Abstract
ADHD (Attention Deficit Hyperactivity Disorder) has a complex, heterogeneous phenotype only partially captured by Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria. In this report, latent class analyses (LCA) are used to identify ADHD phenotypes using K-SADS-IVR (Schedule for Affective Disorders & Schizophrenia for School Age Children-IV-Revised) symptoms and symptom severity data from a clinical sample of 500 ADHD subjects, ages 6–18, participating in an ADHD genetic study. Results show that LCA identified six separate ADHD clusters, some corresponding to specific DSM-IV subtypes while others included several subtypes. DSM-IV comorbid anxiety and mood disorders were generally similar across all clusters, and subjects without comorbidity did not aggregate within any one cluster. Age and gender composition also varied. These results support findings from population-based LCA studies. The six clusters provide additional homogenous groups that can be used to define ADHD phenotypes in genetic association studies. The limited age ranges aggregating in the different clusters may prove to be a particular advantage in genetic studies where candidate gene expression may vary during developmental phases. DSM-IV comorbid mood and anxiety disorders also do not appear to increase cluster heterogeneity; however, longitudinal studies that cover period of risk are needed to support this finding.
Keywords: Children, DSM-IV, Oppositional defiant disorder
1. Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is one of the most common neuropsychiatric disorders with estimated prevalence rates of 5% to 10% in school age children (Scahill and Schwab-Stone, 2000), 4% in college students (Heiligenstein et al., 1998) and ~2.5% in adults (Heiligenstein et al., 1998; Kooij et al., 2005).
The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) defines three ADHD clinical phenotypes, inattentive (IA), hyperactive–impulsive (HI) and combined (C) based on symptom count (6 for either IA or HI and 6 in each category for C) (Association, 2004). In an epidemiological sample of 1480 Swedish twins, parental assessments at ages 8–9, and later at ages 13–14 indicated a high stability of ADHD symptoms over this 5-year period (Larsson et al., 2004). Subsequent follow-up at ages 16–17 indicated that hyperactivity–impulsivity decreased while inattention remained the same (Larsson et al., 2006). In a Finnish population study, the most prevalent ADHD subtypes were combined for childhood and inattentive for adolescents (Hurtig et al., 2007) and a later age of onset for IA is also reported by another study (Willoughby et al., 2000). Changes in subtypes are also reported in clinical samples. A longitudinal ADHD study of 4–6 year olds found that 37% of combined (C), 50% of inattentive (IA) met criteria for a different subtype at least twice during an 8-year time span while almost all hyperactive–impulsive (HI) children remitted or shifted to another subtype (Lahey et al., 2005).
Statistical methods are currently being used to identify more distinct homogenous ADHD subgroups. Latent Class Analysis (LCA), a non-parametric variant of cluster analysis that combines the probability of reported symptoms (without using symptom number cutoffs) and overall symptom profile, has been applied to ADHD data derived from U.S. (Hudziak et al., 1998; Neuman et al., 1999; Neuman et al., 2001, 2005; Todd et al., 2001; Volk et al., 2005, 2006), Australian (Rasmussen et al., 2002, 2004), and Dutch (Althoff et al., 2006) population-based twin studies. In the U.S. and Australian (Althoff et al., 2006) population based twin studies. In the USA and Australian twin samples, six to eight distinct heritable ADHD classes (few symptoms; mild inattentive; talkative/impulsive; mild combined; mild hyperactive/inattentive; severe inattentive; severe combined; severe hyperactive impulsive) have been identified. The three severe classes (severe combined, severe inattentive, severe hyperactive impulsive) overlap with the DSM-IV clinical subtypes. The DSM-IV inattentive subtype was found in several latent classes and the severe inattentive latent class contained some but not all DSM-IV identified cases as well as some subjects without a DSM-IV diagnoses (Rasmussen et al., 2002; Todd et al., 2001; Volk et al., 2005). Over half of the subjects with mild combined subtype also did not meet criteria for DSM-IV ADHD suggesting the presence of a subtype not detected by DSM-IV criteria (Volk et al., 2006). In the twin sample from the Netherlands that utilized parent and teacher ratings (CPRS-R:S and CTRS-R:S), three to four classes were identified that corresponded to the mild and severe forms of inattentive, hyperactive-impulsive and combined groups (Althoff et al., 2006). In another population study of adolescents based on self-reported ADHD symptoms, Rohde et al. (2001) identified eight LCA clusters, one which was unaffected, one with mild hyper-activity and the others with combined symptoms.
LCA applied to a large sample of 4422 clinically referred 6–18 year old ADHD children, deNijs and colleagues identified five classes; three of those classes had high, medium and low levels of both inattentive and hyperactive symptoms while two classes had high IA and lower HI scores. No hyperactive–impulsive cluster was identified that questioned the validity of this subgroup (de Nijs et al., 2007).
Twin studies with heritability estimates of 51% to 90% (Faraone et al., 2005) indicate a high genetic contribution to ADHD. Numerous genetic association studies to date have identified several high-quality candidate ADHD genes (Faraone and Khan, 2006), although none have been confirmed unequivocally. Reasons for this include small sample sizes (leading to low statistical power), genetic heterogeneity and limited genotyping of variants in candidate genes. Most studies have also used only DSM-IV defined diagnostic phenotypes. In one study utilizing both DSM-IV and LCA criteria, Todd et al. (2003) reported a significant association for a CHRNA4 polymorphism for both phenotype classifications. However, in a later study reanalyzing data from three studies investigating associations between polymorphisms of DRD4 and DAT genes and DSM-IV subtypes, a significant association was reported between the 3′DAT VNTR and LCA-defined severe combined ADHD, whereas no significant associations were previously found (Todd et al., 2005).
