SUMMARY
Leptin, a pleiotropic protein hormone produced mainly by fat cells, regulates metabolic activity and many other physiological functions. The intrinsic circadian rhythm of blood leptin is modulated by gender, development, feeding, fasting, sleep, obesity, and endocrine disorders. Hyperleptinemia is implicated in leptin resistance. To determine the specificity and sensitivity of leptin concentrations in sleep disorders, we summarize here the alterations of leptin in four conditions in animal and human studies: short duration of sleep, sleep fragmentation, obstructive sleep apnea (OSA), and after use of continuous positive airway pressure (CPAP) to treat OSA. The presence and causes of contradictory findings are discussed. Though sustained insufficient sleep lowers fasting blood leptin and therefore probably contributes to increased appetite, obesity and OSA independently result in hyperleptinemia. Successful treatment of OSA by CPAP is predicted to decrease hyperleptinemia, making leptin an ancillary biomarker for treatment efficacy. Current controversies also call for translational studies to determine how sleep disorders regulate leptin homeostasis and how the information can be used to improve sleep treatment.
Keywords: Leptin, Circadian rhythm, Sleep fragmentation, Sleep duration, Obstructive sleep apnea, Continuous positive airway pressure
Introduction
Leptin is a 16 kDa polypeptide cytokine that is produced mainly in adipocytes. It inhibits feeding, increases sympathetic activation, modulates immune functions, influences synaptic activities, and often promotes inflammation. Many effects are mediated by the central nervous system (CNS), as leptin crosses the blood–brain barrier (BBB) by a saturable transport mechanism [1]. Leptin concentrations in blood correlate with body weight and body mass index (BMI). Hyperleptinemia in obesity is part of the intriguing phenomenon of leptin resistance. Like insulin resistance in type II diabetes, leptin resistance is a universal finding in obesity and metabolic syndrome [2]. The underlying mechanisms involve upregulation of leptin antagonists such as the soluble leptin receptor and counteracting hormones, reduced efficiency of leptin uptake by organs including the brain, desensitization of leptin signaling in target organs, and development of antagonistic cellular signaling pathways. These factors modulate the physiological response to leptin across time and condition. Partial saturation of leptin transport across the BBB is already present at physiological conditions. In obesity, the BBB becomes a rate limiting factor to reduce the efficacy of leptin in the CNS [3]. By contrast, 48 h fasting decreases blood leptin and its transport across the BBB in lean mice [4].
Sleep, circadian rhythm, and sleep disorders all affect leptin concentrations in blood. The rhythm of leptin in constant conditions differs from that in entrained conditions. There is entrainment by meals [5–7] and regulation by gender and adiposity [8,9]. In most studies, human subjects (and animals) live in an environment with feeding-fasting and wake–sleep cycles, both of which influence the concentration of leptin. Under a constant routine protocol with dim light and 38 h of wakefulness, the circadian rhythm of endogenous leptin peaks around the usual time of waking. This contrasts with the effects of sleep and fasting to lower leptin and with those of wakefulness and feeding to increase leptin. Results from this well-controlled study of six healthy human subjects indicate combined effects from the endogenous circadian pacemaker and day/night patterns on leptin concentrations [7]. While the sleep/wake schedule causes a leptin nadir upon awakening, the entrained rhythm peaks earlier (midnight) and reaches a minimum at 11:40 h, before lunch at 12:30 h. Plasma leptin is not shifted by acute sleep deprivation, but shows a rhythm shift of 5–7 h when meals are shifted 6.5 h without changing the light or sleep cycle. Furthermore, there is a 12 ± 2 h shift induced by day/night reversal (time zone shift). The results indicate meal entrainment, rather than an immediate effect of the circadian clock [5].
In patients with narcolepsy who show fragmented sleep, abnormal rapid eye movement (REM) sleep, and excessive daytime sleepiness (EDS), there is a reduction of mean 24-h plasma leptin concentration and loss of the nocturnal acrophase [10]. This is not unusual since narcolepsy involves degeneration of orexin neurons and probably affects other areas of the hypothalamus involved in neuroendocrine control of feeding. Obesity and its associated leptin resistance also result in hypothalamic astrogliosis [11–13]. However, it is not yet clear whether leptin resistance plays a causal role in metabolic and neurobehavioral changes in subjects with sleep disorders.
The analysis of a relationship between leptin concentration and sleep is important since sleep disturbance contributes greatly to poor health. Sleep durations of five hours (h) or less per night are associated with a significantly increased risk of obesity [14]. Obesity is a main risk factor for obstructive sleep apnea (OSA); a recent analysis with epidemiological modeling from the Wisconsin Sleep Cohort indicates that the estimated prevalence rates increased substantially over the last two decades, from 14% to 55% among different age groups [15]. Sleep fragmentation is also a main feature of OSA and leads to EDS. Hyperleptinemia is a consequence of obesity, and it might serve a biochemical link between sleep disorders and impaired physiological functions.
Here, we review the conflicting literature about leptin and sleep within the last decade (2002–2012) in each of four areas: short sleep duration, sleep fragmentation, OSA, and the use of continuous positive airway pressure (CPAP) for treatment of OSA. The results, including reduction, elevation, or lack of change of leptin, show the complexity of the leptin system that can be influenced by biological behavior and efficacy of treatment. Taking into consideration circadian rhythm changes, adiposity, and the rigor of control of experimental conditions, the most consistent conclusion is that effective treatment of OSA reduces hyperleptinemia. This makes leptin a biomarker for treatment efficacy.
Effects of short sleep duration on leptin in human studies (Table 1)
Table 1.
Effects of sleep duration on blood leptin concentrations in human studies.
