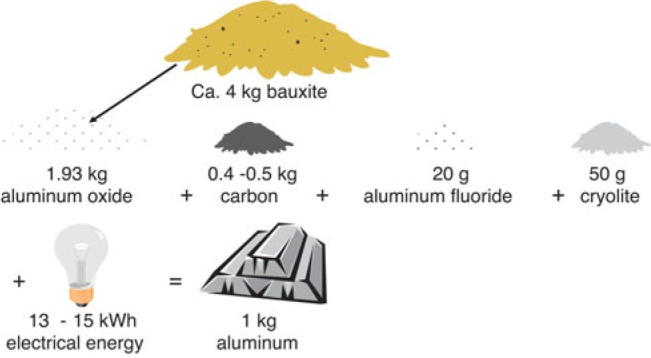
The amounts of raw materials needed to produce 1 kg of aluminum.
Alumina must be added regularly to the electrolyte to keep the normal electrolytic production going on continuously. Older aluminum electrolysis cell designs had large and infrequent additions of alumina, while modern cells are equipped with so-called point feeders. Alumina is then supplied automatically from an overhead bin or hopper, which is built into the superstructure of the cell. Two to six volumetric feeders successively add about 1 kg of alumina to the electrolyte every minute or so. These small additions increase the ability for the alumina powder to dissolve, mix, and disperse rapidly in the electrolyte. The average alumina concentration in the electrolyte is usually kept within the narrow range of 2 to 4 wt% alumina. Higher concentrations may lead to the formation of excessive amounts of undissolved alumina, which in the industry is called sludge. Because of its higher density, the sludge is collected at the bottom of the molten metal. Sludge has no useful purpose in the cell, and it is unwanted, mainly because it contributes to increase the electrical resistance in the cell and thereby the cell voltage.
On the contrary, low alumina concentrations in the electrolyte can give a dramatic change in the anode process, which leads to a so-called anode effect. An anode effect causes a very high cell voltage, perhaps up to 30 to 40 V instead of the normal 4.0 to 4.5 V, by forming an electrically insulating layer of gas underneath the anodes. The anode gas composition then changes abruptly from almost pure CO2 (g) to mainly CO (g) and also some gaseous perfluorocarbon compounds, CF4 (g) and smaller amounts of C2F6 (g). These are greenhouse gases with high global-warming potential and extremely long atmospheric life times (of the order of 10,000 years).
The formation of these gases can be lowered by reducing the anode effect frequency (the number of anode effects per cell per day) and the anode effect duration (given in minutes). All aluminum producers have now made significant progress in reducing their emissions of perfluorocarbon gases. Most modern prebake cells can now be controlled to operate for more than 1 week and even for several months without an anode effect.
Before leaving the topic of anode effects, it should be mentioned that 70% to 80% of the anode gas evolved is then CO (g). In some cases, termination of anode effects may require manual intervention, and the operators may then breathe in this poisonous gas. Nevertheless, even if this effect has probably not been studied in detail, the concentration of CO (g) in the working atmosphere in potlines may be so low that it is not harmful to humans.
In addition to being the raw material for production of aluminum, alumina also acts as a thermal insulator when it is placed on top of the self-formed solid crust above the electrolyte, thereby reducing heat losses. Alumina is also used for covering the top of the anodes, which conserves heat and minimizes air burning of the carbon anodes. More frequently, a mixture of alumina powder and crushed pieces of solid electrolyte is used.
The third major role fulfilled by alumina is a very important one. Alumina is used to capture fluoride emissions from the cells by anode gas cleaning, by use of the so-called dry scrubbing method. Alumina powder adsorbs the hydrogen fluoride (HF) gas evolved, and it also entraps fluoride condensates, mainly particulate sodium tetrafluoroaluminate (NaAlF4). The resulting alumina is called secondary alumina and is then used as feed material to the cells. The cleaned exhaust gas, containing CO2 and smaller amounts of perfluorocarbon gases, is discharged to the atmosphere.
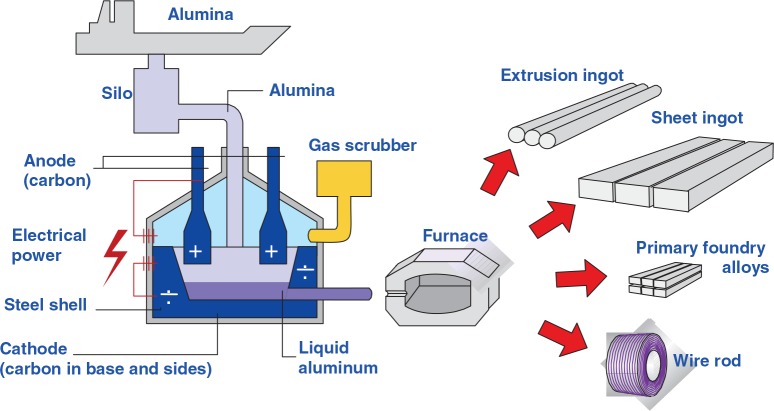
Figure 3 shows a flow sheet of the industrial aluminum production process. The processes made before the metal is sent to the cast house are called upstream processes, while the processes in the cast house to make extrusion ingots, sheet ingots, primary foundry alloys, and/or wire rods are called downstream processes. A much more detailed description of the electrolysis process can be found in several textbooks, for example, in Grjotheim and Kvande2 and Thonstad et al.3
FIGURE 3.
Flow sheet of the aluminum production process.
RAW MATERIALS USED IN THE ALUMINUM PRODUCTION PROCESS
Bauxite Mining
Aluminum is the most abundant metallic element (8 wt%) in the earth's crust. It is found in nature in a wide variety of minerals combined with oxygen, silicon, and other metals. Because all the aluminum compounds are very stable chemically, aluminum is never found as a metal in nature.