Comorbid conditions, including oppositional defiant disorder (35%), conduct disorder (30–50%), anxiety disorders (25%), mood disorders (15–75%) and learning disabilities (10–92%), noted in clinical ADHD samples (Biederman et al., 1991; Brown et al., 2001; Cantwell 1996; Jensen et al., 1997; Spencer, 2006) add another layer of complexity to the ADHD phenotype. High rates of comorbid disorders also occur in epidemiological samples suggesting that this is not an artifact of referral bias (Angold et al., 1999; Caron and Rutter, 1991). A population study of female twins assessing ADHD comorbid patterns using LCA, identified nine significant clusters of which three were highly heritable; (1) IA without comorbidity; (2) IA with ODD; (3) Combined ADHD with ODD, separation anxiety and depressive symptoms (Neuman et al., 2001). In a second population-based ADHD study that included male and female twin pairs (ages 7–19), LCA identified the following five significant clusters; (1) no comorbidity; (2) depression; (3) ODD with CD; (4) ODD; (5) ODD, CD and depression (Volk et al., 2006). Higher levels of comorbid ODD, CD and to a lesser degree mood and anxiety problems were reported in clusters with the higher levels of ADHD symptoms by de Nijs et al. (2007). In multigenerational families in a genetically isolated Paisa community in Colombia that identified seven significant ADHD LCA clusters, ADHD was also found to segregate with ODD, CD, ODD and CD, and CD and alcohol use and dependence. These comorbid ADHD phenotypes were found to have a significant linkage at loci 8q24, 2p21–22.3, 5p13.1–p13.3, 12p11.23–13.3, 8q15 and 14q21.1–22.2 providing support for pleiotropy (Jain et al., 2006).
In this study, we report latent class clusters identified in a cohort of 500 ADHD probands, ages 6–18, who along with their biological parents, participated in an ADHD genetic study. These clusters, representing more phenotypically refined sub-groups than those identified using broad DSM-IV categories, will be used in genetic association studies.
2. Methods
2.1. Participants
This sample includes 500 ADHD probands consecutively recruited in an ongoing ADHD genetic study aimed at recruiting 500 parent/child trios with one or more ADHD probands (ages 6–18). All subjects were of European descent. Individuals of other ancestries were not included because haplotype frequencies vary substantially across major world populations (Chang et al., 1996), lowering power of the study to detect genetic association if multiple ethnic groups were included. The study was approved by the Institutional Review Boards of The Children’s Hospital of Philadelphia (Protocol #2003-1-3125) and the University of Pennsylvania School of Medicine (Protocol #707843). Parents provided consent and children assent.
2.2. Procedures and exclusionary criteria
Families were recruited from pediatric and behavioral health clinics in the Philadelphia area. Phone screenings were conducted to determine age range of 6–18, presence of ADHD symptoms, ancestry, availability and willingness to participate in a genetic study from both biological parents. Exclusionary criteria included gestational age <36 weeks, IQ scores <75, inability to understand and complete the K-SADS interview, major medical (excluding asthma), neurological (e.g. seizures, fetal alcohol syndrome, plumbism), and neuropsychiatric disorders (pervasive developmental disorder, bipolar disorder, major depressive disorder with symptoms starting prior to ADHD or where ADHD symptoms were found to occur primarily during depressed episodes, psychotic disorders). Disruptive behavioral disorders, other mood disorders and anxiety disorders were not excluded. Siblings meeting inclusion and exclusion criteria were also invited to participate in the study, but their participation was not required.
The cohort of 500 subjects was recruited from 2003–2008. Thirty-six subjects who had signed consent/assent were excluded from the study. Twenty subjects passed the phone screen but did not meet ADHD criteria on K-SADS. Five subjects met criteria for ADHD, however in three subjects these symptoms were considered to be due to a major depression and in two subjects anxiety symptoms were significantly contributing to the ADHD. Three other subjects who also met criteria for ADHD were excluded, one meeting criteria for cyclothymia, one for bipolar disorder, and one for psychotic symptoms. Five subjects were excluded due to medical history that became evident during the office visit and included one subject each for sleep apnea, IQ<70, severe hypoglycemia at birth, absence seizures and febrile seizures. Two children agreed to participate and signed assent but then did not want to answer K-SADS questions and I interpreted this as their way of retracting assent while one child had severe social anxiety that prevented him from completing the interview.
2.3. Measures
A child psychiatrist (JE), trained in the administration of the K-SADS, assessed diagnostic status by administering a K-SADS-P IVR interview to the parent(s) and child separately. This semi-structured interview provides diagnoses occurring within the last twelve months of the present episode (PE) and for the last week (LW). K-SADS-P IVR rates each symptom on a graded severity scale thus allowing for a composite severity rating score. The domains of the K-SADS IVR include behavioral, mood, anxiety, psychotic disorders. Each symptom is rated on a graded severity scale (2 — slight; 3 — mild to moderate; 4 — severe), thus allowing for a composite severity rating score and it has modules for both current and lifetime diagnosing (Ambrosini, 2000). Permission to videotape K-SADS interviews was included in the informed consent and 10% of the videotapes were randomly chosen and reliability maintained with the senior K-SADS trainer (PA). Cognitive ability was assessed by reviewing prior IQ assessments completed before study participation or the Wechsler Abbreviated Scale of Intelligence (WASI) was administered to children not previously tested. Other children, included but not formally tested, were performing at academic grade level and were able to understand and complete the K-SADS. Socioeconomic status (SES) was measured by the four factor Hollingshead Scale (Hollingshead, 1975).
2.4. Data analysis
Consistent with DSM-IV criteria, subjects with 6 or more symptoms of inattention (K-SADS-P IVR severity scores >3) but fewer than 6 symptoms of hyperactivity and impulsivity were identified as inattentive subtype. Subjects with 6 or more hyperactive–impulsive symptoms (severity scores >3) and fewer than 6 symptoms of inattention were categorized as Hyperactive–Impulsive subtype and subjects with 6 or more symptoms in both dimensions were categorized as combined subtypes. Age of onset was not used and children with impairing ADHD symptoms who did not meet age criteria were included.
For comorbid mood and anxiety disorders, the K-SADS-P IVR diagnostic domain is keyed to the Research Diagnostic Criteria (RDC) (Spitzer et al., 1978) for those syndromes similar in youths and adults. All other diagnoses are DSM IVR based and were made excluding the DSM hierarchical requirements.