| Study | Sleep duration | Leptin level | Experiment type | Population sampled | Method to measure sleep |
|---|---|---|---|---|---|
| Benedict 2012 [20] | 24 h no sleep | No change | Prospective with | 14 men, 22.6 ± 0.8 y old, | Laboratory environment |
| Charles 2011 [24] | <5 h or >8 h vs 5–7 h |
↑ | crossover design Cross sectional |
BMI 23.9 ± 0.5 kg/m2 443 police |
Questionnaire |
| Klingenberg 2012 [23] |
4 h × 3 nights | No change | Prospective with crossover design |
21 teenagers (15–19 y old), BMI < 25 kg/m2 |
Laboratory environment |
| Knutson 2011 [19] | 6.5 h of sleep at home |
No change; blood drawn 8–10 am |
Prospective | 80 habitual short sleepers, BMI 38.2 kg/m2 |
Actigraphy |
| Mullington 2003 [17] |
88 h sleep loss | ↓Circadian amplitude | Time series | 10 healthy men | Sleep lab environment |
| Nedeltcheva 2009 [29] |
5.5 h vs 8.5 h × 14 nights with overeating |
No difference | Prospective | 11 people; overeating dominates the effect, 39 ± 5 y old, |
EEG and EMG for sleep staging |
| Omisade 2010 [28] | 4 h × 5 nights | ↑Morning leptin 0.2 ng/ml (7.7%) |
Prospective | BMI 26.5 ±1.5 kg/m2 136 people, 18–25 y old (21.6 ± 2.23), BMI 18.3–51.9 kg/m2 (24.47 ± 8.09) |
Laboratory environment |
| Reynolds 2012 [25] | 4 h × 5 nights | ↑0.5 ng/ml (13.7%) | Prospective | 14 men, 27 y old, BMI 23.5 kg/m2 | Laboratory environment |
| Schmid 2008 [21] | 4.5 or 0 h vs 7 h | No change | Prospective | 10 healthy men, BMI 23.8 kg/m2 1 night |
PSG |
| Schmid 2009 [22] | 4.5 h × 2 nights | No change | Prospective with crossover design |
15 healthy young adults (age 27.1 ± 1.3), BMI 22.9 kg/m2 |
accelerometry |
| Simpson 2010 [27] | 3h | ↑33% | Prospective | 15 young women | Laboratory environment |
| Spiegel 2004 [18] | 4 h vs 12 h for 6 d | ↓ 26% | Prospective | 11 healthy men (22 ± 1 y old), BMI 23.4 kg/m2 |
PSG |
| Taheri 2004 [16] | 5 h vs 8 h | ↓ 15.5% | Prospective cohort |
1017 people from Wisconsin Sleep Cohort, BMI 29.7 kg/m2 |
PSG in combination with questionnaires and sleep diary |
| Van Leeuwen 2010 [26] |
4 h × 5 nights | ↑0.35 ng/ml (163.3 ± 42.4% at 5th day of sleep restriction, 123.1 ± 7.0% at 2nd night of recovery sleep) |
Prospective | 23 men, 23.1 ± 2.5 y old, BMI 23.2 kg/m2 |
Laboratory environment |
Human studies showing decreased leptin in short sleepers
Overnight polysomnography (PSG) is the gold standard for evaluation of sleep duration and quality. By use of PSG, the Wisconsin Sleep Cohort showed that subjects sleeping 5 h had fasting blood leptin concentrations 15.5% lower than those sleeping 8 h [16]. The significant correlation between sleep duration and leptin was independent of BMI, age, sex, or the presence/extent of sleep-disordered breathing. This appears counterintuitive, especially that short sleepers in this study tended to have a higher BMI (usually associated with higher leptin), making the reduction of leptin concentrations even more significant. Blood sampling time, in relation to sleep–wake cycle and nycthemeral effects on blood leptin concentrations, therefore, might provide a feasible explanation (Fig. 2).
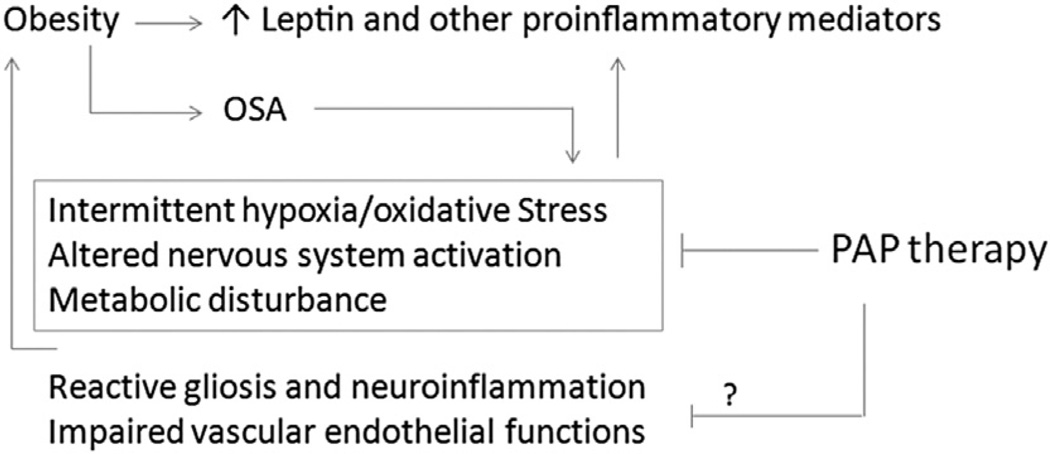
Fig. 2.
Proposed major links between obstructive sleep apnea (OSA) and blood leptin concentrations, and how positive airway pressure (PAP) may be effective. Obesity, a major risk factor of OSA and systemic inflammation, increases adiposity and leptin production by adipocytes. Once OSA has developed, intermittent hypoxia, oxidative stress, and related metabolic and organ/system stress further worsen obesity. Some of the factors are more readily modifiable by PAP treatment which stabilizes sleep, whereas others may be more difficult to resolve and perhaps only partially reversible.
Prolonged sleep loss decreases the circadian amplitude of leptin, as shown in 10 healthy men after 88 consecutive hours of sustained sleeplessness [17]. Multiple consecutive nights of shortened sleep also decreases leptin concentrations. After a week of nightly sleep restriction to 4 h, the maximal blood leptin concentrations were 26% lower in 11 healthy men. This occurred without change in caloric intake, physical activity, body weight, or BMI. Both daytime and nighttime leptin concentrations decreased, as did the amplitude of the diurnal variation. The acrophase of the circadian rhythm was also advanced; i.e., the time it took for the usual increase of blood leptin from a low in the early morning to the nocturnal peak was decreased by about 1.5 h [18].
Human studies showing unchanged leptin in short sleepers
The effect of habitual short sleep duration (less than 6.5 h by actigraphy worn at home) on leptin concentrations was tested in 80 obese subjects (BMI 38.2). When blood leptin was normalized by percent body fat, it showed no correlation with sleep time [19]. The lack of change may be influenced by the following limitations: first, normalization using % fat as the denominator tended to mask hyperleptinemia; second, no control subjects with normal sleep duration were included; third, blood sampling occurred between 8:00 h and 10:00 h (even for the short sleepers awakening earlier), rather than upon awakening of these subjects at home (not in a controlled laboratory environment); fourth, the subjects were selected for obesity and short sleep duration, rather than from the general population as in the Wisconsin Sleep Cohort that found decreased leptin concentrations; lastly, the subjects were morbidly obese; they probably had large variation of their sleeping time and quality that the home recording failed to identify, contributing to variable responses of leptin concentrations to overnight fasting.