It is mainly bauxite that is used as raw material for the aluminum industry. Bauxite contains typically between 40 and 60 wt% alumina, with smaller amounts of iron, silicon, and titanium compounds, as well as many other trace impurities.2
One of these impurities is beryllium, the concentrations of which vary from less than 1 parts per million to several parts per million in different bauxite mines. Because beryllium is toxic to humans and can be found to some extent in the potroom atmosphere (usually in concentrations less than 100 ng/m3), some concerns have been raised. Nevertheless, studies in various aluminum plants have found a very low incidence of sensitization against beryllium among the potroom workers.4,5
Alumina
In alumina refineries, bauxite is processed into pure alumina. The Bayer process extracts alumina by caustic digestion of crushed bauxite at high temperature and high pressure in an autoclave, followed by clarification, precipitation, washing, and finally calcination to produce pure anhydrous alumina. This is a white powder that looks like ordinary table salt. Alumina has a high melting point, more than 2050°C, and is chemically a very stable compound. This is why so much energy is required to produce aluminum from alumina.
Electric Power
A large amount of electrical energy is, therefore, required to reduce alumina to aluminum. The most modern aluminum smelters need close to 13 kWh to produce 1 kg of aluminum, while the world average value for the direct current energy consumption now may be close to 14 kWh/kg Al.2
Data for the mix of power sources for aluminum production in 2009 show the following percentages, when including Chinese aluminum production*:
| Coal | 51% |
| Hydro | 39% |
| Natural gas | 8% |
| Nuclear | 2% |
Whereas hydropower traditionally has been the dominant electricity source for aluminum production, we see that coal now accounts for more than 50% of the world production.
Energy typically counts for roughly 30% of the aluminum production cost,† and its price is, therefore, highly significant for the economy of the process. Energy consumption of aluminum production has decreased in recent years by means of technological improvements of the process. Nevertheless, with the global demand for electric energy increasing steadily, energy savings in all parts of the production process will be a very important task for aluminum producers in the coming years. New aluminum plants will be built only in areas with available and cheap electric power.
Prebaked Carbon Anodes
Today, all aluminum smelters use carbon anodes in their electrolysis cells. Carbon is a reasonably good electrical conductor, and more importantly, it is able to withstand the action of the corrosive fluoride-containing molten electrolyte at about 960°C. Furthermore, carbon is an active part of the electrochemical reaction, and thereby, it contributes to reduce the cell voltage by 1.0 V. As such, electrical energy is saved by burning carbon. On the basis of Equation 1, we may consider carbon as a raw material in aluminum production, because carbon is consumed by the anode reaction.
A typical prebaked anode is made from a mixture of petroleum coke, coal tar pitch, and butts. An anode butt is the rest of the used anode removed from the cell during anode changing. The butts content in the new anodes can vary, but normally it is between 15% and 25%.2
The main constituent of prebaked carbon anodes is calcined petroleum coke. When crude oil is refined, there is a residue of about 30% from the distillation unit. This residue is treated at about 450°C and 4 to 5 bar pressure to form what we call green coke. The process is known as delayed coking. This implies that coking is used to upgrade waste products from oil refineries that would otherwise have to be sold as low-value fuels.
The coke residue from petroleum refining is quite pure, and therefore, it has been the major source of carbon for anodes. This coke requires calcining at about 1200°C to remove volatile constituents and increase its density, strength, and porosity before it is blended into the anode mix. The product, now called calcined petroleum coke, is then ready to be shipped to the anode production plant in the aluminum smelter.
In addition, the carbon anodes contain 13 to 16 wt% coal tar pitch to be used as a binder material, thus binding the coke and butts particles together in the anode.2 The pitch is distilled from the coal tar produced when coke for the iron and steel industry is made from coking of bituminous coal. Coal tar pitch is a complex hydrocarbon mixture consisting of thousands of compounds, of which only a few hundred have been identified chemically. Liquid pitch can be kept at about 200°C and transported by ship to the aluminum smelters.
In the anode production process, the petroleum coke and the recycled anode material (butts) are crushed and sieved into fractions, which are then blended to obtain an optimum particle size composition. This blend is mixed with sufficient coal tar pitch (usually between 13 and 16 wt%) to allow molding into green anode blocks by pressing or by vibrating. Before these green anodes can be used in the electrolysis cells, they have to be prebaked in a special anode baking furnace at about 1150 to 1200°C, causing the pitch to carbonize and forming strong and dense anode blocks.
To provide electrical contact and physical support, an aluminum or copper rod with an iron yoke and from one to six iron stubs are attached to the anode. The stubs are placed into cavities on the top of the carbon anode and are attached by applying molten cast iron around the stubs. The purpose of the cast iron is to make a good mechanical and electrical connection between the carbon anode and the stubs. This process is called anode rodding.
The Søderberg Anode
There are two basic anode designs presently in use. Prebaked anodes are the dominating type now. The other main anode type is the Søderberg anode, invented by the Norwegian engineer Carl Wilhelm Søderberg (1876 to 1955). The Søderberg anode can be characterized as a monolithic, continuous, and self-baking anode. This type of anode is also made from a mix of petroleum coke and coal tar pitch, but here the mix typically contains between 25 and 28 wt% pitch,2 which is about twice the pitch content used for making prebaked anodes. Small briquettes of Søderberg anode paste are then made, and these are added regularly to the top of the Søderberg anode.
Although the anode paste passes slowly downward through a rectangular steel casing, it is baked into an electrically conducting solid composite by pyrolysis of the pitch from the waste heat generated in the electrolyte and in the anode itself. The baked portion of the anode extends past the steel casing and into the molten electrolyte. The briquettes added on the top replace the part of the anode that is being consumed at the working surface on the bottom.
Electric current usually enters the Søderberg anode through vertical spikes or studs, although in some older Søderberg cells, side-entry horizontal studs are used. These spikes are pulled and reset to a higher level when they approach the lower anode surface. Søderberg anodes have an electrical resistivity that is about 30% higher than that of prebaked anodes. Søderberg anodes suffer from resulting lower efficiency and great difficulty in collecting and disposing of anode baking fumes, especially polycyclic aromatic hydrocarbons (PAHs). These hydrocarbons are mainly volatiles from the pitch used in the anode paste, but the PAH emissions can also depend on the conditions of the anode top. Polycyclic aromatic hydrocarbons consist of many different organic compounds, which have been shown to be carcinogenic. Benzo(a)pyrene is considered as the most dangerous compound here. Its concentration is, therefore, measured regularly both in the working atmosphere and in the air outside of the Søderberg potroom.