Latent Class Cluster Analysis (LCCA) models containing one through twelve classes were fitted to the data using Latent GOLD 3.0.1 software (Statistical Innovations, Belmont, MA). Latent GOLD uses both Expectation/Maximization (EM) and Newton–Raphson algorithms to find the maximum likelihood of each model after estimating model parameters (Vermunt et al., 2002). To avoid ending up with local solutions (a well-known problem in LCA), we used multiple sets of starting values as automatically implemented in Latent GOLD. Because we were dealing with sparse contingency tables, we estimated P-values associated with L2 statistics by means of parametric bootstrap (500 replicates) rather than relying on asymptotic P-values.
To obtain a bootstrap estimate of the P value corresponding to the difference in log-likelihood value between two nested models, such as two models with different numbers of latent classes or different number of discrete factors, we followed a procedure where the −2LL-difference statistic is defined (LLH0−LLH1), where H0 refers to the more restricted hypothesized model (say a K-class model) and H1 to the more general model (say a model with K+1 classes) (Vermunt and Magidson, 2005) Replication samples were generated from the probability distribution defined by the ML estimates under H0. The estimated bootstrap P-value is defined as the proportion of bootstrap samples with a larger −2LL-difference value than the original sample (Vermunt and Magidson, 2005). Overall, this approach was comparable with the selection of the best fitting model when using parsimony criteria such as the BIC.
As covariates for the model, we used gender, age, and the fact of being part of an extended sibship. Age was analyzed as a continuous covariate to define clustering membership in the whole group without establishing any conditional age-based stratification. As the effect of age on cluster definitions was very significant we performed several analyses to determine correlation of age with each cluster defined by the model fitting best the data. In addition, we also performed analyses on individuals with an age below 12 years since 75% of the sample was below this age range. Similarly, as our sample included sib groups in addition to trios, we tested the effect of aggregation by family membership while contrasting this fact as a covariate following the same approach that was used for age. Significant differences among models including or excluding these two covariates (i.e. age and familial aggregation) were compared between them by both standard comparisons of likelihood ratio between hypothesis and bootstrapping as described above. Initially, we did not consider the presence of interactions between variables and the basic assumption of local independence of the standard latent class model was supported. Next, we relaxed the local independence assumption by allowing for interactions between variables, as well as for direct effects of covariates on variables (Hagenaars, 1988; Vermunt, 1997). Latent GOLD calculates bivariate variable–variable and variable–covariate residuals that can be used to detect which pairs of observed variables are more strongly related. Therefore, bivariate residuals greater than 3.84 were included iteratively for each model to identify significant correlations between the associated variable–variable and variable–covariate pairs inside each class (for 1 degree of freedom, bivariate residuals greater than 3.84 indicate statistical significance at the 0.05 level).
Latent class analysis utilized the K-SADS-P IVR summary scores for the past year. Severity scores of 2 or less indicated absence of symptoms and severity scores 3 and higher indicated presence of symptoms. For ADHD, 9 inattentive symptoms, 5 impulsive symptoms and 6 hyperactive symptoms were included. The K-SADS includes impulsive symptoms that cover both DSM-III and DSM-IV criteria; hence, there are 5 impulsive symptoms rather than the 3 noted in DSM-IV criteria.
Although LCA is being done on a family-study sample such that the non-independence assumption of the LCA is violated, analyses were applied only to affected children independently of the parents’ status. Therefore such a bias does not apply for these particular analyses.
3. Results
3.1. Descriptive
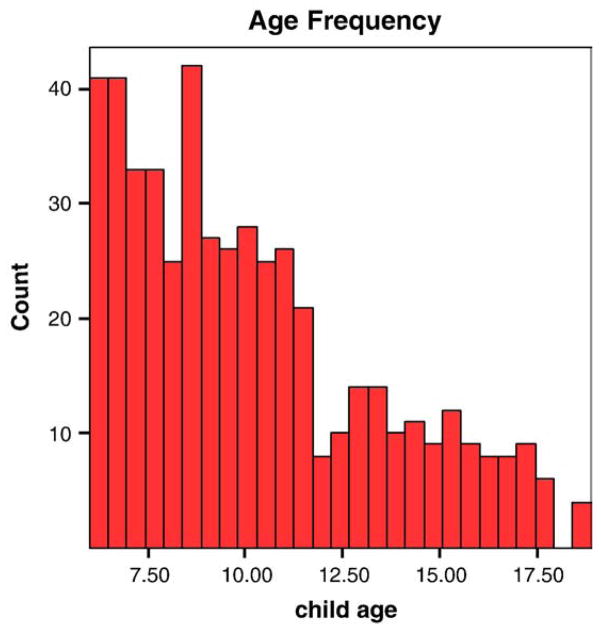
The cohort consists of 500 subjects (398 families; 39 families with 2 siblings and 8 families with 3 siblings). The age range is 6–18 years with a mean of 10.2 (S.D. 3.2). A histogram of age frequency indicates that at the time of assessment, 50% of the cohort was below 9.5 years; 75% and 90% were below 12.1 years and 15.2 years, respectively, at the time of assessment. Birth weight range was 5.25–12 lbs (mean 7.84 lbs; S.D. 1.1). IQ scores are available for 347 subjects (71% of the sample) and range from 75 to 147 (mean 104.4; median 109; S.D.: 13.8). SES data are available for 459 subjects (92.6%) with mean of 42.2; median 49; S.D. 10.7 (Fig. 1).
Fig. 1.
Histogram of age frequency of 500 ADHD cases.
3.2. Latent class analyses
Latent class analyses (LCA) that included 500 ADHD cases and all age ranges (6–18 years) identified 6 statistically significant clusters. These are summarized in Table 1 and Fig. 2 includes cluster 6 with severe, clusters 1 and 4 with moderate and cluster 5 with mild combined; cluster 2 with moderate inattentive and mild hyperactivity; cluster 3 with severe inattentive and moderate hyperactivity.
Table 1.