In a laboratory environment despite the lack of PSG or actigraphy recording, overnight total sleep deprivation (no sleep for 24 h) for 14 healthy men did not change the circadian rhythm of blood leptin [20]. Peak leptin concentrations occurred during the first half of the night, as expected for an inactive period of low energy expenditure. Altered stress hormones and autonomic nervous system activity might have contributed to a compensatory elevation of leptin. In a cross-over design spanning two weeks, controlled sleep durations of 0, 4.5 h, or 7 h of sleep in nine healthy men also failed to change blood leptin, though there was increased hunger sensation and higher ghrelin concentrations [21]. A follow-up study, attempting to overcome insufficient statistical power and a sub-threshold duration for leptin concentrations to fall, did not detect a change in leptin after two nights of restricted sleep (bed time 02:45 h–07:00 h) in comparison with the 8 h sleep controls [22]. Consistent with this, a more recent Danish study of three consecutive nights of 4 h sleep restriction in 21 adolescent boys also failed to show a change of leptin concentrations [23]. These nights of sleep restriction were still shorter than in the studies finding decreased blood leptin concentrations.
Human studies showing increased leptin after sleep loss
Higher leptin concentrations were seen in police officers with both long (>8 h) and short (<5 h) sleep than in those sleeping 5–7 h nightly, influenced by gender, BMI, abdominal width, and shift work [24]. Leptin concentrations were also increased by five nights of sleep restriction to 4 h/night in 14 healthy men who had adequate energy intake and reduced energy expenditure, without change in appetite or hunger [25]. In this design, a similar increase of leptin was seen in 23 healthy young men [26] and 136 subjects [27]. Blood samples were not obtained until between 10:30 h and 12:00 h several hours after being awakened, and there was free access to food throughout the study.
In 15 healthy young women with sleep restricted to 3 h for a single night, leptin was increased in the morning from 2.6 to 2.8 ng/ ml (08:30 h) but not evening (20:00 h). Unusually, morning concentrations were higher than evening concentrations [28]. This might indicate a transient diurnal effect. In a sleep study with an overeating experimental maneuver, two weeks of overeating had a greater effect than reduced sleep duration, as the 5.5 h and 8.5 h groups did not show a difference [29]. The increased leptin was related to increased body weight and fat resulting from enhanced caloric intake.
In general, sleep duration shows a U-shaped curvilinear association with BMI, but habitual short sleepers have sustained elevation of leptin independent of BMI, suggesting that sleep duration may override the influence of circadian rhythm [16]. Most of the studies on sleep duration and leptin did not measure sympathetic nervous system activity. Leptin is known to increase sympathetic outflow [30,31], although reciprocal changes have not been reported to our knowledge. Sleep loss also induces sympathetic activation [32]. It is possible that short sleep duration might also act through autonomic nervous system activity and stress hormones to increase leptin concentrations in certain circumstances.
Overall, the effect of sleep duration on blood leptin has been examined by many groups, with variation in sleep duration, number of nights, laboratory vs home environment, normal vs obese population, food intake intervention, activity level, circadian time of sampling vs time of sleep, as well as sample size. The concurrent use of glucose tolerance tests in place of a breakfast meal may also contribute to restricted or abnormal eating patterns [18,25,33]. Table 1 summarizes findings from the sleep duration maneuvers, mainly with sleep restriction or deprivation. We apologize if we inadvertently missed any studies in the last decade from 2003. It is safe to conclude that PSG-verified sleep restriction reduces fasting leptin concentrations, and that caution should be taken in future experimental designs to control factors that may interfere with feeding, circadian rhythmicity, and leptin production.
Effects of short sleep duration on leptin in animal studies (Table 2)
Table 2.
Effects of sleep duration on blood leptin concentrations in rodent studies.
Rodent studies showing decreased leptin
In rats after 96 h of REM sleep deprivation by the inverted flowerpot platform method, blood leptin is decreased regardless of chow feeding, high fat diet, or liquid diet. Weight loss is significant and most marked during the first 24 h, suggesting the presence of major stress [34]. In a sustained REM deprivation study, leptin concentration was 35% of controls by day 5, before any significant change of food consumption or fat depletion, and it remained low throughout the remainder of the 20 d but returned to normal after two days of rest [35]. In a “weekday” sleep deprivation study, leptin decreased at the end of each five-day period and after the 4 h period of rest, before any significant change in food ingestion [36]. Overall, four days or more of sleep deprivation reduces blood leptin concentration, regardless of diet or deprivation protocol, and this is accompanied by weight loss. The rodent studies did not involve frequent sampling of blood so that no data are yet available about how sleep deprivation changes the known circadian rhythm of blood leptin concentrations or the rhythm of leptin transport across the blood–brain barrier [37].
Rodent studies showing unchanged leptin
Five hours of sleep deprivation at the beginning of the light cycle did not affect leptin concentrations in rats, though there was a rapid increase of ghrelin, elevation of corticosterone, and increased feeding [38]. The increase in feeding probably contributed to the higher level of leptin, indicating the importance of controlling food intake in the experimental design. Overall, the influence of feeding, obesity, and duration of sleep restriction all contribute to the outcome of the studies.
Lack of effect of sleep fragmentation on leptin in both human and rodent studies
In a 24 h cross-over design, 12 healthy men (mean age 23 and mean BMI 24.4 kg/m2) received five wakeup calls at about 90 min intervals during the 8 h allotted time in bed [39]. As a result of the fragmentation, there was reduced REM sleep and increased stage 2 non-REM sleep. There were no changes in total sleep time, sleep latency, time awake, sleep stage 1, or leptin. Thus, a single night of sleep fragmentation does not appear to affect blood leptin concentration. However, this amount of sleep disruption is far less that that seen in patients with severe sleep apnea (e.g., every 2 min).
Increased sleep fragmentation as well as increased sleep duration have been observed in obese mice with genetic mutations, namely the ob/ob mice lacking leptin production [40] and the db/db mice with a mutant ObRb receptor subtype [41]. Sleep fragmentation alone, however, did not affect leptin production in the rodents. In a study spanning 72 d, rats underwent six cycles of 10 d sleep fragmentation by platform rotation followed by two days of recovery. The decrease of leptin was not significantly different even though there was weight loss [42].
In summary, both human and rodent experimental sleep fragmentation did not affect leptin concentrations. Fragmentation interval, altered locomotor activity, stress hormone production, and feeding might have contributed to the overall outcome.
Effects of OSA on leptin concentrations (Table 3)
Table 3.