In Søderberg plants, epidemiological studies have found an increased incidence of bladder cancer, which is considered to be caused by PAH exposures. Some studies have also found an increase in lung cancer among Søderberg potroom workers. This is also believed to be caused by PAH exposures.1,6
The trend now is that Søderberg cells are gradually being replaced by prebaked anode cells, even though the former save the capital cost, labor, and energy required to manufacture the latter. Particularly in the recent 5 to 10 years, many Søderberg potlines have been shut down, because they cannot cope with the new stringent emissions limit values to air for total fluorides, gaseous hydrogen fluoride, particulate fluorides, and dust. Nevertheless, there are still several Søderberg plants in operation in Russia, Europe, Brazil, and the United States.
ELECTROLYTE MATERIALS
The four main functions of the electrolyte are as follows:
To be the solvent for alumina to enable its electrolytic decomposition, forming molten aluminum and CO2
To pass electricity from the anode to the cathode
To provide a physical separation between the cathodically produced aluminum metal and the anodically evolved CO2 gas
To provide a heat-generating resistor that allows the cell to be self-heating
Cryolite usually comprises 75 to 80 wt% of the molten electrolyte, which typically also contains excess aluminum fluoride (9% to 12%), calcium fluoride (4% to 7%), and alumina (2% to 4%). These three additives lower the melting point of the electrolyte, as well as the cell operating temperature, and they increase the efficiency of the process.
Cryolite
The mineral cryolite is a double fluoride of sodium and aluminum and has a stoichiometric composition very close to the formula Na3AlF6 and a melting point of about 1011°C. It has been found in substantial quantities only in Greenland. Cryolite was mined extensively there in the early twentieth century, but the mine is now essentially exhausted. Cryolite, thus, has to be made synthetically now. It can be produced by reacting hydrofluoric acid with an alkaline sodium aluminate solution according to the overall reaction:
| [2] |
Aluminum Fluoride
Aluminum fluoride, AlF3, may comprise as much as 9 to 12 wt% of the electrolyte, when it is recorded in excess of the amount represented by the cryolite composition. Aluminum fluoride is consumed during normal operation by three major mechanisms. First and foremost, aluminum fluoride reacts with sodium oxide that is always added as an impurity material in alumina. This amount has to be replaced, and it requires addition of about 20 kg of aluminum fluoride per metric ton of aluminum produced to keep the AlF3 concentration in the electrolyte constant.
The second consumption mechanism is that aluminum fluoride can be depleted by hydrolysis due to moisture in different forms in the cell:
| [3] |
Gaseous hydrogen fluoride is extremely hazardous. Fortunately, fume capture and gas scrubbing efficiencies have been improved strongly in aluminum smelters, and very little HF (g) is emitted now to the potroom and the surrounding atmosphere.
Finally, losses of aluminum fluoride by vaporization from the electrolyte are appreciable. The most volatile species evolved from the electrolyte is sodium tetrafluoroaluminate vapor, NaAlF4 (g). It has a partial pressure of 400 to 600 Pa over the operating electrolyte, depending on its composition and temperature. Fortunately, more than 98% of the fluorides, including HF (g), are collected by the gas cleaning process in the fume-treatment plant and are returned to the cell together with the secondary alumina.
Exposures to dust and fluorides in the prebake potrooms are typically over short periods with extremely high exposures for certain tasks, followed by longer periods with very low exposures. HF (g) may reach high concentrations, up to 100 parts per million, during short episodes during certain job procedures. These peak exposures are considered to be a risk factor in causing occupational asthma. Occupational asthma among smelter workers has been extensively reported in several epidemiological studies. Recent reports from Australia and Norway, however, have shown a considerable decrease in the incidence of occupational asthma among potroom workers.7,8 Recent methods of simultaneous exposure measures and video surveillance when the operators carry out their jobs visualize the exposure during work performance and help to mitigate exposure through work practice changes.9
Calcium fluoride is seldom added intentionally to the electrolyte. Because of the small amount of calcium oxide impurity in the alumina (typically only about 0.035 wt%), it attains a stable steady-state concentration of calcium fluoride of 4 to 7 wt% in the melt. At this level, a minor amount of calcium is codeposited into the aluminum, while some is emitted as a calcium compound, maybe CaCO3 vapor, in the off-gas at a rate equal to its rate of introduction with the alumina.
Finally, a few words are needed about safety when working with molten cryolite. Many reactive substances can result in danger by contact with electrolyte and metal, and moisture is the most hazardous. The molten electrolyte can give splashes and must be treated with respect and awareness. The electrolyte and also the metal have a temperature of about 950°C. In addition, the fluoride-containing electrolyte is corrosive. Remember that electrolyte burns must be cooled immediately with temperate water for at least an hour.
THE CATHODE AND CATHODE MATERIALS
In the cell, the electrolyte and the molten aluminum are contained in a preformed carbon lining that has refractory and thermally insulating materials inside a steel shell. Graphitic or semigraphitized materials are now used extensively as prebaked carbon cathode blocks. The other materials used are silicon carbide (SiC) sidewall bricks and carbonaceous ramming paste. Several steel current collector bars are embedded in the carbon cathode and conduct the electric current away from the cell. Figure 4 shows a schematic drawing of an aluminum electrolysis cell.
FIGURE 4.
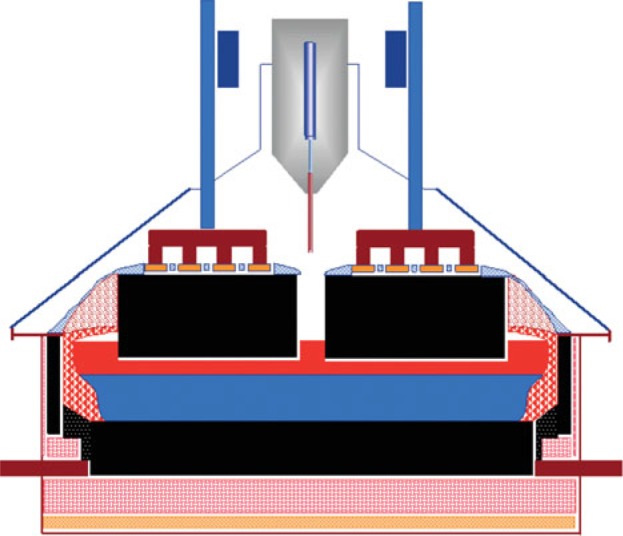
Schematic drawing of an aluminum electrolysis cell.