Latent class cluster profiles in 500 ADHD subjects ages 6–18 (367 males/133 females).
| Class | ADHD profile
|
Member (%) | Age (yrs) | Gender distribution (%)
|
|||
|---|---|---|---|---|---|---|---|
| Inattention | Impulsivity | Hyperactivity | Boys | Girls | |||
| 1 | Moderate | Mild | Moderate | 0.39 | 10.4 | 0.70 | 0.30 |
| 2 | Moderate | Mild | Mild | 0.17 | 12.4 | 0.70 | 0.30 |
| 3 | Severe | Moderate | Moderate | 0.15 | 10.8 | 0.80 | 0.20 |
| 4 | Moderate | Moderate | Moderate | 0.15 | 8.3 | 0.76 | 0.24 |
| 5 | Mild | Mild | Mild | 0.07 | 7.8 | 0.84 | 0.16 |
| 6 | Severe | Severe | Severe | 0.07 | 9.1 | 0.70 | 0.30 |
This table lists the 6 ADHD latent class clusters (1–6), their ADHD symptom profile (inattention, impulsivity, hyperactivity), size, age and gender distribution.
Fig. 2.
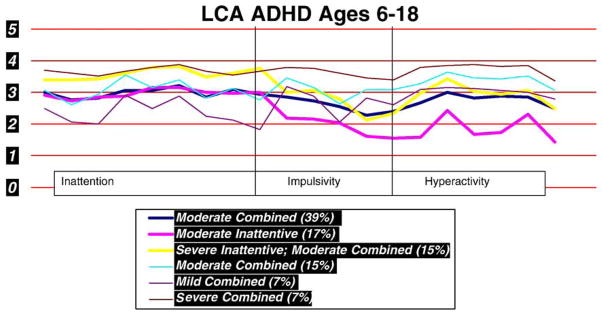
Latent class analyses identified 6 ADHD clusters that utilized the K-SADS-P IVR summary scores for the past year. Severity scores of 2 or less indicated absence of symptoms and severity scores 3 and higher indicated presence of symptoms.
Gender and age were used as covariates. Their distribution within the various clusters is summarized in Table 1. Clusters with mild, moderate and severe combined symptoms had mean ages of 7.8, 8.3 and 9.1 years respectively while clusters with higher inattentive than hyperactive symptoms (clusters 1, 2 and 3) had the older children. The ratio of boys to girls is 2.3 to 1 in clusters 1, 2 and 6 and climbs to 3.1, 4 and 5.25 to 1 in clusters 4, 3 and 5, respectively. Females appear to be represented at similar levels in the moderate and severe clusters, are underrepresented in the severe inattentive cluster and overrepresented in the mild clusters.
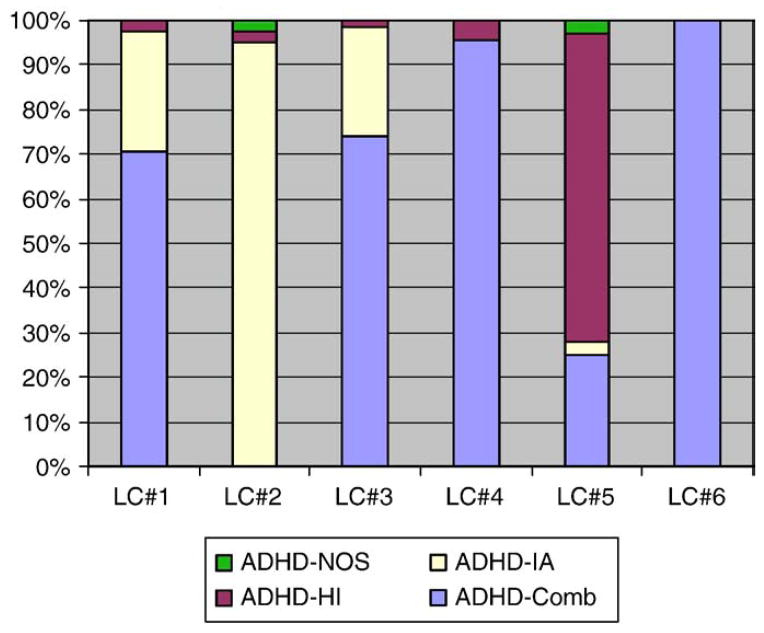
Ascertainment of the cohort using DSM-IV diagnostic ADHD criteria for subtypes indicates that 60% of the cohort met criteria for combined subtypes; 31% for the Inattentive subtype and 9% for the Hyperactive–Impulsive subtype. Table 2 and Fig. 3 indicate that the Combined Subtypes comprise most of clusters 4 and 6, the Inattentive subtype corresponds to cluster 2 and small parts of clusters 1 and 3 while the hyperactive–impulsive subtype is located predominantly within cluster 5.
Table 2.
DSM-IV ADHD subtypes in LCA ADHD clusters.
| LC#1
|
LC#2
|
LC#3
|
LC#4
|
LC#5
|
LC#6
|
Total
|
||
|---|---|---|---|---|---|---|---|---|
| 204 | 84 | 73 | 70 | 36 | 33 | 500 | ||
| ADHD-Comb | 1441 | 02 | 541 | 673 | 94 | 333 | 307 | P<.000 |
| 70.6% | 74.0% | 95.7% | 25.0% | 100.0% | 61.4% | |||
| ADHD-IA | 551 | 802 | 181 | 03 | 13 | 03 | 154 | P<.000 |
| 27.0% | 95.2% | 24.7% | 2.8% | 30.8% | ||||
| ADHD-HI | 51 | 21 | 11 | 31 | 252 | 01 | 36 | P<.000 |
| 2.5% | 2.4% | 1.4% | 4.3% | 69.4% | 7.2% | |||
| ADD-NOS | 0 | 2 | 0 | 0 | 1 | 0 | 3 | NS |
| 2.4% | 2.8% | 0.6% |
ADHD IA — inattentive subtype; HI — hyperactive–impulsive subtype; Comb —combined inattentive and hyperactive–impulsive: NOS (sub-threshold ADHD with impairment); cells with similar superscripts are equivalent.
This table summarizes the DSM-IV ADHD subtypes found within clusters 1–6.
Fig. 3.
Clusters 4 and 6 correspond completely to DSM-IV ADHD combined subtype; Cluster 2 corresponds primarily to the inattentive subtype while clusters 1, 3 and 5 have mixed subtypes.