Effects of OSA on blood leptin concentrations.
| Study | OSA group BMI (kg/m2) (sample size) |
OSA AHI (event/h) |
OSA blood leptin (ng/ml) |
Control BMI (kg/m2) (sample size) |
Control AHI (event/h) |
Control blood leptin (ng/ml) |
Change from control |
|---|---|---|---|---|---|---|---|
| Basoglu 2011 [79] | 33.5 (36) | 27.7 ± 19.6 | 13.1 | 34.5 (34) | <5 | 9.0 | Higher |
| Fredheim 2011 [50] | 47.2 (84) | 25 | 5.74 | 46.3 (53) | 2 | 6.66 | Lower |
| Hargens 2013 [82] | 32.4 (12) | 25.4 | 11.9 | 22.2 (16), 31.6 (18) | 2.0, 2.2 | 4.1, 9.7 | Higher or no change |
| Harsch 2003 [54] | 30.6 (30) | 58 | 12.4 | 32.6 (30) | 2 | 4.4 | Higher |
| Kapsimalis 2008 [47] | 28.7 (26) | Severe | 20.2 | 29.4 (26), 30.6 (15) | Mild-moderate, none |
23.1, 9.4 | Higher |
| McArdle 2007 [44] | 28 (46) | 40 | 7.8 | 28 (46) | 3 | 4.1 | Higher |
| Ozturk 2003 [81] | 30.8 (8) | 43 | 21.2 | 31.0 (12, mild OSA), 28.5 (12, non-OSA) |
12, 2 | 16.2 (mild OSA), 10.6 (non-OSA) |
Higher |
| Papaioannou 2011 [83] | 30 (68) | 22.4 | 9.6 | 28 (37) | 2.5 | 7.9 | Higher – correlates with BMI |
| Sharma 2007 [85] | 29.8 (40) | 32.2 | 10.65 | 29.0 (40) | 1.4 | 8.52 | No difference |
| Tatsumi 2005 [46] | 24.7 (96) | 32.5 | 6.0 | 24.7 (52) | 2.3 | 3.8 | Higher |
| Ulukavak 2005 [80] | 33 (30) | 44 | 29.4 | 31 (22) | 1.55 | 20.2 | Higher |
| Ursavas 2010 [84] | 32.5 (55) | 43.5 | 10.9 | 31.6 (15) | 2.8 | 9.4 | No difference |
OSA is commonly correlated with obesity, and it poses severe metabolic and vascular consequences resulting from increased sympathetic activation, vascular endothelial dysfunction, oxidative stress, inflammation, increased coagulability, and metabolic dysregulation [43–45]. OSA patients often have increased sleep fragmentation, secondary insomnia, and reduction of slow wave and REM sleep. Intermittent hypoxia further exacerbates the metabolic changes related to sleep disturbance and obesity. The severity of OSA is collectively evaluated by clinical symptoms, comorbidities, extent of desaturation, sleep fragmentation, impairment of sleep stage distribution, reduction of sleep efficiency, apnea–hypopnea index (AHI), and respiratory disturbance index (RDI). However, most studies have to depend mainly on AHI for classification, with a value higher than 30 events/h defined as severe OSA, 15–30 for moderate severity, and 5–15 as mild.
As shown in Table 3, there is a large variation of basal leptin concentrations as well as changes in OSA groups among different studies. OSA has been reported to be associated with higher, the same, or even lower leptin concentrations.
In most studies showing hyperleptinemia, OSA was associated with higher circulating leptin independent of BMI. The regression line between leptin and BMI was significantly shifted higher in the OSA group [46]. It also appears that the presence of OSA, regardless of its severity, led to elevated leptin in comparison with BMI-matched non-OSA controls [47]. Both morning and evening leptin concentrations were higher in 138 OSA patients with a wide range of AHI [48]. This correlation between leptin level and AHI disappeared after adjustment for BMI. Nevertheless, in patients with an AHI >15, the ratio of evening to morning leptin was increased 23%. In these studies, hyperleptinemia is a main feature in the patients with OSA regardless of BMI.
There are also reports that obese patients with OSA do not show high blood leptin concentrations. Blood leptin in the morning did not differ among 28 obese patients with OSA, 10 obese controls without OSA, 21 non-obese patients with OSA, and 20 non-obese controls [49]. Here, obesity alone was sufficient to induce hyperleptinemia even without OSA.
There is even a report that leptin concentrations are lower in OSA patients with morbid obesity (57.4 ± 20.3 ng/ml) than non-OSA controls with morbid obesity (66.6 ± 16.4 ng/ml) [50]. It is not clear whether the 18% of severe OSA patients (24 subjects) had ongoing positive airway pressure (PAP) treatment. The gender differences in this study were not controlled; there were fewer males in the non-OSA group (7 vs 29) and a gender difference in leptin might also have contributed to the anomaly.
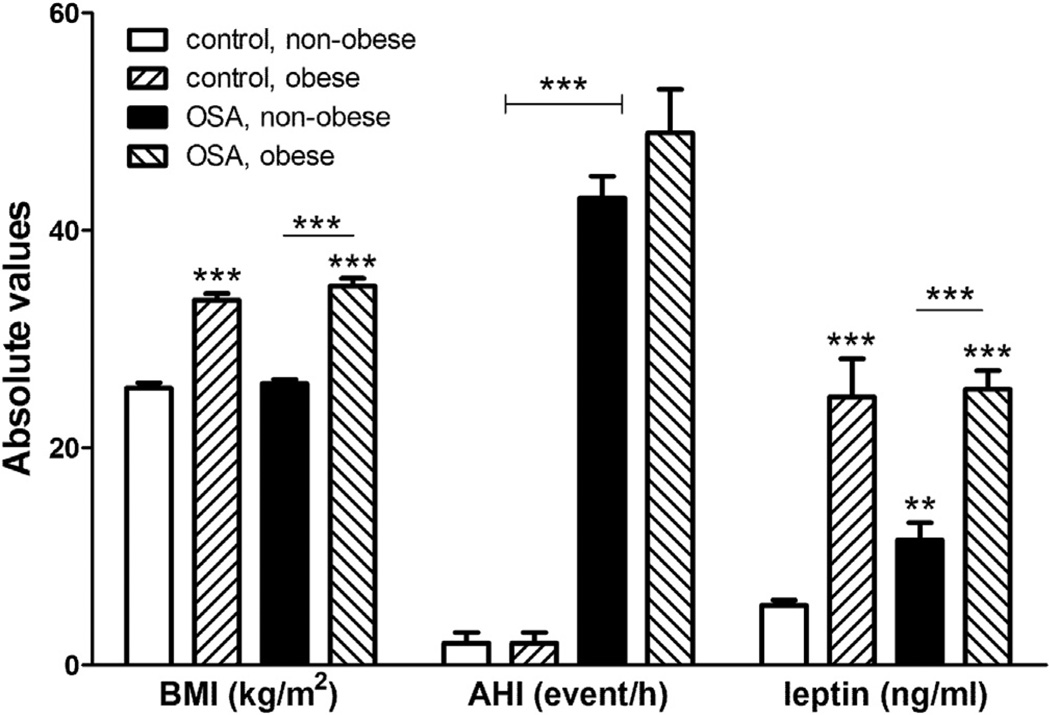
It should be noted that factors such as sampling methods, meal patterns, sleep duration and cohesiveness, or blunted circadian rhythm induced by obesity can all modulate leptin concentrations. Furthermore, blood leptin in OSA patients is influenced by the presence or absence of obesity. In patients with relatively normal BMI, OSA is more likely to induce hyperleptinemia in comparison with the BMI-matched controls. Fig. 1 plots relevant data from Barcelo et al. in their study of four groups of subjects [51]; the results are consistent with those summarized above showing that obesity appears to play a greater role in hyperleptinemia than OSA. The interplay of obesity and OSA on blood leptin is somewhat similar to that described above on obesity and sleep restriction. Because of the distinct circadian rhythm of leptin (and the shift of rhythm by disrupted sleep), studies with small sample size may not have sufficient statistical power to detect the actual difference. On one hand, leptin is a main adipocytokine, the blood level of which correlates with adiposity and therefore is a better indicator of adiposity than body weight. On the other hand, persistent elevation of leptin and its associated leptin resistance are better indicators of sustained neuroinflammation. In OSA patients, intermittent hypoxia and oxidative stress occur both in the periphery and the brain. Leptin aggravates reactive gliosis in obesity [11,12,52] as well as inflammatory status, and thus might further worsen the control of OSA and the neurological sequelae.