Insulation bricks are used to insulate the cathode thermally. These bricks are porous and vulnerable to penetration of electrolyte components through the cathode blocks. The insulation materials are protected by using refractory bricks, and sometimes a special barrier material made of steel, glass, or other materials can be added. Refractory bricks also have some insulating effect, so that the temperature in the insulation materials does not become too high.
It should be noted here that the word cathode is used in the aluminum industry to describe the whole container of electrolyte and metal. Nevertheless, the real acting cathode from an electrochemical point of view is the top surface of the molten aluminum pool. Thus, the aluminum atoms are formed from aluminum-containing ions that are reduced at the electrolyte–aluminum interface.
Cells are now typically 9 to 18 m long, 3 to 5 m wide, and 1 to 1.5 m deep. The depth of the operating cell cavity is relatively low, however, only 0.4 to 0.5 m. Although carbon is the material known to withstand best the combined corrosive action of molten fluorides and molten aluminum, even carbon would have a very limited life time in contact with the electrolyte at the sides of the cell if it was not protected by a layer of frozen electrolyte. Now silicon carbide is used as sidewall material, but this material is also corroded by the electrolyte and needs to be protected.
TECHNOLOGICAL KEY OPERATIONAL PARAMETERS FOR THE ALUMINUM SMELTING PROCESS
Current efficiency (CE) is a very important technological parameter used to describe the performance of the process. One may simply say that current efficiency is the part of the current that is used to produce aluminum. According to Faraday's first law, 1 kAh of electric current should theoretically produce 0.335 kg of aluminum, but only 90% to 96% of this amount can be obtained in industrial cells. Loss in metal production is typical for all electrolytic processes and is, therefore, very difficult to avoid completely. The principal loss mechanism in aluminum electrolysis is recombination of the anodic and cathodic products, the so-called “back reaction,” where aluminum back-reacts with CO2 to form alumina and carbon monoxide.
To account for these losses and to measure the electrochemical efficiency of the process, the concept of current efficiency has been introduced in the industry as the ratio between measured and theoretical production rates:
| [4] |
Here, p is the measured production rate (kg/h), and po is the theoretical production rate (kg/h), calculated from Faraday's first law.
In addition to the “back reaction,” there are several other mechanisms accounting for additional small losses in current efficiency. A new cell lining will absorb sodium. Fortunately, the cell lining becomes saturated early in the cell's life, but until this occurs, current efficiency will be low. When a metal dissolves in a molten salt, it usually imparts electronic conductivity to the melt, thereby lowering the current efficiency. This is because the electrons “steal” current without producing any metal. Some investigators have found a small electronic conductivity for cryolite-based melts in contact with molten aluminum in laboratory cells, while others have not; so more studies are needed here. In any case, this contribution is small compared to the “back reaction.”
Energy consumption (EC) is given in kWh/kg Al and can be calculated by the following equation:
| [5] |
Here Voltage is the operational cell voltage given in volts (V), and CE is current efficiency given as a fraction (and not in percent here). Energy consumption is the best technological parameter in aluminum production, because it also includes current efficiency.
Energy efficiency is defined as the part of the electrical energy (amperage multiplied by voltage) that is used to produce aluminum. Typical values are only between 45% and 50%, even in modern cells. The rest of the energy produces heat, which is lost to the surroundings. One important task for the industry in the future is to reduce the energy consumption and thereby increase energy efficiency.
CELL OPERATION
The following operational procedures have to be performed regularly, although at different time intervals in the potlines:
Alumina feeding
Anode change and anode covering
Metal tapping
Addition of aluminum fluoride
Rack raising
Alumina feeding is nowadays automated through the use of point feeders, and therefore, anode changing is now the most labor-intensive manual routine operation. Prebaked anodes must be replaced at regular intervals when they have reacted down to about one fourth of their original size. This occurs after 25 to 30 days. Because each cell may have between 16 and 40 prebaked anodes, this means that one anode in average has to be changed approximately each day in every cell. Modern potlines are equipped with sophisticated overhead cranes that allow the operator to sit in an air-conditioned cabin and perform the anode-changing operation by manipulating robotic arms. Alternatively, the use of anode-changing vehicles is also common in many plants.
Anode changing causes the largest operating disturbance in cells with prebaked anodes. When a new, cold anode is inserted, weighing about 1 ton, a layer of solid electrolyte quickly freezes on the underside of the anode, and it can take up to 24 hours to melt this layer completely. This reduces the temperature of the electrolyte locally, as the new anode draws very little current during this remelting process. The solid electrolyte layer is a poor electrical conductor. It also disturbs the anodic current distribution in the cell. Several aluminum plants are now changing two anodes simultaneously, and this introduces an even larger thermal and electrical disturbance in the cell.
There will unavoidably be some dust present in the air inside the potrooms. This potroom dust consists both of alumina and fluorides from the electrolyte and gives exposure of fine particulates to operators. The presence and compositional nature of these airborne particles have been discussed recently by Wong et al.10
Particularly during anode change, significant densities of nanoparticles with a median particle size smaller than 20 nm can be recorded in the vicinity of the cells. They are possibly produced when the molten mass is exposed to the colder environment. The surface of these particles is large, and HF, SO2, Be, and other particles on the surface represent potential health hazards of which there is limited knowledge at present. After being released into the air, the nanoparticle mode is subject to ageing, leading to a shift in the size distribution toward larger particles.11
Individual aerosol particles of aluminum oxide/cryolite with a high cryolite content immediately become surrounded by a surface water film when exposed to relative high humidity (such as in the upper respiratory tract). Because gaseous HF and SO2 are highly soluble in water, the aerosol particles may act as carrier for these gases into the lower respiratory tract.12
Anode covering is usually done about 4 hours after anode changing. Because the anodes are hot, we need a method of protecting them from air oxidation (air burn) and heat loss. The anode cover material must not introduce any metal contamination, and hence a mixture of alumina and recycled electrolyte is used. The composition of the anode cover can play an important part by reacting with the fume and fusing the under surface of the crust. Poor anode cover practice can result in air burn of the anodes.