LCA analyses that included ADHD and Oppositional Defiant Disorder (ODD) symptoms and Conduct Disorder (CD) symptoms define 4 clusters. As can be seen in Table 3 and Fig. 4, these include cluster 6 with severe combined ADHD and moderate ODD; cluster 1 with moderate combined and moderate ODD, cluster 5 with moderate combined ADHD and mild ODD and cluster 3 with severe inattentive and mild ODD, cluster 4 with sub-threshold ODD and moderate inattentive and cluster 2 with sub-threshold ODD and moderate combined. DSM-IV ascertained ODD occurred in all ADHD LCA clusters with the greatest representation in clusters 4 and 6 (61.4% and 69.7%) 59.9%) and the least in cluster 2 (14.3%). CD was represented only in ADHD clusters 1, 3 and 4.
Table 3.
DSM-IV comorbid ODD and CD in LCA ADHD Clusters.
| Total n= | LC#1
|
LC#2
|
LC#3
|
LC#4
|
LC#5
|
LC#6
|
Total
|
|
|---|---|---|---|---|---|---|---|---|
| 204 | 84 | 73 | 70 | 36 | 33 | 500 | ||
| ODD | 921 | 122 | 321 | 433 | 161,3 | 233 | 218 | P<.000 |
| 45.1% | 14.3% | 43.8% | 61.4% | 44.4% | 69.7% | 43.6% | ||
| CD | 4 | 0 | 1 | 1 | 0 | 0 | 6 | NS |
| 2.0% | 1.4% | 1.4% | 1.2% |
ODD — oppositional defiant disorder; CD — conduct disorder. Cells with similar superscripts are similar.
This table summarizes comorbid ODD, with the greatest representation in clusters 4 and 6 and least representation in Cluster 2. CD comorbidity is minimal.
Fig. 4.
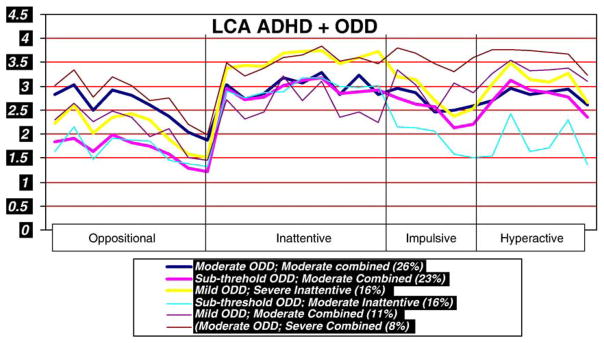
Latent Class Cluster Analyses that included ADHD and ODD symptoms identified 6 clusters, two with moderate and two with mild ODD.
Table 4 summarizes comorbid mood disorders in the different clusters. There were no statistically significant differences in any of the 6 clusters.
Table 4.
Comorbid mood disorders in latent class clusters.
| n= | LC#1
|
LC#2
|
LC#3
|
LC#4
|
LC#5
|
LC#6
|
Total
|
|
|---|---|---|---|---|---|---|---|---|
| 204 | 84 | 73 | 70 | 36 | 33 | 500 | ||
| MDD | 8 | 7 | 7 | 2 | 1 | 1 | 26 | NS |
| 3.9% | 8.3% | 9.6% | 2.9% | 2.8% | 3.0% | 5.2% | ||
| MD | 18 | 13 | 10 | 5 | 3 | 3 | 52 | NS |
| 8.8% | 15.5% | 13.7% | 7.1% | 8.3% | 9.1 | 10.4 | ||
| IMD | 6 | 1 | 0 | 2 | 0 | 0 | 9 | NS |
| 2.9% | 1.2 | 2.9% | 1.8 | |||||
| DD | 361 | 32 | 101 | 81,2 | 31,2 | 41,2 | 64 | 0.02 |
| 17.6% | 3.6% | 13.7% | 11.4% | 8.3% | 12.1% | 12.8% | ||
| IDD | 3 | 1 | 0 | 1 | 0 | 1 | 6 | NS |
| 1.5% | 1.2% | 1.4% | 3.0% | 1.2% | ||||
| DDN | 0 | 1 | 0 | 0 | 0 | 0 | 1 | NS |
| 1.2% | 0.2% | |||||||
| CYCLO | 1 | 0 | 0 | 1 | 0 | 0 | 2 | NS |
| 0.5% | 1.4% | 0.4% | ||||||
| MDDD | 63 | 18 | 20 | 16 | 6 | 8 | 131 | NS |
| 30.9% | 21.4% | 27.4% | 22.9% | 16.7% | 24.2% | 26.2% | ||
| ANYDEP | 69 | 24 | 22 | 19 | 7 | 9 | 150 | NS |
| 33.8% | 28.6% | 30.1% | 27.1% | 19.4% | 27.3% | 30.0% |
(MDD — major depressive disorder; MD — minor depression; IMD — irritable minor depression; DD — dysthymic mood disorder; IDD — irritable dysthymic mood disorder; DDN — dysthymic disorder nos; Cyclo — cylothymic mood disorder; MDDD (minor depression and dysthymic disorder); AnyDep — any mood disorder.
This table summarizes mood disorders which appear to be dispersed across all clusters.
Table 5 summarizes comorbid anxiety disorders. With the exception of generalized anxiety disorder, none of the other anxiety disorders were statistically significantly different in the different clusters.
Table 5.