Fig. 1.
Differential effects of obesity and obstructive sleep apnea (OSA) on severity of sleep apnea (determined by apnea-hypopnea index, AHI) and leptin concentrations. Graphed from data of Barcelo et al. 2005 [51]. **: p < 0.01; ***: p < 0.005.
Effects of CPAP treatment on hyperleptinemia (Table 4)
Table 4.
Effects of CPAP treatment duration, efficacy, and baseline BMI on hyperleptinemia.
| Study | Sample size and treatment duration |
Pre-BMI (kg/m2) | Post-BMI (kg/m2) | Pre-AHI (event/h) | Post-AHI (event/h) | Leptin concentration |
|---|---|---|---|---|---|---|
| Barcelo 2005 [51] | 4 groups of 18–24/group, 3 or 12 m |
25.9 in non-obese and 34.9 in obese groups |
nd | 43 in non-obese and 49 in obese groups |
nd | ↓ |
| Cuhadaroglu 2009 [55] | 31 patients, 8 wk | 32 | nd | 43 | Nd | ↓ |
| Drummond 2008 [62] | 98 patients, 1 wk and 6 m | 33.2 | nd | 51.7 | 2.7 ± 1.7 | No change |
| Garcia 2011 [63] | 20 patients × 6 m | 36.5 | 37.1 | >15 | Not specified | No change |
| Harsch 2003 [54] | 30 men, 8 wk | 32.6 | nd | 58 ± 16 | nd | ↓ |
| Huang 2004 [60] | 8 patients, 1 d and 7.5 m | nd | nd | nd | nd | ↓ |
| Nakra 2008 [57] | 25 children with OSA and 9 without |
z score of 2.8 with OSA and z score of 2.57 without |
No change | 8.68 | nd | ↓ by CPAP |
| Rubinsztajn 2006 [61] | 29 men, 3 wk | nd | nd | nd | nd | No change |
| Sanchez-de-la-Torre 2011 [49] | 28 obese men and 21 non-obese men, 3 m |
>30 as obese and <27 as control |
nd | >20 | ↓ | |
| Sanner 2004 [59] | 53 patient, 6 m | nd | nd | 29.4 | 1.6 | ↓ |
| Sanner 2004 [59] | 26 patient, 6 m, ineffectively treated |
nd | nd | 24.8 | 13.7 | ↑ |
| Trenell 2007 [56] | 19 patients > 3 m | 36 | nd | 64 | nd | ↓ |
| Yee 2006 [53] | 14 patients, 1.6–3 y | 40.9 | nd | 44 (RDI) | nd | ↓ |
nd: no data.
As shown in Table 4, the response of leptin concentrations to CPAP treatment also has some inconsistency; most studies showed reduction but some showed a lack of change. The change of leptin concentration is more sensitive than the reduction of body weight or BMI.
In studies showing that CPAP lowers leptin levels, Yee et al. reported a reduction of blood leptin from 47.1 to 29.8 ng/ml over a course of 2.3 y (range of 1.6–3 y , as the sampling interval between the first and last measurement differed among subjects) in nine regular PAP users who were morbidly obese and hypercapneic. Six of the patients were on CPAP whereas three were on bilevel positive airway pressure (BiPAP). This contrasts with the lack of change in five non-users in the same study. In the non-users, the concentration of leptin was 19.6 ± 17.8 ng/ml and re-testing showed 20.4 ± 19.3 ng/ml after the same observation interval. Neither group had a change in BMI [53]. The reduction of hyper-leptinemia may occur rather quickly. There are reports of effectiveness after eight weeks of CPAP use (ranging from 6.8 to 12.7 ng/ml) unaccompanied by a decrease in BMI or body fat [54,55], 3 mo [56–58], 6 mo [59], 7.5 mo [60], a year [51], or 1.6– 3 y [53].
While Barcelo et al. reported a reduction of leptin concentration only in the non-obese OSA group (from 11.0 to 9.2 ng/ml after a year) without accompanying BMI changes [51], Sanchez et al. found that CPAP significantly reduced blood leptin only in the obese OSA patients but not in the non-obese ones [49]. Both have sound explanations; the former may be influenced by more cohesive sleep after effective treatment of OSA, whereas the latter suggests long-term efficacy in the reduction of adiposity and the inflammatory state by CPAP treatment.
There are also reports showing a lack of change of leptin concentration with CPAP or auto-PAP, although treatment efficacy was not monitored, for instance by symptoms or residual AHI [61,62]. In particular, 40% of 20 obese patients with six months of consistent CPAP use gained weight and leptin concentrations did not change significantly [63]. This raises questions about treatment efficacy that can be influenced by factors such as inadequate PAP setting, insufficient sleeping duration, issues with pressure delivery interface, low adherence to long-term treatment plan, coexisting sleep disorders not yet identified or treated, and lack of a comprehensive management program that includes diet control and increased activity levels. In these circumstances, residual respiratory events and sleep fragmentation would persist. Thus, treatment efficacy should be a key measurement, along with sufficient sleep duration and management of other medical issues.
The effect of CPAP treatment on weight loss also has variable results. There is successful weight reduction in obese and overweight patients with OSA after CPAP use [64]. Concurrent with weight loss, visceral and subcutaneous fat (as measured by computerized tomography) are decreased in six months, whereas leptin concentrations are reduced in 3–4 d. The remaining 59% of the 22 subjects did not have significant weight loss but visceral fat was also decreased [65]. This consistent reduction of visceral fat is an exciting finding, since visceral fat functions as a large endocrine organ and produces a variety of proinflammatory molecules detrimental to vascular and metabolic functions. Assessment at eight weeks also showed no change of body weight but reduction of hyperleptinemia [54]. However, a study with unequal sample size between control and treatment groups suggested weight gain in non-obese OSA patients, particularly in the 16 females out of the 46 subjects (p = 0.06) though males had no difference [66].