The spent anodes, the butts, are cleaned outside the cell in a separate butts-cleaning station. First the adhering electrolyte and alumina are removed and are recycled to the cells. The cleaned butts are then crushed and reused as a carbon raw material in the manufacture of new prebaked anodes.
Removal of molten aluminum from the cells is called tapping, and this is also a labor-intensive routine manual operation. The spout of a vacuum ladle, or crucible, is dipped into the metal pad in the cell, and the metal is then siphoned out and into the crucible by the suction from an air ejector system. The molten metal is then weighed and transported to the cast house. Although overhead cranes are usually used to assist the manual tapping work practices, also specially constructed motorized vehicles can be used. But otherwise, these tapping procedures are identical.
Addition of aluminum fluoride is performed automatically in modern cells. One or more silos for aluminum fluoride are built into the superstructure of the cell, and the addition is done through the hole in the crust made by the point feeder breakers for alumina addition.
The last manual routine operation in the aforementioned list is called rack raising or anode beam raising. Because the anodes are consumed, the anode beam, which is holding all the anodes in position, has to be gradually lowered downward into the cell to maintain a constant anode–cathode distance. The cathode, which is the metal surface, is kept approximately at the same position by regular tapping. Finally, the position of the anode beam becomes so low that it reaches a stopping device that can be electronic or physical. The beam then has to be raised by use of a special anode beam–raising machine carried by an overhead crane. All anodes are first connected electrically to this machine and are held in their correct positions in the electrolyte, while the anode clamps are loosened and the anodes are electrically disconnected from the anode beam. The beam is then raised to its upper position, the anodes are refastened to the anode beam in their correct position again, and the machine is finally removed from the top of the cell superstructure. This operation is carried out every 2 to 3 weeks for each cell, and it then raises the anode beam by about 20 cm.
If potline operators are asked what they think is the most risky or hazardous work they do on the cells, the answer will probably be beam raising. The reason for this is that sparks can occur through electric arcing, if the electrical contact with the anodes is not satisfactory. The possible loss of electrical continuity during beam raising if an anode effect occurs on the cell is the underlying reason for operators' concern here. A potline open circuit, with explosive consequences, can and has indeed occurred, with a high risk of fatality for those in the proximity.
The extensive work to run the potrooms, as well as continuous maintenance, causes much noise at times, and the operators usually have to wear devices for noise protection. In spite of this, hearing loss is a risk that has to be managed by the industry.
CELL PREHEATING AND START-UP
Before a new cell can be started, it has to be preheated. There are two main preheating methods that are used now. One is based on electrical resistance preheating with a thin bed of small coke or graphite particles (2 to 5 mm usually) between the anodes and the cathode. The other main method is flame preheating, where gas burners are used. Resistance preheating is the oldest method here, but both methods are now frequently in use in the aluminum industry.
The objective of preheating is to heat the cell materials as close as possible to the operating temperature of a normal cell, which really means 960°C for the cathode surface and the underside of the anodes. This provides a careful transition from the cold cell to the operating temperature, and it contributes to avoid thermal shock of the cathode materials when the molten electrolyte is added. The higher the preheat temperature, the easier is the cell start-up. Nevertheless, this target is difficult to reach in practice, because unwanted hot spots on the cathode surface may then be hard to avoid. The target for the average cathode surface temperature at the end of the preheating is, therefore, usually around 900°C.
The actual start-up is done by adding molten electrolyte to the cell and raising the anodes carefully when the electric current is cut in. Then the electrolysis process starts. The temperature preferably should be kept less than 1000°C in the first few hours and then lowered gradually to increase the aluminum production efficiency.
During the start-up and early operation of the cell, many of the hoods are removed so that the operators will be able to observe the movement of the molten electrolyte. In this period, there is formation of fluoride vapors and also HF (g), which can cause asthma-like symptoms for the operators when this gas in inhaled. The use of proper respiratory protection is, therefore, very important during this type of work, and certainly also in other work where people are exposed to fluoride vapors from the electrolyte.
MAGNETOHYDRODYNAMICS
The large electric currents used in modern aluminum electrolysis cells (300 to 600 kA) generate strong magnetic fields, both outside and within the cell. These magnetic fields interact with the high electric currents and exert so-called Lorentz forces. These forces are strong enough to produce movement of liquid conductors. Molten aluminum, which in itself is nonmagnetic, is influenced by these strong magnetic fields. The reason is that molten aluminum then acts as a movable current conductor.
The magnetic movement of the metal may give rise to high metal velocities, metal height variations, and metal instabilities. To minimize the adverse consequences of these effects, it is desirable to compensate for the magnetic forces by a special arrangement of the interconnecting electrical current conductor system.
Calculation of the magnetic fields and electric current flow patterns is complicated. Nevertheless, for many years now, at least four decades, powerful computer programs have been designed to make these calculations and describe the fluid-dynamic consequences. These calculations have been refined to the point where cells with amperages up to 600 kA have been designed and such cells are now in operation.
Even if these static magnetic fields usually range from 5 to 15 mT in the potrooms, there is no indication that that they cause any serious health effects.13,14 Nevertheless, during maintenance work in the rectifiers where the static fields may be even higher, there are some operators who experience magnetophosphenes (visual blurring or light flashes) and some operators who have dental fillings with mercury, describe metal taste in the mouth.
SAFETY IN ALUMINUM PRODUCTION PLANTS
Safety is number 1 priority for all aluminum producers. This area has received considerable attention in the last decades and rightly so. Workers' health and safety have become an integrated part of the aluminum business. Protection of the employees is critical, because there are numerous possible exposures to the employees in this industry. Nothing is more important than to send the employees home safely at the end of the working day. Good working environment is crucial, and good housekeeping is a prerequisite here.
In principle, all accidents at work can be avoided. The expression “Accidents don't happen; they are caused” is a good philosophy. Thus, work-related injuries and also illnesses are preventable. The ultimate goal to eliminate accidents with lost-time injuries will hopefully be approached in the future.
ALUMINUM IN THE HUMAN BODY
Aluminum is a metal that is all around us. People wear it, cook in it, and eat and drink it. Much food is packaged in aluminum foil, and beverage cans are usually made from aluminum. Daily intake of aluminum may range from 10 to 100 mg, the majority being through oral routes. Nevertheless, most of this will be excreted. Still the amount of aluminum in the human body ranges between 50 and 150 mg, with an average value of about 65 mg. About half of the aluminum in the human body is stored in the bones, and about one quarter in the lungs.