Comorbid anxiety disorders in latent class clusters.
| Total N | LC#1
|
LC#2
|
LC#3
|
LC#4
|
LC#5
|
LC#6
|
Total
|
||
|---|---|---|---|---|---|---|---|---|---|
| 204 | 84 | 73 | 70 | 36 | 33 | 500 | |||
| AD | 11 | 5 | 4 | 3 | 1 | 1 | 25 | NS | |
| 5.4% | 6.0% | 5.5% | 4.3% | 2.8% | 3.0% | 5.0% | |||
| GAD | 28 | 14 | 12 | 2 | 6 | 8 | 70 | NS | 0.02 |
| 13.7% | 16.7% | 16.4% | 2.9% | 16.7% | 24.2% | 14.0% | |||
| OAD | 27 | 10 | 14 | 4 | 7 | 5 | 67 | NS | |
| 13.2% | 11.9% | 19.2% | 5.7% | 19.4% | 15.2% | 13.4% | |||
| PTSD | 3 | 5 | 0 | 0 | 1 | 1 | 10 | NS | |
| 1.5% | 6.0% | 2.8% | 3.0% | 2.0% | |||||
| PD | 3 | 1 | 1 | 0 | 3 | 1 | 9 | NS | |
| 1.5% | 1.2% | 1.4% | 8.3% | 3.0% | 1.8% | ||||
| SA | 20 | 4 | 6 | 6 | 2 | 3 | 41 | NS | |
| 9.8% | 4.8% | 8.2% | 8.6% | 5.6% | 9.1% | 8.2% | |||
| SPD | 14 | 5 | 4 | 4 | 5 | 5 | 37 | NS | |
| 6.9% | 6.0% | 5.5% | 5.7% | 13.9% | 15.2% | 7.4% | |||
| SP | 3 | 4 | 4 | 0 | 0 | 1 | 12 | NS | |
| 1.5% | 4.8% | 5.5% | 3.0% | 2.4% | |||||
| OCD | 7 | 2 | 5 | 4 | 1 | 1 | 20 | NS | |
| 3.4% | 2.4% | 6.8% | 5.7% | 2.8% | 3.0% | 4.0% | |||
| AnyAD | 74 | 35 | 30 | 15 | 13 | 13 | 180 | NS | |
| 36.3% | 41.7% | 41.1% | 21.4% | 36.1% | 39.4% | 36.0% |
AD — avoidant dis. GAD — generalized anxiety dis.; OAD — overanxious dis.; PTSD —posttraumatic stress dis.; PD — panic dis.; SA — separation anxiety; SPD — simple phobic dis.; SP — social phobia: OCD — obsessive compulsive dis.: Any AD — any anxiety disorder.
This table summarizes comorbid anxiety disorders. With the exception of generalized anxiety disorder, none of the other anxiety disorders were statistically significantly different in the different clusters.
4. Discussion
This is one of the first studies investing the aggregation of ADHD and other neuropsychiatric symptoms in a clinical sample of ADHD children and adolescents using K-SADS-IVR data. Six significant ADHD clusters were identified in the age 6–18 range, similar to that reported by other investigators in population-based studies that also used DSM-IV diagnoses (Hudziak et al., 1998; Rasmussen et al., 2002; Rohde et al., 2001; Volk et al., 2005, 2006). A separate twin study utilizing teacher and parent Conner ratings identified fewer clusters possibly due to the limited impulsive symptoms captured by those rating scales (Althoff et al., 2006).
Our clusters are also similar to those identified in a very large sample of clinically referred 6–18 year old ADHD (de Nijs et al., 2007) that identified 5 clusters. Two of our clusters (1 and 2) correspond to one of those clusters (medium inattentive and hyperactive–impulsive). In contrast to this study, one of our clusters did correspond to a hyperactive–impulsive subtype, albeit this cluster had the smallest number of subjects. In both deNijs’s study and ours, clusters with the more severe inattentive symptoms appear to include the older age ranges while clusters with hyperactive–impulsive symptoms include younger cohorts. These results suggest that membership in a particular LC clusters may change over time and may reflect some of the same developmental instability noted over time in longitudinal studies using DSM-IV criteria (Lahey et al., 2005) and the later age of onset for IA reported by other studies (Willoughby et al., 2000). However, unlike DSM-IV phenotypes, the fact that relatively distinct age ranges aggregate in different clusters suggests that these may hold advantages in genetic studies, which to date have included all age ranges. Gender also appears to affect class membership and in our study, Cluster 5 with mild hyperactivity has one of the highest ratios of boys to girls (5.25 to 1) and girls do not appear to aggregate to the inattentive clusters, a finding different from that of de Nijs and others that have showed an over-representation of girls in the inattentive subtypes.
Comorbidity adds another layer of heterogeneity to ADHD, and in our sample 40.6% met DSM-IV criteria for ODD, 0.3% for CD. The number of subjects meeting DSM-IV criteria for ODD were unevenly scattered in the ADHD LC clusters with the highest membership being in the severe and moderate ADHD combined cluster and the lowest in cluster 2 (moderate inattention). LCA was also able to identify 6 distinct comorbid ODD-ADHD clusters; These included two clusters with moderate ODD, one aggregating with moderate and one with severe combined ADHD, two clusters with sub-threshold ODD, one aggregating with moderate combined and one with moderate inattention and two clusters with mild ODD, one aggregating with severe inattentive and one with moderate combined.
Comorbid anxiety disorders occurred in 32.2% of the sample and comorbid mood disorders in 23.2%. One third of our sample without comorbid disorders did not aggregate with any one of the six ADHD clusters. DSM-IV mood disorders were scattered throughout all the six ADHD LCA clusters. Anxiety Disorders, with the exception of generalized anxiety that occurred less frequently in cluster 4, were also scattered throughout the different clusters. However, this may be a reflection of development given that this cluster had one of the younger cohorts and higher rates (although not statistically significant) of separation anxiety. It is important to note however that at the time of evaluation many of our subjects had not passed through the ages of risk of some of the comorbid conditions. Therefore we cannot generalize about ADHD or comorbidity from data using cross-sectional phenotypes that include a broad age range. Longitudinal data is essential in order to determine whether individuals retain membership in any particular cluster or whether there is a trajectory of clusters for ADHD and the comorbid conditions.
Twin studies indicate that subtypes defined by DSM-IV categories as well as by latent-class analyses are highly heritable (Todd et al., 2001) with the possible exception of the hyperactive–impulsive group (Rasmussen et al., 2004). However, ADHD symptoms change with age. In a longitudinal twin study, ADHD symptoms were found to be moderately stable across the ages studied (18 months; 2, 3, 4, 7 and 8 years) however this was thought to be due mainly to shared genetic influences emerging during later stages of development not shared with those acting during earlier years (Kuntsi et al., 2005). The differing ages in the LCA clusters also suggest that these are also subject to developmental instability. However, since LC clusters, unlike DSM-IV subgroups appear to have some distinct age ranges that could prove useful in identifying corresponding genes whose expression may be age dependent.