It appears that effective treatment of OSA can help to decrease body weight, adiposity, and leptin concentrations, but the incidence of successful weight reduction is probably less than 50%. However, the loss of fat and increase of muscle mass may cancel each other, resulting in variable changes of body weight. Analysis of treatment outcome should involve monitoring of residual respiratory events and other measures of sleep quality, including insufficient sleep time, persistent sleep fragmentation, low sleep efficiency resulting from chronic insomnia, parasomnia, circadian phase delay sleep disorders, drug effects, and comorbidities. Finally, weight loss and resolution of hyperleptinemia are dependent on restoration of energy homeostasis. Normalizing sleep indicates a reversal of the increased energy expenditure due to frequent nocturnal arousals; thus, if caloric intake and activity levels remain the same, it is possible that the successfully treated OSA patient can gain weight. Therefore counseling for normal feeding behavior and increase of physical activity should be provided for every obese OSA patient.
A translational point linking the human and animal study evidence discussed above
Fig. 2 proposes potential interactions between OSA and blood leptin concentrations, and how CPAP may be effective. A role of leptin in sleep regulation is shown by its ability to decrease REM sleep and increase slow wave sleep [67]. However, leptin is not the main driving factor for increased total sleep time and NREM sleep in obesity, as seen in mice fed with a high-fat diet [68], ob/ob mice lacking leptin [40,41] and obese Zucker rats with defective ObR [69,70].
Astrocytic leptin signaling, however, may serve as a link. In the brain, the cellular targets for leptin show dynamic changes. Obesity induces upregulation of astrocytic leptin receptors [11,71], as do autoimmune disorders such as experimental autoimmune encephalomyelitis [72]. Astrocytes respond to leptin by induction of multiple signaling pathways and calcium influx [11–13], which in turn modulate neuronal activities. In obesity, activation of astrocytic leptin signaling further exacerbates metabolic functions [73]. Gliotransmitters released by astrocytes, such as adenosine, have been shown to modulate sleep pressure [74–76]. Thus, leptin might act through astrocytes to be a key regulator of sleep drive in obesity.
There are many unanswered questions about sleep and leptin. For example, what drives a reduction of leptin during sleep restriction? What mediates hyperleptinemia in OSA in the absence of obesity? Sleep fragmentation alone does not induce significant elevation of leptin; does it support a long-term relationship in the pathophysiological regulation leading to hyperleptinemia, or is it an experimental bias resulting from the fragmentation design? An immediate response of cytokines to OSA events [77] and effects of CPAP treatment have been demonstrated [78]. How does leptin interact with the proinflammatory cytokines during this dynamic process?
Conclusions
There is considerable disagreement among studies during the last decade examining the relation of sleep to blood leptin concentrations. The outcome would be more consistent once the study design takes into consideration the circadian rhythm of leptin and implants rigorous control of experimental conditions (such as sleep time, caloric intake, energy expenditure, adequate control groups, and efficient experimental interventions, particularly PAP treatment).
In most of the studies in the past decade summarized here, short sleep duration decreases fasting leptin, OSA frequently results in increased leptin, and effective PAP treatment decreases leptin in the long run. As hyperleptinemia is associated with leptin resistance and metabolic syndrome in obesity, it is conceivable that successful treatment of sleep disorders in the obese subjects or OSA-associated inflammation is marked by a reduction of leptin. In this sense, leptin can be a useful marker for treatment efficacy.
Practice points.
Short sleep duration usually decreases fasting blood leptin, a change that may facilitate feeding behavior and promote obesity in the long run.
While OSA alone increases leptin in less obese patients, obesity plays a greater role in inducing hyperleptinemia.
Successful long-term treatment of OSA with positive airway pressure is predicted to lower hyperleptinemia over time.
Research agenda.
To use the reduction of hyperleptinemia as a biomarker for efficient treatment of OSA, a standardized sampling protocol should be developed that takes into consideration the circadian rhythm, activity level, meal, sleep time, and absence of concurrent interfering measures such as glucose tolerance tests.
More studies are needed to determine disease mechanisms that can explain how sleep disturbance leads to abnormal leptin regulation, and how this is tied to metabolic dysregulation and autonomic nervous system activation.
Acknowledgments
The authors receive grant support from NIH (DK54880 and DK92245 to AJK, and NS62291 to WP). There are no conflicts of interest.
Abbreviations
- AHI
apnea—hypopnea index
- BBB
blood—brain barrier
- BMI
body mass index
- CNS
central nervous system
- CPAP
continuous positive airway pressure
- EDS
excessive daytime sleepiness
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- REM
rapid eye movement
References
*The most important references are denoted by an asterisk.
- 1.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 2.Banks WA. The many lives of leptin. Peptides. 2004;25:331–338. doi: 10.1016/j.peptides.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol. 2000;278:E1158–E1165. doi: 10.1152/ajpendo.2000.278.6.E1158. [DOI] [PubMed] [Google Scholar]
- 4.Kastin AJ, Akerstrom V. Fasting, but not adrenalectomy, reduces transport of leptin into the brain. Peptides. 2000;21:679–682. doi: 10.1016/s0196-9781(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 5.Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100:1882–1887. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci. 2003;73:2467–2475. doi: 10.1016/s0024-3205(03)00628-3. [DOI] [PubMed] [Google Scholar]
- 7.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90:2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saad MF, Riad-Gabriel MG, Khan A, Sharma A, Michael R, Jinagouda SD, et al. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab. 1998;83:453–459. doi: 10.1210/jcem.83.2.4532. [DOI] [PubMed] [Google Scholar]
- 9.Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, et al. Sex differences in circulating human leptin pulse amplitude: clinical implications. J Clin Endocrinol Metab. 1998;83:4140–4147. doi: 10.1210/jcem.83.11.5291. [DOI] [PubMed] [Google Scholar]
- 10.Kok SW, Meinders AE, Overeem S, Lammers GJ, Roelfsema F, Frolich M, et al. Reduction of plasma leptin levels and loss of its circadian rhythmicity in hypocretin (orexin)-deficient narcoleptic humans. J Clin Endocrinol Metab. 2002;87:805–809. doi: 10.1210/jcem.87.2.8246. [DOI] [PubMed] [Google Scholar]
- 11.Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, et al. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009;132:889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan W, Hsuchou H, He Y, Kastin AJ. The astroglial leptin receptors and obesity. In: Preedy VR, editor. Modern insights into disease – from molecules to man: adipokines. Enfield, NH, USA: Science Publishers; 2011. pp. 185–196. [Google Scholar]