Measurements of aluminum in serum of industrial workers have always been difficult because so much sampling equipment contains aluminum, and thus, contamination of the sample may easily occur. Healthy subjects usually have less than 10 μg/L aluminum in serum. Postshift serum aluminum will increase to some extent in potroom workers. With normal renal function, aluminum is readily excreted in the urine. So far, there are no consistent findings of an increased incidence of either aluminum-induced diseases or neurological disorders among potroom workers.
INNOVATIVE ALTERNATIVE ALUMINUM PRODUCTION TECHNOLOGIES
Electrolysis Cells With Inert Anodes
The concept of inert anodes for aluminum electrolysis is by no means a new idea. It was suggested first by Charles Martin Hall already in his famous patent from 1886. Hall tried to use copper anodes, but he soon found out that they did not work in practice. Copper dissolved quickly in the electrolyte, and he had to give up this idea. Carbon anodes have since then been the only possible practical solution for the anode material in industrial alumina reduction cells.
Nevertheless, in 2000, Alcoa announced that it was working hard with inert anodes, and 2 years later, Alcoa's chief executive officer Alain Belda stated that “the science is proved, so we have an inert anode, but we have not proved the commercial aspects.” Of course, this led to highly increased interest, curiosity, and activities, and it inspired several aluminum companies and research institutions to start work to try to find an inert anode material. Much of this work is surely unpublished and will probably remain so for proprietary reasons. Still, the open literature now offers a vast amount of individual publications and patents on inert anode materials. A comprehensive literature review of inert anodes was given by Galasiu et al15 in 2007.
So what is actually meant by an inert anode? The word inert means chemically nonreactive, and a completely inert anode will, therefore, not react chemically or electrochemically in the electrolysis process. This means that it would ideally not be consumed by the anode reaction. An inert anode has been given many names, like dimensionally stable anode, nonconsumable anode, and passive anode.
With inert anodes in the electrolysis cell, the total cell reaction will be very simple:
| [6] |
We see immediately that this reaction is different from Equation 1. Here, oxygen is formed at the anode, which environmentally is a highly favorable gas, compared with CO2.
The dream would of course be to have anodes that lasted as long as the cell life, which now may be up to 5 years or longer. Anode changes would then not be necessary after the cell has been started. Nevertheless, it is a chemical fact that all materials have a finite solubility in the very corrosive cryolitic melts at about 960°C, so a totally inert anode will probably never be found for use in these electrolytes. Thus, what we really are looking for is a slowly consumable anode. But how slow a consumption rate can we tolerate?
The potential inert anode material must have low solubility and low reactivity in the electrolyte and also show good chemical resistance against the anodically produced hot oxygen gas. In addition, the anode material should be physically stable at the operating temperature, mechanically robust and resistant to thermal shock. There will be extreme requirements for keeping the wear rates of these anodes low. A wear rate of the order of 10 mm/y may perhaps be sufficient, but lower values would certainly be beneficial.
There are two main challenges in the development of inert anode materials. In addition to the requirement that the anode material should survive sufficiently long in the electrolyte, the metal produced must be of adequate purity. The impurity metal content in the aluminum can indeed be very significant for the customers, and the need for making pure aluminum will become more stringent in coming years. The corrosion products, caused by the dissolution of the anode material into the electrolyte, predominantly will end up in the metal phase and thereby contaminate the aluminum produced. Hence, the anode corrosion should be low enough to give impurity contents corresponding to the present specifications for smelter grade aluminum.
There are three principal potential advantages in favor of developing a new cell technology with inert anodes:
Cost reduction. All costs directly associated with the consumable carbon anode will then be eliminated, including the capital saving and raw materials costs by eliminating the need for the carbon anode fabrication, baking, and also the anode rodding plant. These cost savings may be significant. It has been indicated that there might be 25% to 30% lower capital costs for a new potline with inert anode cell technology.3
Environmental friendliness. Inert anodes would eliminate all greenhouse gas generation and emissions from the electrolysis cells. Smelters would no longer generate CO2, carbon monoxide, or perfluorocarbon gases (CF4 and C2F6), because carbon would no longer be used as anode material. Carbon residues (butts) will of course disappear. In addition, the fluoride and dust emissions during anode change will also be eliminated.
Improved occupational health issues. Inert anodes would reduce the work practices associated with the present prebaked carbon anode change. The frequency of anode changes will certainly be drastically reduced with inert anodes. Working conditions in the potrooms would also be improved by avoiding all anode effects.
What types of materials are the most promising for inert anodes? Two main paths have emerged so far:
The cermet conducting electrodes, which is used by Alcoa. The word cermet means a combination of ceramics and metals and consists of a mixture of oxides and metals, NiFe2O4 + NiO + Cu + Ag
The so-called “metal anodes” were previously developed by Moltech. These were metal alloys made of Ni + Fe + Cu
Both these groups have reported large-scale trials retrofitting the conventional cell design. In reality, these two types of the electrodes become extremely similar at the anode–electrolyte interface, because this electroactive surface necessarily has to be an oxide, irrespectively of what materials the anode substrate is made of.16
Present Conclusion on Inert Anodes
Several companies and research institutions have studied inert anode materials actively in recent years. It is no doubt that substantial progress has been made in inert anode development during the last decade, particularly regarding the two main challenges: anode wear and metal purity in inert anode cells.
UC Rusal has now started rig testing of a small 3 kA cell with inert anodes. On success of the rig tests, the company plans to begin production tests on its inert anode cells in 2015 at the Krasnoyarsk aluminum smelter.17
Operation of inert anode cells will certainly be very challenging. The commercial aspects of inert anodes have not yet been proven. At present, a number of engineering problems remain to be solved. It is presently impossible to say when, or even if, this may be a proven technology. In any case, there will probably go several years before the issues mentioned previously will be solved satisfactorily. Maybe, cell retrofitting will not be the preferred development path in the future, and it is possible that a completely new cell design will be necessary.15
Carbothermic Production of Aluminum
History
The idea of carbothermic reduction of alumina to aluminum is also an old dream. Aluminum–copper alloys with about 15% aluminum were produced industrially by this method already in 1886,18 the same year as the present industrial process was invented. In the 1920s, Al–Si alloys with 40% to 60% aluminum were produced in Germany, and about 10,000 tons of these alloys were produced annually up to 1945.