4.1. Clinical and research implications
The latent clusters identified in this report include cases that meet criteria for DSM-IV subgroups. While the DSM-IV subgroups have predictive validity (Lee et al., 2008) with clinical implications, this is yet unknown for the latent clusters. LC may have clinical value in potentially identifying subjects that fall below the threshold for DSM-IV criteria but who may still have impairment (Smalley et al., 2007). LCA ascertained phenotypes may provide more phenotypically refined sub-groups than those identified using broad DSM-IV categories and may better correspond with the complex genetics underlying ADHD. The differing age ranges and gender representation in the different clusters may provide more homogenous groups necessary for genetic studies. Comorbidity for mood and anxiety disorders appear to be relatively similar across all the clusters however this needs to be shown in longitudinal data before their contribution to heterogeneity is dismissed.
4.2. Limitations
This sample is not representative of the general ADHD population or even clinical ADHD groups because it only included ADHD probands of European descent where both biological parents were available and willing to participate in a genetic study. The entire cohort was recruited at a single tertiary pediatric center (The Children’s Hospital of Philadelphia) and may reflect biased referrals. For example, the center does not provide treatment for substance use limiting the referrals with this comorbidity by clinicians in the area. The majority of the K-SADS IVR interviews were completed by one rater, (JE) and this could introduce an information collection bias to this data set. Although impairment was assessed for several areas of functioning, informants included only parents and children. Environmental measures of support or adversity that may impact cluster assignments are also lacking. The young mean age of the subjects (90% below age 15) also implies that many subjects have yet to pass through syndrome specific age of onset time frames for comorbid conditions.
Acknowledgments
We want to thank all the families that have participated, the referring clinicians and Tamika Scott, research coordinator. This research is supported by NIMH (K23MH066275-01), CTR (UL1-RR-024134) and NHGRI.
References
- Althoff RR, Copeland WE, Stanger C, Derks EM, Todd RD, Neuman RJ, Van Beijsterveldt TC, Boomsma DI, Hudziak JJ. The latent class structure of ADHD is stable across informants. Twin Research and Human Genetics. 2006;9:507–522. doi: 10.1375/183242706778025008. [DOI] [PubMed] [Google Scholar]
- Ambrosini PJ. Historical development and present status of the schedule for affective disorders and schizophrenia for school-age children (K-SADS) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:49–58. doi: 10.1097/00004583-200001000-00016. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1999;40:57–87. [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. American Journal of Psychiatry. 1991;148:564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Brown RT, Freeman WS, Perrin JM, Stein MT, Amler RW, Feldman HM, Pierce K, Wolraich ML. Prevalence and assessment of attention-deficit/hyperactivity disorder in primary care settings. Pediatrics. 2001;107:E43. doi: 10.1542/peds.107.3.e43. [DOI] [PubMed] [Google Scholar]
- Cantwell DP. Attention deficit disorder: a review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:978–987. doi: 10.1097/00004583-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Caron C, Rutter M. Comorbidity in child psychopathology: concepts, issues and research strategies. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1991;32:1063–1080. doi: 10.1111/j.1469-7610.1991.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Human Genetics. 1996;98:91–101. doi: 10.1007/s004390050166. [DOI] [PubMed] [Google Scholar]
- de Nijs PF, Ferdinand RF, Verhulst FC. No hyperactive–impulsive subtype in teacher-rated attention-deficit/hyperactivity problems. European Child and Adolescent Psychiatry. 2007;16:25–32. doi: 10.1007/s00787-006-0572-1. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Khan SA. Candidate gene studies of attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry. 2006;67 (Suppl 8):13–20. [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Hagenaars J. Latent structure models with direct effects between indicators: local dependence models. Sociological Methods and Research. 1988;16:379–405. [Google Scholar]
- Heiligenstein E, Conyers LM, Berns AR, Miller MA. Preliminary normative data on DSM-IV attention deficit hyperactivity disorder in college students. Journal of the American College of Health. 1998;46:185–188. doi: 10.1080/07448489809595609. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status; New Haven. 1975.1975. [Google Scholar]
- Hudziak JJ, Heath AC, Madden PF, Reich W, Bucholz KK, Slutske W, Bierut LJ, Neuman RJ, Todd RD. Latent class and factor analysis of DSM-IV ADHD: a twin study of female adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:848–857. doi: 10.1097/00004583-199808000-00015. [DOI] [PubMed] [Google Scholar]
- Hurtig T, Ebeling H, Taanila A, Miettunen J, Smalley SL, McGough JJ, Loo SK, Jarvelin MR, Moilanen IK. ADHD symptoms and subtypes: relationship between childhood and adolescent symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1605–1613. doi: 10.1097/chi.0b013e318157517a. [DOI] [PubMed] [Google Scholar]
- Jain M, Palacio LG, Castellanos FX, Palacio JD, Pineda D, Restrepo MI, Munoz JF, Lopera F, Wallis D, Berg K, Bailey-Wilson JE, Arcos-Burgos M, Muenke M. Attention-deficit/hyperactivity disorder and comorbid disruptive behavior disorders: evidence of pleiotropy and new susceptibility loci. Biological Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Martin D, Cantwell DP. Comorbidity in ADHD: implications for research, practice, and DSM-V. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1065–1079. doi: 10.1097/00004583-199708000-00014. [DOI] [PubMed] [Google Scholar]