- 13.Pan W, Hsuchou H, Jayaram B, Khan RS, Huang EYK, Wu X, et al. Leptin action on non-neuronal cells in the CNS: potential clinical implications. Ann N Y Acad Sci. 2012;1264:64–71. doi: 10.1111/j.1749-6632.2012.06472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 15.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013 doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–854. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel K, Leproult R, L’hermite-Baleriaux M, Copinschi G, Penev PD, Van CE. Leptin levels are dependent on sleep duration: relationships with sym-pathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 19.Knutson KL, Galli G, Zhao X, Mattingly M, Cizza G. No association between leptin levels and sleep duration or quality in obese adults. Obesity (Silver Spring) 2011;19:2433–2435. doi: 10.1038/oby.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benedict C, Shostak A, Lange T, Brooks SJ, Schioth HB, Schultes B, et al. Diurnal rhythm of circulating nicotinamide phosphoribosyltransferase (Nampt/visfatin/PBEF): impact of sleep loss and relation to glucose metabolism. J Clin Endocrinol Metab. 2012;97:E218–E222. doi: 10.1210/jc.2011-2241. [DOI] [PubMed] [Google Scholar]
- 21.Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17:331–334. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 22.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–1482. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 23.Klingenberg L, Chaput JP, Holmback U, Jennum P, Astrup A, Sjodin A. Sleep restriction is not associated with a positive energy balance in adolescent boys. Am J Clin Nutr. 2012;96:240–248. doi: 10.3945/ajcn.112.038638. [DOI] [PubMed] [Google Scholar]
- 24.Charles LE, Gu JK, Andrew ME, Violanti JM, Fekedulegn D, Burchfiel CM. Sleep duration and biomarkers of metabolic function among police officers. J Occup Environ Med. 2011;53:831–837. doi: 10.1097/JOM.0b013e31821f5ece. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds AC, Dorrian J, Liu PY, Van Dongen HP, Wittert GA, Harmer LJ, et al. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS One. 2012;7:e41218. doi: 10.1371/journal.pone.0041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Leeuwen WM, Hublin C, Sallinen M, Harma M, Hirvonen A, Porkka-Heiskanen T. Prolonged sleep restriction affects glucose metabolism in healthy young men. Int J Endocrinol. 2010;2010:108641. doi: 10.1155/2010/108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biol Res Nurs. 2010;12:47–53. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–656. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scarpace PJ, Matheny M, Pollock BH, Tumer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol. 1997;273:E226–E230. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- 32.Carter JR, Durocher JJ, Larson RA, DellaValla JP, Yang H. Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am J Physiol Heart Circ Physiol. 2012;302:H1991–H1997. doi: 10.1152/ajpheart.01132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegel K, Tasali E, Penev P, Van CE. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 34.Martins PJ, Fernandes L, de Oliveira AC, Tufik S, D’Almeida V. Type of diet modulates the metabolic response to sleep deprivation in rats. Nutr Metab (Lond) 2011;8:86. doi: 10.1186/1743-7075-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koban M, Swinson KL. Chronic REM-sleep deprivation of rats elevates metabolic rate and increases UCP1 gene expression in brown adipose tissue. Am J Physiol Endocrinol Metab. 2005;289:E68–E74. doi: 10.1152/ajpendo.00543.2004. [DOI] [PubMed] [Google Scholar]
- 36.Barf RP, Desprez T, Meerlo P, Scheurink AJ. Increased food intake and changes in metabolic hormones in response to chronic sleep restriction alternated with short periods of sleep allowance. Am J Physiol Regul Integr Comp Physiol. 2012;302:R112–R117. doi: 10.1152/ajpregu.00326.2011. [DOI] [PubMed] [Google Scholar]
- 37.Pan W, Kastin AJ. Diurnal variation of leptin entry from blood to brain involving partial saturation of the transport system. Life Sci. 2001;68:2705–2714. doi: 10.1016/s0024-3205(01)01085-2. [DOI] [PubMed] [Google Scholar]
- 38.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 39.Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr. 2012:1–9. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- 40.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R894–R903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 41.Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2059–R2066. doi: 10.1152/ajpregu.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everson CA, Szabo A. Repeated exposure to severely limited sleep results in distinctive and persistent physiological imbalances in rats. PLoS One. 2011;6:e22987. doi: 10.1371/journal.pone.0022987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. J Am Med Assoc. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 44.McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med. 2007;175:190–195. doi: 10.1164/rccm.200602-270OC. [DOI] [PubMed] [Google Scholar]
- 45.Seif F, Patel SR, Walia H, Rueschman M, Bhatt DL, Gottlieb DJ, et al. Association between obstructive sleep apnoea severity and endothelial dysfunction in an increased background of cardiovascular burden. J Sleep Res. 2013;22:443–451. doi: 10.1111/jsr.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatsumi K, Kasahara Y, Kurosu K, Tanabe N, Takiguchi Y, Kuriyama T. Sleep oxygen desaturation and circulating leptin in obstructive sleep apnea-hypopnea syndrome. Chest. 2005;127:716–721. doi: 10.1378/chest.127.3.716. [DOI] [PubMed] [Google Scholar]
- 47.Kapsimalis F, Varouchakis G, Manousaki A, Daskas S, Nikita D, Kryger M, et al. Association of sleep apnea severity and obesity with insulin resistance, C-reactive protein, and leptin levels in male patients with obstructive sleep apnea. Lung. 2008;186:209–217. doi: 10.1007/s00408-008-9082-x. [DOI] [PubMed] [Google Scholar]
- 48.Patel SR, Palmer LJ, Larkin EK, Jenny NS, White DP, Redline S. Relationship between obstructive sleep apnea and diurnal leptin rhythms. Sleep. 2004;27:235–239. doi: 10.1093/sleep/27.2.235. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-de-la-Torre M, Mediano O, Barcelo A, Pierola J, de la Pena M, Esquinas C, et al. The influence of obesity and obstructive sleep apnea on metabolic hormones. Sleep Breath. 2012;16:649–656. doi: 10.1007/s11325-011-0552-7. [DOI] [PubMed] [Google Scholar]
- 50.Fredheim JM, Rollheim J, Omland T, Hofso D, Roislien J, Vegsgaard K, et al. Type 2 diabetes and pre-diabetes are associated with obstructive sleep apnea in extremely obese subjects: a cross-sectional study. Cardiovasc Diabetol. 2011;10:84. doi: 10.1186/1475-2840-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barcelo A, Barbe F, Llompart E, de la Pena M, Duran-Cantolla J, Ladaria A, et al. Neuropeptide Y and leptin in patients with obstructive sleep apnea syndrome: role of obesity. Am J Respir Crit Care Med. 2005;171:183–187. doi: 10.1164/rccm.200405-579OC. [DOI] [PubMed] [Google Scholar]