The first attempt to produce pure aluminum by carbothermic reduction of alumina was made around 1955. In France, Pechiney worked on the process from 1955 to 1967, but terminated the program for technical reasons. Reynolds worked on an electric arc furnace to produce aluminum from 1971 to 1984. Alcan acquired information from Pechiney and continued their research, but stopped in the early 1980s. Alcoa tried to develop the process to produce Al–Si alloys from 1977 to 1982.
Nevertheless, in 1998, Alcoa started the carbothermic production project again together with Elkem R&D in Norway. Elkem had a long-time experience with modern silicon furnace technology and came up with the idea to use their experience to design a new type of tailor-made high-temperature electric reduction reactor for carbothermic production of aluminum. Alcoa had a strong fundamental understanding and a long-time experience with carbothermic production of aluminum from the work in the 1960s, 1970s, and 1980s. Together Alcoa and Elkem then agreed to try this again, and the work is still going on, as we will see later.
But what is really meant by carbothermic aluminum production? As the name says, the carbothermic method is to use carbon and heat to reduce alumina to aluminum, according to the overall reaction:
| [7] |
The reaction proceeds close to and higher than 2000°C and produces CO as the primary gas. From Equation 7, it is easily seen that the carbothermic process is different from the present electrolytic process. No electrolysis is involved here, but electrical energy is of course required. In the carbothermic process, alternating current is used to heat up the raw materials alumina and carbon.
The Three Main Steps in the Carbothermic Process
The reduction of alumina to aluminum must take place in three stages (see Kvande et al,18 Bruno,19 and Johansen et al20), which are characterized by three different phase combinations:
| [8] |
| [9] |
| [10] |
Nevertheless, reaction equations with pure condensed solid phases will give only an approximate description of the chemical system involved here. Therefore, these reactions do not give a correct description of the reactions that will actually occur. Equations 8 and 9 will really give production of a molten slag, which contains a molten mixture of alumina and aluminum carbide.
The molten aluminum phase will contain some dissolved carbon, and therefore, it can be considered chemically as an Al–C alloy. Equation 10, therefore, actually gives production of a molten aluminum–carbon–(carbide) alloy rather than pure aluminum. This molten alloy will float on top of the molten slag. An additional reaction step required would then be the production of pure aluminum (refining) from the alloy containing aluminum–carbon–(carbide).
The two most difficult steps here are the production of the aluminum–carbon alloy and the subsequent refining of the alloy. In addition, a gas scrubber is needed for collection of all the aluminum-containing gases that will evaporate from the furnace at these high temperatures. This is also an engineering challenge. A simplified flowchart of the process is shown in Figure 5.
FIGURE 5.
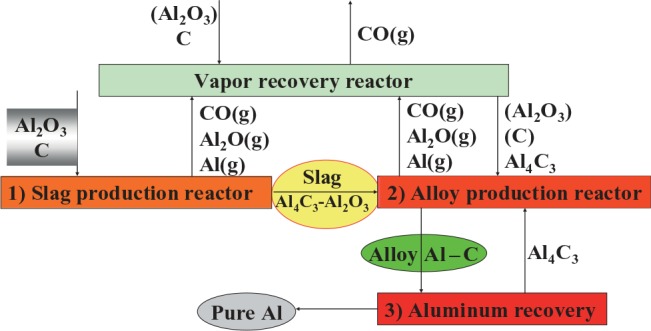
Flowchart of the aluminum carbothermic technology–advanced reactor process concept of Alcoa and Elkem.20,21
Greenhouse Gas Emissions From Carbothermic Aluminum Production
The carbothermic reaction produces CO as the primary gas, and the gaseous by-product is, therefore, different from the present industrial process. Equation 7 shows that theoretically 1.5 mol of CO are formed per mole of aluminum, which on a mass basis means 1.56 kg of CO per kilogram of aluminum produced.
In the atmosphere, CO generally has a lifetime of several months before it converts to CO2 by natural atmospheric processes. Still it is obvious, from both health and environmental reasons, that poisonous CO gas cannot be emitted directly into the atmosphere from the carbothermic aluminum production. The gas will have to be burnt to CO2, and this reaction can be written as follows:
| [11] |
This means that 1.56 kg of CO will form 2.44 kg CO2 per kilogram of aluminum. This value does not include any CO2 resulting from electrode consumption during carbothermic reduction, but this is expected to be small here. Thus, the theoretical production of CO2 is then increased by about 60% in the carbothermic process, compared with the present process.
In practice, this means that the entire amount of CO produced has to be captured. In a recent article by White et al,21 it is reported that the CO generated from the process is more than 90% pure and it can then potentially be collected and used as a chemical. The CO gas can, for example, be used industrially as raw material for several different chemical products. According to these authors,21 this can include use as a reductant for removing Fe2O3 from bauxite, or as a reductant in direct reduced iron processes. If CO is used as fuel, it would produce CO2, which then will have to be stored by carbon capture and sequestration.
The conclusion is then that the carbothermic process itself will increase the specific greenhouse gas emissions by about 60%. Nevertheless, the process promises to reduce energy consumption from 13 to 11 kWh/kg Al. For hydroelectric power, this does not matter much for the overall greenhouse gas emissions. Nevertheless, if the energy used to produce aluminum is coal-fired power and if the energy consumption can be reduced from 13 to 11 kWh/kg Al, this in itself can contribute to reduce the overall greenhouse gas emissions by about 20%. Even if this reduction may be considered to be significant, it can be concluded that unless all the CO (g) is captured and used industrially, the carbothermic process is not the solution to minimize the carbon footprint from aluminum production.
CONCLUDING REMARKS
The outlook of the primary aluminum industry may be summarized as follows: This is now a mature industry, which presently (2013) suffers severely from low aluminum prices and a very challenging market situation.