- Kooij JJ, Buitelaar JK, van den Oord EJ, Furer JW, Rijnders CA, Hodiamont PP. Internal and external validity of attention-deficit hyperactivity disorder in a population-based sample of adults. Psychological Medicine. 2005;35:817–827. doi: 10.1017/s003329170400337x. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Rijsdijk F, Ronald A, Asherson P, Plomin R. Genetic influences on the stability of attention-deficit/hyperactivity disorder symptoms from early to middle childhood. Biological Psychiatry. 2005;57:647–654. doi: 10.1016/j.biopsych.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Archives of General Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Larsson JO, Larsson H, Lichtenstein P. Genetic and environmental contributions to stability and change of ADHD symptoms between 8 and 13 years of age: a longitudinal twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1267–1275. doi: 10.1097/01.chi.0000135622.05219.bf. [DOI] [PubMed] [Google Scholar]
- Larsson H, Lichtenstein P, Larsson JO. Genetic contributions to the development of ADHD subtypes from childhood to adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:973–981. doi: 10.1097/01.chi.0000222787.57100.d8. [DOI] [PubMed] [Google Scholar]
- Lee SI, Schachar RJ, Chen SX, Ornstein TJ, Charach A, Barr C, Ickowicz A. Predictive validity of DSM-IV and ICD-10 criteria for ADHD and hyperkinetic disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49:70–78. doi: 10.1111/j.1469-7610.2007.01784.x. [DOI] [PubMed] [Google Scholar]
- Neuman RJ, Todd RD, Heath AC, Reich W, Hudziak JJ, Bucholz KK, Madden PA, Begleiter H, Porjesz B, Kuperman S, Hesselbrock V, Reich T. Evaluation of ADHD typology in three contrasting samples: a latent class approach. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:25–33. doi: 10.1097/00004583-199901000-00016. [DOI] [PubMed] [Google Scholar]
- Neuman RJ, Heath A, Reich W, Bucholz KK, Madden PAF, Sun L, Todd RD, Hudziak JJ. Latent class analysis of ADHD and comorbid symptoms in a population sample of adolescent female twins. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2001;42:933–942. doi: 10.1111/1469-7610.00789. [DOI] [PubMed] [Google Scholar]
- Neuman RJ, Sitdhiraksa N, Reich W, Ji TH, Joyner CA, Sun LW, Todd RD. Estimation of prevalence of DSM-IV and latent class-defined ADHD subtypes in a population-based sample of child and adolescent twins. Twin Research and Human Genetics. 2005;8:392–401. doi: 10.1375/1832427054936646. [DOI] [PubMed] [Google Scholar]
- Rasmussen ER, Neuman RJ, Heath AC, Levy F, Hay DA, Todd RD. Replication of the latent class structure of attention-deficit/hyperactivity disorder (ADHD) subtypes in a sample of Australian twins. Journal of Child Psychology and Psychiatry, and allied disciplines. 2002;43:1018–1028. doi: 10.1111/1469-7610.00229. [DOI] [PubMed] [Google Scholar]
- Rasmussen ER, Neuman RJ, Heath AC, Levy F, Hay DA, Todd RD. Familial clustering of latent class and DSM-IV defined attention-deficit/hyperactivity disorder (ADHD) subtypes. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2004;45:589–598. doi: 10.1111/j.1469-7610.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- Rohde LA, Barbosa G, Polanczyk G, Eizirik M, Rasmussen ER, Neuman RJ, Todd RD. Factor and latent class analysis of DSM-IV ADHD symptoms in a school sample of Brazilian adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:711–718. doi: 10.1097/00004583-200106000-00017. [DOI] [PubMed] [Google Scholar]
- Scahill L, Schwab-Stone M. Epidemiology of ADHD in school-age children. Child and Adolescent Psychiatric Clinics of North America. 2000;9:541–555. vii. [PubMed] [Google Scholar]
- Smalley SL, McGough JJ, Moilanen IK, Loo SK, Taanila A, Ebeling H, Hurtig T, Kaakinen M, Humphrey LA, McCracken JT, Varilo T, Yang MH, Nelson SF, Peltonen L, Jarvelin MR. Prevalence and psychiatric comorbidity of attention-deficit/hyperactivity disorder in an adolescent Finnish population. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1575–1583. doi: 10.1097/chi.0b013e3181573137. [DOI] [PubMed] [Google Scholar]
- Spencer TJ. ADHD and comorbidity in childhood. Journal of Clinical Psychiatry. 2006;67 (Suppl 8):27–31. [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Archives of General Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Todd RD, Rasmussen ER, Neuman RJ, Reich W, Hudziak JJ, Bucholz KK, Madden PA, Heath A. Familiality and heritability of subtypes of attention deficit hyperactivity disorder in a population sample of adolescent female twins. American Journal of Psychiatry. 2001;158:1891–1898. doi: 10.1176/appi.ajp.158.11.1891. [DOI] [PubMed] [Google Scholar]
- Todd RD, Lobos EA, Sun LW, Neuman RJ. Mutational analysis of the nicotinic acetylcholine receptor alpha 4 subunit gene in attention deficit/hyperactivity disorder: evidence for association of an intronic polymorphism with attention problems. Molecular Psychiatry. 2003;8:103–108. doi: 10.1038/sj.mp.4001257. [DOI] [PubMed] [Google Scholar]
- Todd RD, Huang H, Smalley SL, Nelson SF, Willcutt EG, Pennington BF, Smith SD, Faraone SV, Neuman RJ. Collaborative analysis of DRD4 and DAT genotypes in population-defined ADHD subtypes. Journal of Child Psychology and Psychiatry, and allied disciplines. 2005;46:1067–1073. doi: 10.1111/j.1469-7610.2005.01517.x. [DOI] [PubMed] [Google Scholar]
- Vermunt J. LEM: a general program for the analysis of categorical data Tilberg University 1997 [Google Scholar]
- Vermunt J, Magidson J. Technical Guide for Latent GOLD 4.0: Basic and Advanced. Belmont. Massachusetts: Statistical Innovations Inc; 2005. [Google Scholar]
- Volk HE, Neuman RJ, Todd RD. A systematic evaluation of ADHD and comorbid psychopathology in a population-based twin sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:768–775. doi: 10.1097/01.chi.0000166173.72815.83. [DOI] [PubMed] [Google Scholar]
- Volk HE, Henderson C, Neuman RJ, Todd RD. Validation of population-based ADHD subtypes and identification of three clinically impaired subtypes. American Journal of Medical Genetics; B Neuropsychiatric Genetics. 2006;141:312–318. doi: 10.1002/ajmg.b.30299. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Curran PJ, Costello EJ, Angold A. Implications of early versus late onset of attention-deficit/hyperactivity disorder symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1512–1519. doi: 10.1097/00004583-200012000-00013. [DOI] [PubMed] [Google Scholar]