- 52.Jayaram B, Pan W, Wang Y, Hsuchou H, Mace A, Cornelissen-Guillaume GG, et al. Astrocytic leptin-receptor knockout mice show partial rescue of leptin resistance in diet-induced obesity. J Appl Physiol. 2013;114:734–741. doi: 10.1152/japplphysiol.01499.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yee BJ, Cheung J, Phipps P, Banerjee D, Piper AJ, Grunstein RR. Treatment of obesity hypoventilation syndrome and serum leptin. Respiration. 2006;73:209–212. doi: 10.1159/000088358. [DOI] [PubMed] [Google Scholar]
- 54.Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour SS, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22:251–257. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 55.Cuhadaroglu C, Utkusavas A, Ozturk L, Salman S, Ece T. Effects of nasal CPAP treatment on insulin resistance, lipid profile, and plasma leptin in sleep apnea. Lung. 2009;187:75–81. doi: 10.1007/s00408-008-9131-5. [DOI] [PubMed] [Google Scholar]
- 56.Trenell MI, Ward JA, Yee BJ, Phillips CL, Kemp GJ, Grunstein RR, et al. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab. 2007;9:679–687. doi: 10.1111/j.1463-1326.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 57.Nakra N, Bhargava S, Dzuira J, Caprio S, Bazzy-Asaad A. Sleep-disordered breathing in children with metabolic syndrome: the role of leptin and sympathetic nervous system activity and the effect of continuous positive airway pressure. Pediatrics. 2008;122:e634–e642. doi: 10.1542/peds.2008-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zirlik S, Hauck T, Fuchs FS, Neurath MF, Konturek PC, Harsch IA. Leptin, obestatin and apelin levels in patients with obstructive sleep apnoea syndrome. Med Sci Monit. 2011;17:CR159–CR164. doi: 10.12659/MSM.881450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanner BM, Kollhosser P, Buechner N, Zidek W, Tepel M. Influence of treatment on leptin levels in patients with obstructive sleep apnoea. Eur Respir J. 2004;23:601–604. doi: 10.1183/09031936.04.00067804. [DOI] [PubMed] [Google Scholar]
- 60.Huang R, Huang XZ, Wang HG, Li M, Xiao Y. Effects of nasal continuous positive airway pressure on serum leptin concentration and the metabolic parameters in obstructive sleep apnea hypopnea syndrome. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004;26:168–171. [PubMed] [Google Scholar]
- 61.Rubinsztajn R, Kumor M, Byskiniewicz K, Chazan R. The influence of 3 weeks therapy with continuous positive airway pressure on serum leptin and ho-mocysteine concentration in patients with obstructive sleep apnea syndrome. Pneumonol Alergol Pol. 2006;74:63–67. [PubMed] [Google Scholar]
- 62.Drummond M, Winck JC, Guimaraes JT, Santos AC, Almeida J, Marques JA. Autoadjusting-CPAP effect on serum leptin concentrations in obstructive sleep apnoea patients. BMC Pulm Med. 2008;8:21. doi: 10.1186/1471-2466-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia JM, Sharafkhaneh H, Hirshkowitz M, Elkhatib R, Sharafkhaneh A. Weight and metabolic effects of CPAP in obstructive sleep apnea patients with obesity. Respir Res. 2011;12:80. doi: 10.1186/1465-9921-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight less in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97:896–897. doi: 10.1016/s0002-8223(97)00220-4. [DOI] [PubMed] [Google Scholar]
- 65.Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–712. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 66.Redenius R, Murphy C, O’Neill E, Al-Hamwi M, Zallek SN. Does CPAP lead to change in BMI? J Clin Sleep Med. 2008;4:205–209. [PMC free article] [PubMed] [Google Scholar]
- 67.Sinton CM, Fitch TE, Gershenfeld HK. The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res. 1999;8:197–203. doi: 10.1046/j.1365-2869.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- 68.Jenkins JB, Omori T, Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased in mice with obesity induced by high-fat food. Physiol Behav. 2006;87:255–262. doi: 10.1016/j.physbeh.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 69.Danguir J. Sleep patterns in the genetically obese Zucker rat: effect of acar-bose treatment. Am J Physiol. 1989;256:R281–R283. doi: 10.1152/ajpregu.1989.256.1.R281. [DOI] [PubMed] [Google Scholar]
- 70.Megirian D, Dmochowski J, Farkas GA. Mechanism controlling sleep organization of the obese Zucker rats. J Appl Physiol. 1998;84:253–256. doi: 10.1152/jappl.1998.84.1.253. [DOI] [PubMed] [Google Scholar]
- 71.Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, et al. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology. 2008;149:2798–2806. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu X, Mishra PK, Hsuchou H, Kastin AJ, Pan W. Upregulation of astrocytic leptin receptor in mice with experimental autoimmune encephalomyelitis. J Mol Neurosci. 2013;49:446–456. doi: 10.1007/s12031-012-9825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan W, Hsuchou H, Xu CL, Wu X, Bouret SG, Kastin AJ. Astrocytes modulate distribution and neuronal signaling of leptin in the hypothalamus of obese Avy mice. J Mol Neurosci. 2011;43:478–484. doi: 10.1007/s12031-010-9470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–629. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alberti A, Sarchielli P, Gallinella E, Floridi A, Floridi A, Mazzotta G, et al. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res. 2003;12:305–311. doi: 10.1111/j.1365-2869.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 78.Steiropoulos P, Kotsianidis I, Nena E, Tsara V, Gounari E, Hatzizisi O, et al. Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. Sleep. 2009;32:537–543. doi: 10.1093/sleep/32.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Basoglu OK, Sarac F, Sarac S, Uluer H, Yilmaz C. Metabolic syndrome, insulin resistance, fibrinogen, homocysteine, leptin, and C-reactive protein in obese patients with obstructive sleep apnea syndrome. Ann Thorac Med. 2011;6:120–125. doi: 10.4103/1817-1737.82440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ulukavak CT, Kokturk O, Bukan N, Bilgihan A. Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome. Respiration. 2005;72:395–401. doi: 10.1159/000086254. [DOI] [PubMed] [Google Scholar]
- 81.Ozturk L, Unal M, Tamer L, Celikoglu F. The association of the severity of obstructive sleep apnea with plasma leptin levels. Arch Otolaryngol Head Neck Surg. 2003;129:538–540. doi: 10.1001/archotol.129.5.538. [DOI] [PubMed] [Google Scholar]
- 82.Hargens TA, Guill SG, Kaleth AS, Nickols-Richardson SM, Miller LE, Zedalis D, et al. Insulin resistance and adipose-derived hormones in young men with untreated obstructive sleep apnea. Sleep Breath. 2013;17:403–409. doi: 10.1007/s11325-012-0708-0. [DOI] [PubMed] [Google Scholar]
- 83.Papaioannou I, Patterson M, Twigg GL, Vazir A, Ghatei M, Morrell MJ, et al. Lack of association between impaired glucose tolerance and appetite regulating hormones in patients with obstructive sleep apnea. J Clin Sleep Med. 2011;7:486–492. doi: 10.5664/JCSM.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ursavas A, Ilcol YO, Nalci N, Karadag M, Ege E. Ghrelin, leptin, adiponectin, and resistin levels in sleep apnea syndrome: role of obesity. Ann Thorac Med. 2010;5:161–165. doi: 10.4103/1817-1737.65050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma SK, Kumpawat S, Goel A, Banga A, Ramakrishnan L, Chaturvedi P. Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med. 2007;8:12–17. doi: 10.1016/j.sleep.2006.06.014. [DOI] [PubMed] [Google Scholar]