Technologically, the present aluminum production process can be a close-to-zero greenhouse gas producer. The first step, which is actually ongoing, is to focus on lower specific energy consumption, and also to eliminate the occurrence of anode effects. Furthermore, it is possible to reduce the inherent production of CO2 by reducing the net carbon anode consumption, although this reduction can only be perhaps 10% or even less with the existing carbon anode technology. Here, an inert anode, if such a material can be found and developed for use in industrial aluminum production, would represent a remarkable technological breakthrough, because then oxygen is formed at the anodes instead of CO2. On the contrary, another alternative process, carbothermic production of aluminum, would increase the CO2 emissions if the produced CO is not captured and stored.
A natural step to save energy in the present electrolysis process would be to recover energy from the main heat loss sources of the cells, the cathode sidewalls and the anode gas exhaust systems. A future step may be CO2 gas capture and sequestration related to the electric power generation. Finally, collection and cleaning of the CO2 from the electrolysis process itself may perhaps be a technical possible scenario in the future.
Data reported to the International Aluminium Institute in 2009.
Source: CRU, based on average weighted global aluminum production.
The authors declare no conflicts of interest.
REFERENCES
- 1.Romundstad P, Haldorsen T, Andersen A. Lung and bladder cancer among workers in a Norwegian aluminium reduction plant. Occup Environ Med. 2000;57:495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grjotheim K, Kvande H, eds. Introduction to Aluminium Electrolysis—Understanding the Hall–Héroult Process. 2nd ed. Düsseldorf, Germany: Aluminium-Verlag; 1993:199–217 [Google Scholar]
- 3.Thonstad J, Fellner P, Haarberg GM, et al. Aluminium Electrolysis—Fundamentals of the Hall–Héroult Process. 3rd ed. Düsseldorf, Germany: Aluminium-Verlag, Marketing and Kommunikation GmbH; 2001:1–359 [Google Scholar]
- 4.Nilsen AM, Vik R, Behrens C, et al. Beryllium sensitivity among workers at a Norwegian aluminium smelter. Am J Ind Med. 2010;53:724–732 [DOI] [PubMed] [Google Scholar]
- 5.Taiwo OA, Slade MD, Cantley LF, et al. Beryllium sensitization in aluminum smelter workers. J Occup Environ Med. 2008;50:157–162 [DOI] [PubMed] [Google Scholar]
- 6.Armstrong B, Hutchinson E, Fletcher T. Cancer Risk Following Exposure to Polycyclic Aromatic Hydrocarbons (PAHs): A Meta-Analysis. Research Report 068. London, England: London School of Hygiene and Tropical Medicine for the Health and Safety Executive; 2003. ISBN 0-7176-2604-0. [Google Scholar]
- 7.Kongerud J, Grønnesby JK, Magnus P. Respiratory symptoms and lung function of aluminum potroom workers. Scand J Work Environ Health. 1990;16:270–277 [DOI] [PubMed] [Google Scholar]
- 8.Donoghue AM, Frisch N, Ison M, et al. Occupational asthma in the aluminum smelters of Australia and New Zealand: 1991–2006. Am J Ind Med. 2011;54:224–231 [DOI] [PubMed] [Google Scholar]
- 9.Rosen G, Andersson I-M, Walsh PT, et al. A review of video exposure monitoring as an occupational hygiene tool. Ann Occup Hyg. 2005;49:201–217 [DOI] [PubMed] [Google Scholar]
- 10.Wong DS, Tjahyono NI, Hyland MM. Visualising the sources of potroom dust in aluminium smelters. In: Suarez CE, ed. Light Metals 2012. Orlando, FL: TMS (The Minerals, Metals and Materials Society); 2012:833–838 [Google Scholar]
- 11.Thomassen Y, Kock W, Dunkhorst W, et al. Ultrafine particles at workplaces of a primary aluminium smelter. J Environ Monit. 2006;8:127–133 [DOI] [PubMed] [Google Scholar]
- 12.Weinbruck S, Benker N, Kock W, et al. Hygroscopic properties of the workroom aerosol in aluminium smelter aerosols: a case for transport of HF and SO2 into the lower airways. J Environ Monit. 2010;12:448–454 [DOI] [PubMed] [Google Scholar]
- 13.Moen BE, Drabløs PA, Pedersen S, et al. Symptoms of the musculoskeletal system and exposure to magnetic fields in an aluminium plant. Occup Environ Med. 1995;52:524–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moen BE, Drabløs PA, Pedersen S, et al. Absence of relation between sick leave caused by musculoskeletal disorders and exposure to magnetic fields in an aluminium plant, Bioelectromagnetics. 1996;17:37–43 [DOI] [PubMed] [Google Scholar]
- 15.Galasiu I, Galasiu R, Thonstad J. Inert Anodes for Aluminium Electrolysis. Düsseldorf, Germany: Aluminium-Verlag, Marketing & Kommunikation GmbH; 2007:1–207 [Google Scholar]
- 16.Welch B. Inert anodes—the status of the materials science, the opportunities they present and the challenges that need resolving before commercial implementation. In: Bearne G, ed. Light Metals 2009. Orlando, FL: TMS (The Minerals, Metals and Materials Society); 2009:971–977 [Google Scholar]
- 17.4-Traders. AlCircle Newsletter. February 7, 2013 [Google Scholar]
- 18.Kvande H, Huglen R, Grjotheim K. Carbothermal production of aluminium—technically possible, but today economically impossible? In: Kvande H, ed. Proceedings of the International Symposium Arranged in Honour of Professor Ketil Motzfeldt. Trondheim, Norway: NTH; 1991:75–102 [Google Scholar]
- 19.Bruno M J. Aluminum carbothermic technology comparison to Hall–Héroult process. In: Crepeau PN, ed. Light Metals 2003, San Diego, CA: TMS (The Minerals, Metals and Materials Society); 2003:395–400 [Google Scholar]
- 20.Johansen K, Aune JA, Bruno M, et al. Aluminum carbothermic technology Alcoa–Elkem advanced reactor process. In: Crepeau PN, ed. Light Metals 2003. San Diego, CA: TMS (The Minerals, Metals and Materials Society); 2003:401–406 [Google Scholar]
- 21.White CV, Mikkelsen Ø, Roha D. Status of the Alcoa carbothermic aluminum project. In: Downey JP, Battle TP, White JF, eds. International Smelting Technology Symposium (Incorporating the 6th Advances in Sulfide Smelting Symposium). San Antonio, TX: TMS (The Minerals, Metals and Materials Society); 2012:81–88 [Google Scholar]